Abstract
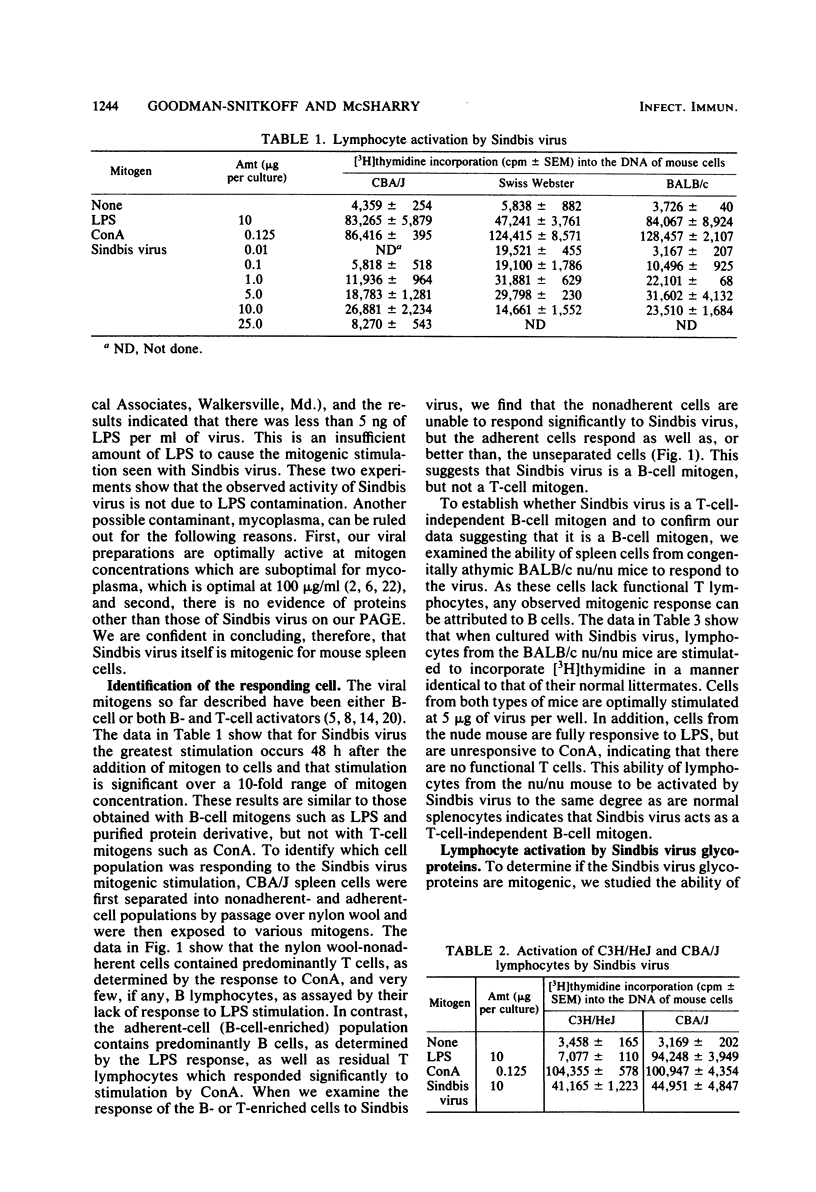
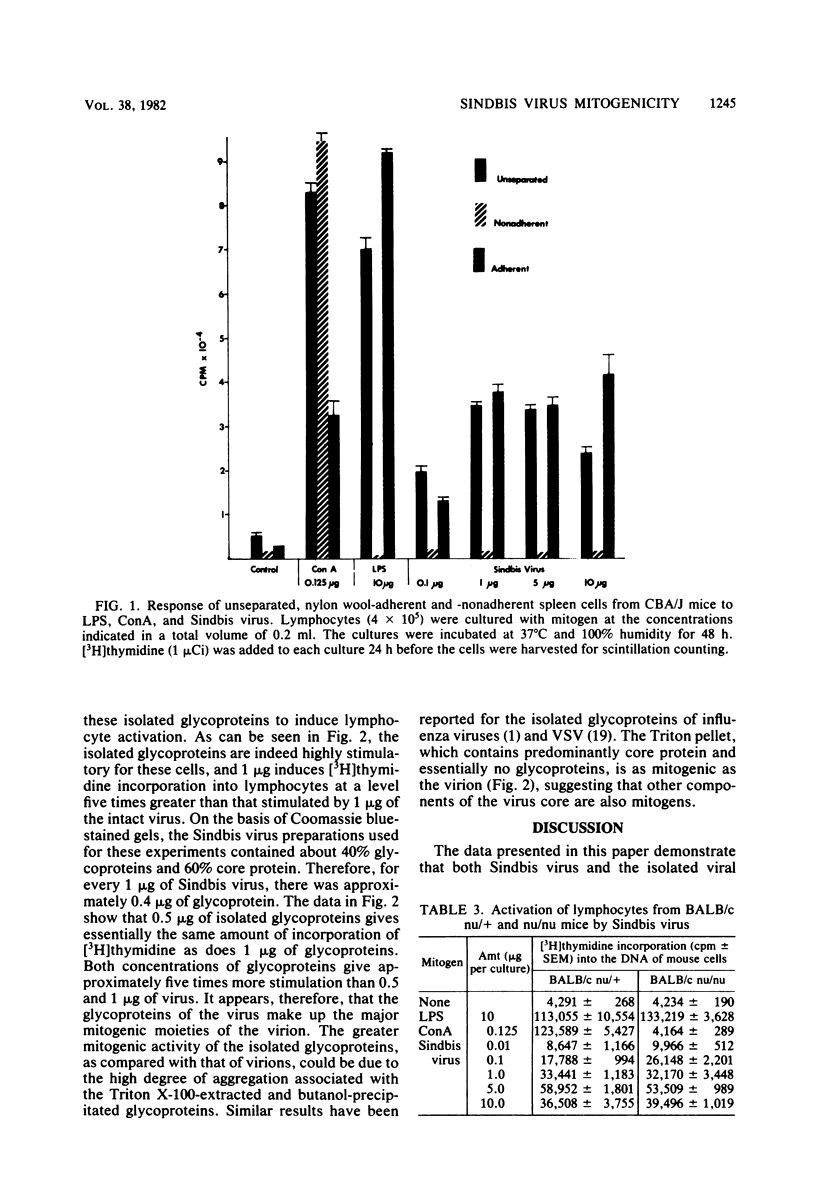
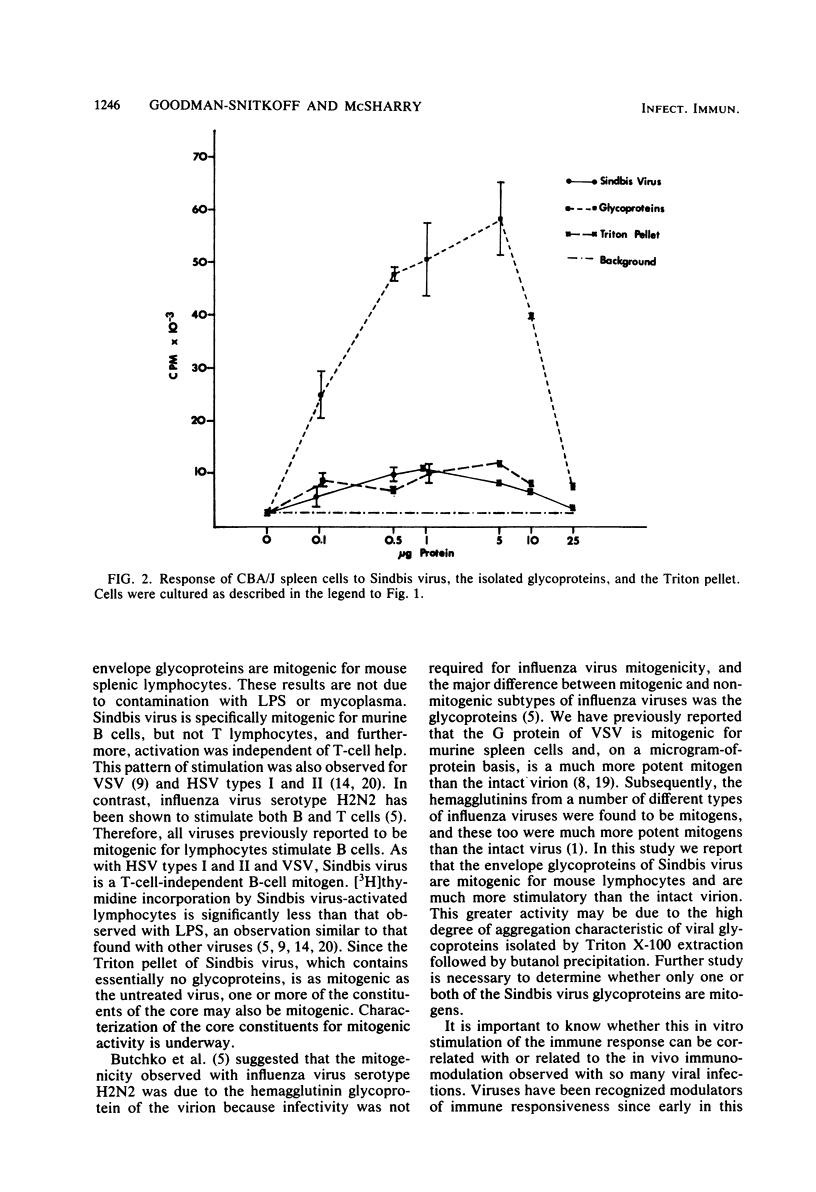
Purified preparations of Sindbis virus, a member of the togavirus family, are mitogenic for lymphocytes from a number of different mouse strains. Cell separation techniques, as well as studies using lymphocytes from the congenitally athymic BALB/c nu/nu mouse, showed that Sindbis virus is a T-cell-independent B-cell mitogen. Additionally, the envelope glycoproteins of Sindbis virus, isolated by Triton X-100 extraction and butanol precipitation, stimulated lymphocytes to incorporate five times as much [3H]thymidine into their DNA as did the Sindbis virion. These results are similar to those previously reported for vesicular stomatitis virus and herpes simplex virus types I and II and for the purified glycoproteins of vesicular stomatitis virus and influenza virus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. B., Butchko G. M., Kiley S. C., Phelan M. A., Ennis F. A. Mitogenicity of influenza hemagglutinin glycoproteins and influenza viruses bearing H2-hemagglutinin. Infect Immun. 1981 Oct;34(1):140–143. doi: 10.1128/iai.34.1.140-143.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberfeld G., Gronowicz E. Mycoplasma pneumoniae is a polyclonal B-cell activator. Nature. 1976 May 20;261(5557):238–239. doi: 10.1038/261238a0. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Jimenez L., Marcus P. I. A plaque assay for enumerating antigen-sensitive cells in delayed-type hypersensitivity. J Exp Med. 1970 Jul 1;132(1):16–30. doi: 10.1084/jem.132.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Senik A., Stoner G., Ju G., Nowakowski M., Kano S., Jimenez L. Studies on the interactions between viruses and lymphocytes. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):73–83. doi: 10.1101/sqb.1977.041.01.011. [DOI] [PubMed] [Google Scholar]
