Abstract
We have previously demonstrated that quercetin, a bioflavonoid, blocks hepatitis C virus (HCV) proliferation by inhibiting NS5A-driven internal ribosomal entry site (IRES)-mediated translation of the viral genome. Here, we investigate the mechanisms of antiviral activity of quercetin and six additional bioflavonoids. We demonstrate that catechin, naringenin, and quercetin possess significant antiviral activity, with no associated cytotoxicity. Infectious virion secretion was not significantly altered by these bioflavonoids. Catechin and naringenin demonstrated stronger inhibition of infectious virion assembly compared to quercetin. Quercetin markedly blocked viral translation whereas catechin and naringenin demonstrated mild activity. Similarly quercetin completely blocked NS5A-augmented IRES-mediated translation in an IRES reporter assay, whereas catechin and naringenin had only a mild effect. Moreover, quercetin differentially inhibited HSP70 induction compared to catechin and naringenin. Thus, the antiviral activity of these bioflavonoids is mediated through different mechanisms. Therefore combination of these bioflavonoids may act synergistically against HCV.
Keywords: HSP70, NS5A, IRES, HCV, Bioflavonoid
Introduction
The hepatitis C virus (HCV) infects 3% of the world population and is mainly responsible for liver transplantation in patients with cirrhosis in developed countries (Shepard et al., 2005). Furthermore, HCV is the most common chronic blood borne pathogen in the United States (U.S.) affecting 1.8% of the population and is the major etiologic factor responsible for the recent doubling of hepatocellular carcinoma (HCC) (El-Serag, 2002).
HCV infection is currently treated by pegylated interferon-α (PEG-IFN) and ribavirin with a sustained virologic response (SVR) of only 50–56% in patients with genotype 1 (Gambarin-Gelwan and Jacobson, 2008). Adverse side effects and contraindications are common for therapy of all genotypes as well (McHutchison and Patel, 2002). Recently, NS3/4A protease inhibitors in combination with PEG-IFN and ribavirin led to an increased SVR, but also increased adverse events including anemia and gastrointestinal symptoms (Ciesek and Manns, 2011). Nevertheless, a significant number of patients cannot receive these treatments as they require PEG-IFN. For these reasons, there is the need to develop adjunct or replacement therapies that are less toxic and more efficacious in terms of higher SVR rates and HCC prevention.
The 5′ non-coding region (NCR) of the viral genome possesses an internal ribosomal entry site (IRES) (Wang et al., 1993), a cis-acting element found in some host RNA transcripts as well as in viruses that allows ribosomal translation initiation to occur internally within a transcript in lieu of 5′ cap dependent translation (Pacheco and Martinez-Salas, 2010). The HCV viral life cycle in a cell can be divided into six phases: 1) binding and internalization, 2) cytoplasmic release and uncoating 3) viral polyprotein translation and processing, 4) RNA genome replication, 5) packaging and assembly, and 6) virus morphogenesis and secretion (Moradpour et al., 2007).
The viral nonstructural protein 5A (NS5A), a 56–59 kDa phosphoprotein, is a multi-functional protein and a component of the viral replicase complex. It has been implicated in regulation of HCV genome replication, viral protein translation, virion assembly, and viral secretion (He et al., 2003; Hughes et al., 2009; Tellinghuisen et al., 2008). We have previously identified, through co-immunoprecipitation, an NS5A/HSP complex composed of NS5A, HSP70, and HSP40 (cofactor of HSP70) and demonstrated their colocalization in Huh-7 cells (Gonzalez et al., 2009). We further showed that both NS5A-augmented IRES-mediated translation and virus production are blocked by 1) HSP70 knockdown, 2) the HSP synthesis inhibitor quercetin, a bioflavonoid, and 3) a small peptide from NS5A domain I that is capable of blocking NS5A/HSP70 interaction, with no associated cytotoxicity (Gonzalez et al., 2009; Khachatoorian et al., 2012). These findings support our hypothesis that HCV utilizes an NS5A/HSP complex to facilitate IRES-mediated translation of its genome and that disruption of this complex by quercetin-mediated knockdown of HSP70 blocks viral proliferation, implicating quercetin as a potential HCV treatment option.