- Butchko G. M., Armstrong R. B., Martin W. J., Ennis F. A. Influenza A viruses of the H2N2 subtype are lymphocyte mitogens. Nature. 1978 Jan 5;271(5640):66–67. doi: 10.1038/271066a0. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Daynes R. A., Ward J. R. Mycoplasma-mediated activation of normal mouse lymphocytes: induction of cytotoxic lymphocytes and lymphocyte transformation by Mycoplasma arthritidis are under Ir gene control. J Immunol. 1981 Mar;126(3):922–927. [PubMed] [Google Scholar]
- Goodman-Snitkoff G. W., McSharry J. J. Activation of mouse lymphocytes by vesicular stomatitis virus. J Virol. 1980 Sep;35(3):757–765. doi: 10.1128/jvi.35.3.757-765.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman-Snitkoff G., Mannino R. J., McSharry J. J. The glycoprotein isolated from vesicular stomatitis virus is mitogenic for mouse B lymphocytes. J Exp Med. 1981 Jun 1;153(6):1489–1502. doi: 10.1084/jem.153.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman G. W., Sultzer B. M. Mild alkaline hydrolysis of lipopolysaccharide endotoxin enhances its mitogencity for murine B cells. Infect Immun. 1977 Jul;17(1):205–214. doi: 10.1128/iai.17.1.205-214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger J. S., Phillips S. M., Stephenson J. R., Aaronson S. A. Induction of mouse type-C RNA virus by lipopolysaccharide. J Immunol. 1975 Jul;115(1):317–320. [PubMed] [Google Scholar]