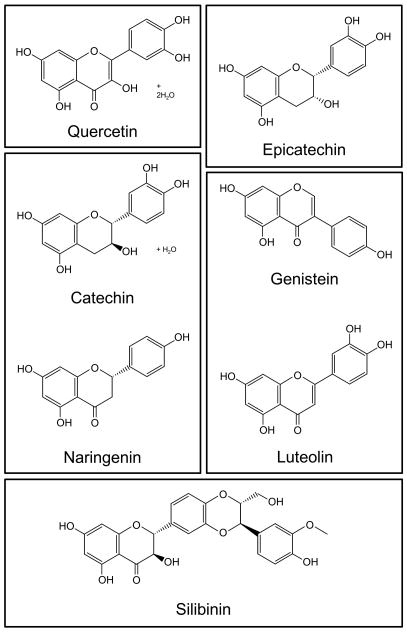
Bioflavonoids are a group of plant secondary metabolites that serve a variety of functions in plants including pigmentation and resistance to predators. The basic structure of bioflavonoids consists of two phenyl moieties linked together by 3 carbons (Figure 1A). A variety of functional groups occur at different positions on this backbone to give rise to the large selection of these naturally occurring compounds. In recent years, bioflavonoids have been extensively studied for their health benefits.
Figure 1.
Molecular structure of the bioflavonoids. Bioflavonoid molecular structures are adapted from Sigma-Aldrich. For naringenin, the structure of the (S) enantiomer is shown; however, the chemical is a racemic mixture of both enantiomers. Silibinin is the major active component of silymarin.
In this study, we sought to examine, in more detail, the effect of quercetin and a number of bioflavonoids structurally related to quercetin on viral proliferation and to obtain further insights into the mechanisms of bioflavonoid-mediated suppression of HCV production.
Results
We have previously shown that quercetin blocks virus production with no associated cytotoxicity (Gonzalez et al., 2009). Here, we sought to determine if a number of other bioflavonoids structurally related to quercetin would also possess antiviral activity. We tested a total of seven compounds from a variety of bioflavonoid groups: catechin and epicatechin (flavanols), genistein (an isoflavone), luteolin (a flavone), naringenin (a flavanone), quercetin (a flavonol), and silymarin (a mixture of flavonolignans, were silibinin is the major active component (Wellington and Jarvis, 2001)) (Figure 1).
Screening of bioflavonoids for cellular toxicity
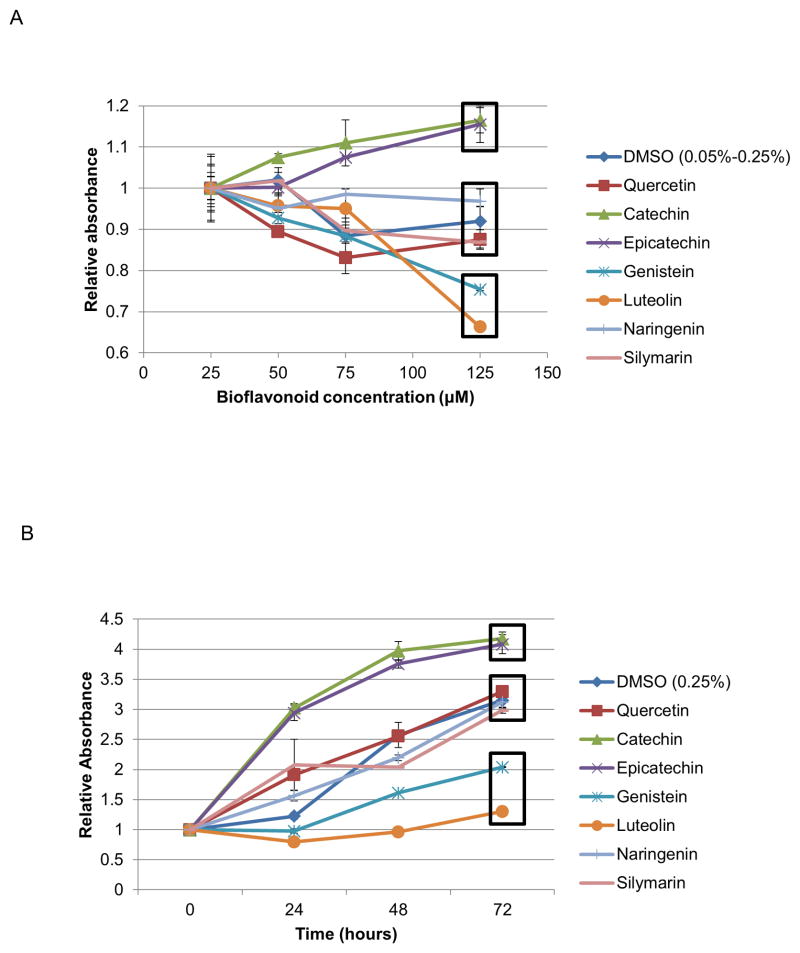
We first determined the cellular toxicity of these seven compounds at a concentration range of 25 and 125μM using standard MTT assays. As shown in Figure 2A, the bioflavonoids can be divided into three groups based on their cytotoxicity profiles. Genistein and luteolin, potential chemotherapeutic agents, display significant cytotoxicity and were not studied further. Naringenin, quercetin, and silymarin possess a cytotoxicity profile similar to DMSO carrier. Interestingly, catechin and epicatechin result in significantly increased absorbance. This may reflect an increase in metabolism, cell division, or cell size; decreased apoptosis; or increased mitochondrial biogenesis. This is a subject of ongoing study. In a separate experiment, we analyzed the toxicity of all bioflavonoids in a time course of 72 hours, and we obtained a similar toxicity profile (Figure 2B).
Figure 2.
Cytotoxicity of bioflavonoids as determined by MTT assays A. Bioflavonoid cellular toxicity profile in a concentration range of 25–125μM. MTT assays were performed 72 hours post treatment. B. Bioflavonoid cellular toxicity profile in a time course of 72 hours at 125μM. All data was normalized to time 0. Boxes indicate flavonoids grouped together based on similar toxicity profiles. (Error bars reflect standard deviation.)
Catechin, quercetin, and naringenin significantly attenuate HCV production in a dose-dependent manner
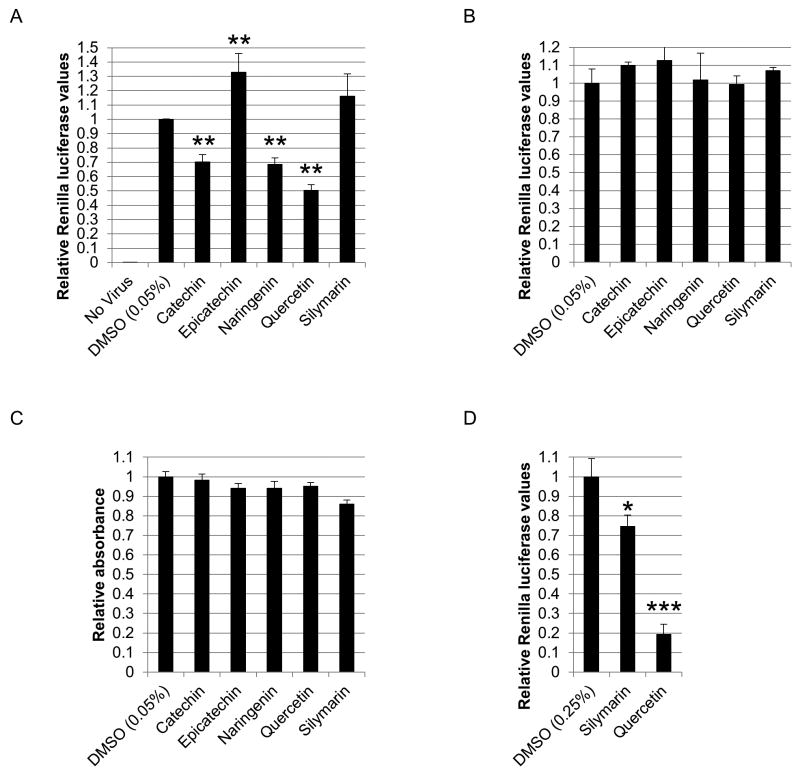
Next, we tested the bioflavonoids for their antiviral activity using the HCV cell culture (HCVcc) system measuring intracellular levels of HCV-driven protein expression. Based on our preliminary analyses, we found the concentration range of 25–125μM bioflavonoids to be the most informative for the assays conducted in this study in terms of distinguishing bioflavonoid effects on the stages of viral life cycle. Initially, huh-7.5 cells were infected with the reporter virus and treated with 25μM bioflavonoid for 48 hours, followed by measuring luciferase activity. Quercetin and naringenin displayed significant antiviral activity in agreement with previous reports (Goldwasser et al., 2011; Gonzalez et al., 2009) (Figure 3A). In addition, we found catechin to possess significant antiviral activity as well (Figure 3A). Interestingly, epicatechin, a diastereoisomer of catechin, displayed a slight increase in virus production (Figure 3A). We are currently investigating the opposite effects of catechin and epicatechin on viral proliferation levels. The observed antiviral activities were not indirect effects of bioflavonoids on luciferase expression as determined independently by transfection of a plasmid expressing Renilla luciferase (Figure 3B). Silymarin did not result in viral attenuation at 25μM dose (Figure 3A). None of the compounds displayed any cytotoxicity in MTT assays (Figure 3C). We speculated that higher concentrations of silymarin may display some antiviral activity as reported previously (Wagoner et al., 2010). As shown in Figure 3D, 125μM silymarin resulted in a modest antiviral activity in a 48 hour assay. However, silymarin was not studied further due to its significantly lower antiviral activity compared with the other bioflavonoids.