- Hodes R. J., Handwerger B. S., Terry W. D. Synergy between subpopulations of mouse spleen cells in the in vitro generation of cell-mediated cytotoxicity: evidence for the involvement of a non-T cell. J Exp Med. 1974 Dec 1;140(6):1646–1659. doi: 10.1084/jem.140.6.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura Y., Takaoki M., Kono Y., Minato N. Augmentation of delayed-type hypersensitivity by vesicular stomatitis virus infection in mice. J Immunol. 1980 Oct;125(4):1459–1462. [PubMed] [Google Scholar]
- Kelsey D. K., Overall J. C., Jr, Glasgow L. A. Correlation of the suppression of mitogen responsiveness and the mixed lymphocyte reaction with the proliferative response to viral antigen of splenic lymphocytes from cytomegalovirus-infected mice. J Immunol. 1978 Aug;121(2):464–470. [PubMed] [Google Scholar]
- Kirchner H., Darai G., Hirt H. M., Keyssner K., Munk K. In vitro mitogenic stimulation of murine spleen cells by herpes simplex virus. J Immunol. 1978 Feb;120(2):641–645. [PubMed] [Google Scholar]
- Kirchner H., Hirt H. M., Kleinicke C., Munk K. Replication of herpes simplex virus in mouse spleen cell cultures stimulated by lipopolysaccharide. J Immunol. 1976 Nov;117(5 PT2):1753–1756. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mathes L. E., Olsen R. G., Hebebrand L. C., Hoover E. A., Schaller J. P., Adams P. W., Nichols W. S. Immunosuppressive properties of a virion polypeptide, a 15,000-dalton protein, from feline leukemia virus. Cancer Res. 1979 Mar;39(3):950–955. [PubMed] [Google Scholar]
- Mochizuki D., Hedrick S., Watson J., Kingsbury D. T. The interaction of Herpes Simplex Virus with murine lymphocytes. I. Mitogenic properties of herpes simplex virus. J Exp Med. 1977 Dec 1;146(6):1500–1510. doi: 10.1084/jem.146.6.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni C., Schumann G. Mitogen induction of murine C-type viruses. II. Effect of B-lymphocyte mitogens. Virology. 1976 Aug;73(1):17–22. doi: 10.1016/0042-6822(76)90056-8. [DOI] [PubMed] [Google Scholar]