Figure 3.
Bioflavonoid antiviral activity. A. Effect of 25μM bioflavonoids on viral proliferation. Huh-7.5 cells were infected with the reporter virus and immediately treated with bioflavonoids. 48 hours post treatment, luciferase activity was assayed. B. Effect of bioflavonoids on Renilla activity. Luciferase assays were performed on cells transfected with pRL-TK plasmid. C. MTT assays performed in parallel with assays in parts A and B. D. Effect of 125μM silymarin on viral proliferation. Huh-7.5 cells were infected with the reporter virus and treated with bioflavonoids for 48 hours as in part A, followed by luciferase assay. (*, **, and *** indicate P<0.05, P<0.005, and P<0.0005, respectively. Error bars reflect standard deviation.)
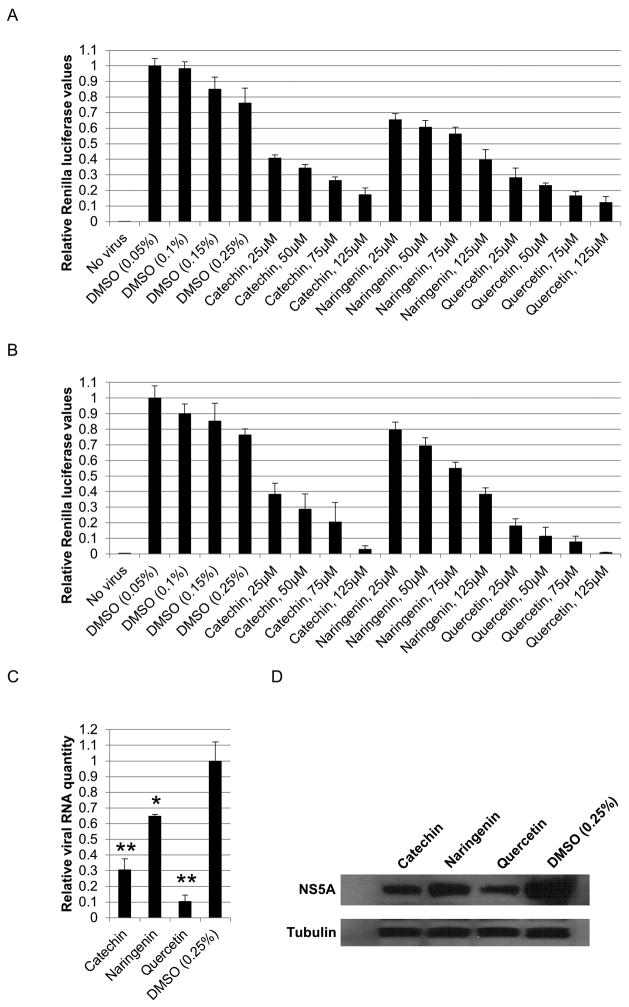
Intracellular viral levels were also determined with a treatment range of 25–125μM catechin, naringenin, and quercetin for 72 hours. All three compounds displayed a dose-dependent antiviral activity with quercetin being the most potent bioflavonoid followed by catechin and naringenin (Figure 4A). To further confirm the antiviral activity of these compounds, the supernatants of these cultures were concentrated 30-fold to remove approximately 97% of bioflavonoids, and the concentrated supernatants were used to infect naïve cells. As shown in Figure 4B, infectious virus production was significantly decreased in a dose-dependent manner.
Figure 4.
Dose dependent antiviral activity of catechin, naringenin, and quercetin. A. Huh-7.5 cells were infected with the Renilla reporter virus and immediately treated with a concentration range of 25-125μM of each bioflavonoid for 72 hours, followed by measuring luciferase activity. B. Luciferase assays performed on cells infected with the concentrated supernatants of the cells in figure 4A. Huh-7.5 cells were infected with supernatants and harvested 72 hours later. C. Bioflavonoid effect on viral genome levels. Huh-7.5 cells were infected with the reporter virus and treated with 125μM bioflavonoid for 72 hours followed by quantitative reverse-transcriptase PCR. D. Bioflavonoid effect on viral protein levels. Western analyses for NS5A and loading control tubulin was performed the same samples from part C. (* and ** indicate P<0.05 and P<0.005, respectively. Error bars reflect standard deviation.)
To further confirm the antiviral activity of catechin, naringenin, and quercetin, huh-7.5 cells were infected and treated with catechin, naringenin, and quercetin for 72 hours as above, followed by measuring viral RNA levels by quantitative reverse transcriptase PCR as well as assaying NS5A protein levels by Western analysis. As shown in Figure 4C and 4D, all three compounds significantly reduced NS5A RNA and protein levels, in agreement with the luciferase reporter levels.
Quercetin markedly inhibits intracellular viral protein production compared to catechin and naringenin
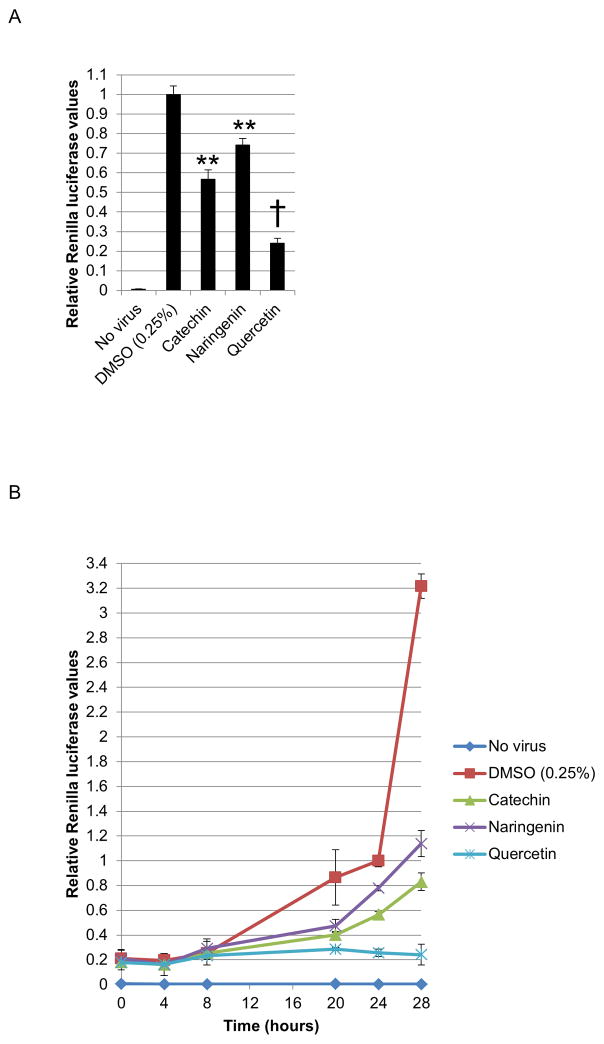
We proceeded to determine the mechanism(s) of action of catechin, quercetin, and naringenin, using the HCVcc system. To test the bioflavonoid inhibition of viral protein production, huh-7.5 cells were infected and immediately treated with 125μM of bioflavonoid. 125μM concentration was chosen as it was optimal for distinguishing the mechanism of antiviral activity of the bioflavonoids. Luciferase assays 20 hours after treatment showed that catechin, quercetin, and naringenin significantly inhibited intracellular viral protein translation, with quercetin demonstrating more than two-fold higher activity than catechin and naringenin (Figure 5A). The 20-hour assay time is crucial to limit the assay to one viral life cycle (see below) and eliminate any possible effects of bioflavonoids on other stages of viral life cycle such as virion assembly and secretion.
Figure 5.
Bioflavonoid effect on intracellular viral protein production. A. Huh-7.5 cells were infected with the reporter virus and immediately treated with 125μM bioflavonoid. 20 hours later, luciferase levels were measured. B. Luciferase assays performed in a similar way, but at different time points after infection. All values were normalized to the 24 hour time point. (** and † indicate P<0.005 and P<0.000005, respectively. Error bars reflect standard deviation).