- Naot Y., Ginsburg H. Activation of B lymphocytes by mycoplasma mitogen(s). Immunology. 1978 Apr;34(4):715–720. [PMC free article] [PubMed] [Google Scholar]
- Okunewick J. P., Meredith R. F., Brozovich B., Weaver E. V. Stimulation of immune response in hybrid mice following Rauscher virus infection. Proc Soc Exp Biol Med. 1978 Mar;157(3):449–452. doi: 10.3181/00379727-157-40074. [DOI] [PubMed] [Google Scholar]
- Phillips S. M., Stephenson J. R., Greenberger J. S., Lane P. E., Aaronson S. A. Release of xenotropic type C RNA virus in response to lipopolysaccharide: acitivity of lipid-A portion upon B lymphocytes. J Immunol. 1976 Apr;116(4):1123–1128. [PubMed] [Google Scholar]
- Semenov B. F. Viruses as nonspecific modulators of immunological reactivity. Acta Virol. 1981 Mar;25(2):122–128. [PubMed] [Google Scholar]
- Sultzer B. M., Goodman G. W. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ lymphocytes. J Exp Med. 1976 Sep 1;144(3):821–827. doi: 10.1084/jem.144.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M., Nilsson B. S. PPD tuberculin--a B-cell mitogen. Nat New Biol. 1972 Dec 13;240(102):198–200. doi: 10.1038/newbio240198a0. [DOI] [PubMed] [Google Scholar]
- Tinghitella T. J., Booss J. Enhanced immune response late in primary cytomegalovirus infection of mice. J Immunol. 1979 Jun;122(6):2442–2446. [PubMed] [Google Scholar]
- Wheelock E. F., Toy S. T. Participation of lymphocytes in viral infections. Adv Immunol. 1973;16:123–184. doi: 10.1016/s0065-2776(08)60297-7. [DOI] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. T lymphocyte interaction with viruses and virus-infected tissues. Prog Med Virol. 1975;19:120–160. [PubMed] [Google Scholar]