We also monitored translation levels within 28 hours after infection. As shown in Figure 5B, with no bioflavonoid treatment, viral translation levels steadily increased followed by sharp burst in viral translation between 24 and 28 hour time points. This was interpreted to result from secondary infection. From this we inferred that the viral cycle duration is between 20–24 hours. Bioflavonoid treatment significantly decreased viral protein production during one viral life cycle and afterwards (Figure 5B). Quite interestingly, quercetin, our most potent bioflavonoid, inhibits any increase in viral protein levels during and after one viral life cycle (Figure 5B).
Infectious virion secretion is not inhibited by catechin, naringenin, and quercetin
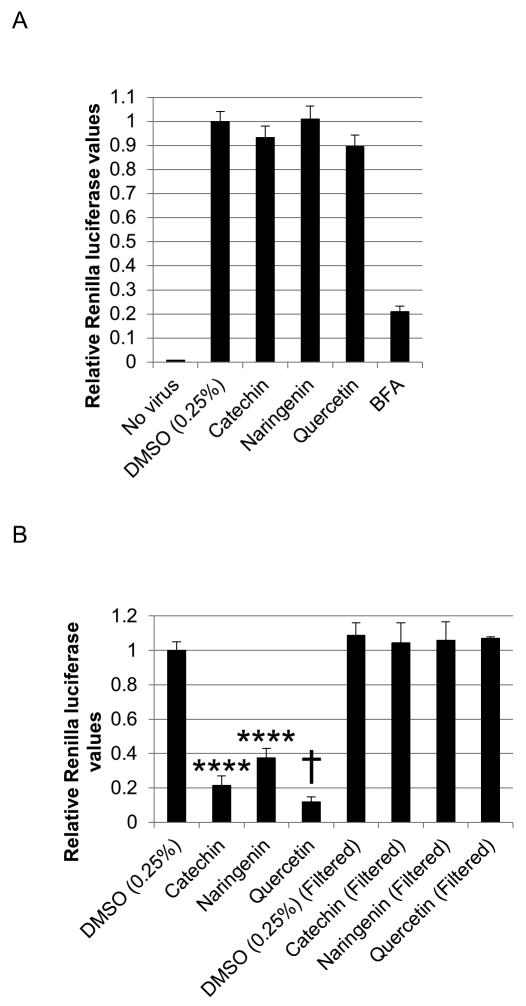
To determine the effect of bioflavonoids on infectious virion secretion, huh-7.5 cells were infected with the reporter virus. After 24 hours, allowing for sufficient accumulation of intracellular virus, cells were washed to remove secreted virions and treated with 125μM bioflavonoid for 5 hours. This limited time of treatment is necessary to minimize their effect on viral protein production. Subsequently, supernatants were concentrated 30-fold to remove approximately 97% of bioflavonoids. Elimination of bioflavonoids is necessary to exclude their potential subsequent effects on infection of naïve cells. The concentrated supernatants were used to infect naïve cells. While catechin and quercetin slightly affected viral secretion compared with DMSO, the differences were not statistically significant and may reflect their potent translation inhibitory effects during the 5 hour infection period (Figure 6A). As a positive control, treatment with Brefeldin A (BFA), an inhibitor of Golgi-dependent secretion including viral secretion (Goldwasser et al., 2011), did significantly reduce viral secretion (Figure 6A).
Figure 6.
Bioflavonoid effect on infectious virion secretion. A. Huh-7.5 cells were infected and 24 hours later, treated with 125μM bioflavonoid for 5 hours. Supernatants were immediately removed and concentrated 30-fold to remove approx. 97% of the bioflavonoids and used to infect naïve cells, followed by luciferase assays 72 hours later. B. Control experiment to determine if concentration effectively removed bioflavonoids from supernatants in part A. Two sets of huh-7.5 cells were infected with the reporter virus. Subsequently, the supernatants of one set of cells were replaced with medium containing 125μM bioflavonoid. For the other set of cells, the media containing 125μM bioflavonoid were filtered and concentrated to remove the bioflavonoids. The cleared medium was then used to replace the culture supernatants. 72 hours later, luciferase activity was measured. (**** and † indicate P<0.00005 and P<0.000005, respectively. Error bars reflect standard deviation.)
We also ensured that concentrating the culture supernatants indeed removes the bioflavonoids. Aliquots of culture medium were prepared containing 125μM of catechin, naringenin, or quercetin. Two sets of huh-7.5 cells were infected. After the infection period, the supernatants of one set of cells were replaced by the media containing the bioflavonoids as done for all assays in this study. However, for the second set of cells, the medium was subjected to filtration in the same manner as above, and the cleared medium was used to replace the culture supernatants. Luciferase activity was assayed for both sets 48 hours after infection. The filtration step abolished the antiviral activity that was observed with no filtration (Figure 6B).
Catechin, naringenin, and quercetin significantly inhibit intracellular infectious virion assembly
We next determined the effect of bioflavonoids on viral assembly. Two sets of huh-7.5 cells were infected and treated with BFA. One set was used for the assembly assay and the ‘control set’ for measuring viral translation (below). BFA treatment was performed to trap all assembled virions in the cytoplasm of infected cells. This step is necessary to limit the effect of bioflavonoid treatment to viral assembly alone, as opposed to viral secretion. 3 hours later, cells were treated with 125μM bioflavonoids for 5 hours. This limited time of bioflavonoid treatment is necessary to minimize the effect of bioflavonoids on viral protein translation. Supernatants were removed and saved to determine infectious virion production (see below). After adding fresh medium, cells were subjected to three cycles of freeze/thaw to release intracellular virions. The medium was cleared of cellular debris and used to infect naïve cells. As shown in Figure 7A, all three bioflavonoids significantly blocked infectious virion assembly; here catechin and naringenin demonstrated more potency compared to quercetin.
Figure 7.
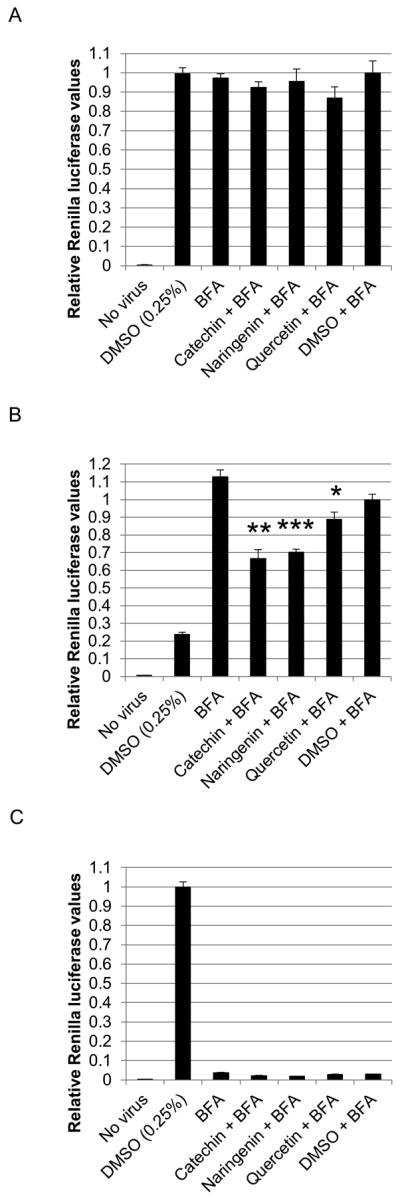
Bioflavonoid effects on assembly. A. Huh-7.5 cells were infected immediately treated with 0.1μg/ml of Brefeldin A (BFA). Thirty-one hours later, cells were treated with 125μM bioflavonoid for 5 hours after which, the medium was removed and saved for further analysis, and cells were washed twice with PBS to remove bioflavonoids. Fresh medium was added, and cells were subjected to three cycles of freeze/thaw. After clearing cellular debris, supernatants were used to infect naïve cells followed by luciferase assays 72 hours later. All values were normalized to ‘DMSO + BFA’. B. Control experiment to determine if bioflavonoids effect viral protein translation in the conditions of part A. Huh-7.5 cells were infected and treated with BFA and bioflavonoids exactly as described in part A. Immediately after the 5-hour bioflavonoid treatment, cells were washed and lysed, and Renilla luciferase assay was performed. C. Control experiment to determine the effect of BFA on viral secretion in the original part A assembly assay. The supernatants saved from the original culture were concentrated 30-fold to remove bioflavonoids and used to infect naïve cells, followed by luciferase assays 72 hours later. All values were normalized to DMSO treatment. (*, **, and *** indicate P<0.05, P<0.005, and P<0.0005, respectively. Error bars reflect standard deviation.)
Luciferase assays of the ‘control set’ of cells showed no significant decrease in viral translation (Figure 7B). BFA inhibition of secretion was verified by infection of naïve cells with the supernatants of the original infection. As shown in Figure 7C, BFA treatment abolished viral secretion as expected, while significant viral secretion occurred when cells were treated with DMSO.
Quercetin blocks NS5A-augmented IRES-mediated translation, whereas naringenin and catechin exhibit mild activity
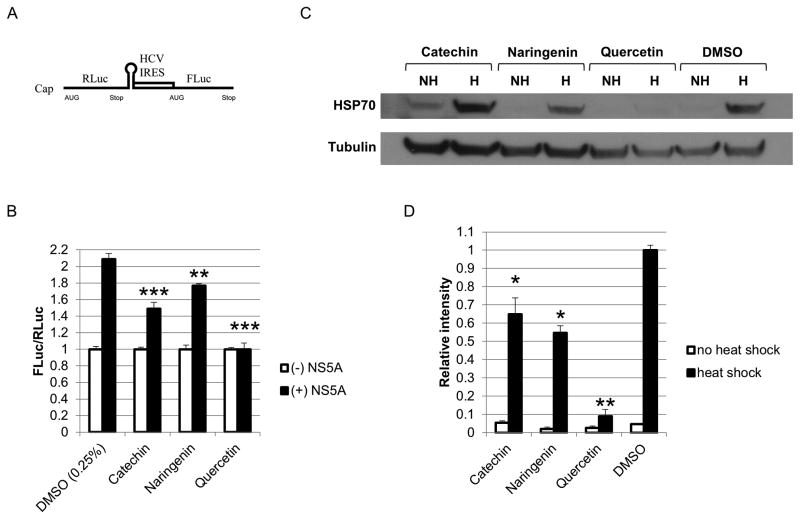
We tested catechin, naringenin, and quercetin in a previously described cell culture-based bicistronic reporter system to measure levels of viral internal ribosomal entry site (IRES)-mediated translation (Gonzalez et al., 2009). This reporter consists of a Renilla luciferase (RLuc) open reading frame (ORF) and a Firefly luciferase (FLuc) ORF driven by a 5′-cap and HCV IRES, respectively (Figure 8A). The ratio of Firefly to Renilla luciferase values (FLuc/RLuc) reflects the levels of HCV IRES-mediated translation. We have previously shown that quercetin suppresses the NS5A-driven increase in IRES-mediated translation (Gonzalez et al., 2009). In this study, 293T cells were transfected with the reporter construct and either NS5A or GFP (control) and treated with 125μM bioflavonoid for 72 hours after which, cells were assayed for dual luciferase activity. All bioflavonoids significantly decreased IRES-mediated translation compared with DMSO (Figure 8B). Quercetin completely blocked NS5A-augmented IRES activity in contrast to catechin and naringenin which demonstrated mild inhibition.
Figure 8.
Bioflavonoid effects on IRES-mediated translation. A. Schematic of the bicistronic reporter construct used to measure IRES-mediated translation. Renilla luciferase (RLuc) and Firefly luciferase (FLuc) are driven by a 5′ cap and the HCV IRES, respectively. Firefly to Renilla ratios reflect changes in IRES-mediated translation. B. Bioflavonoid effects on IRES-mediated translation. 293T Cells were transfected with the IRES reporter construct and either NS5A or GFP. 12 hours later, cells were treated with 125μM bioflavonoid, followed by luciferase assays 72 hours later. C. Western analysis of the effect of bioflavonoids on HSP70 levels after heat shock. Huh-7.5 cells were treated with 125μM bioflavonoids for 2 hours and subjected to heat shock at 42°C for 30 minutes. After 6 hours of recovery at 37°C, cells were lysed, and Western analysis was performed with antibody against HSP70 (HSPA1A). D. Densitometry of the Western blot in panel C. HSP70 quantities were normalized to tubulin. (*, **, and *** indicate P<0.05, P<0.005, and P<0.0005, respectively. Error bars reflect standard deviation.)
Quercetin strongly inhibits heat shock induced HSP70 expression compered to catechin, naringenin
Quercetin has been reported to inhibit HSP70 expression through different mechanisms (Elia and Santoro, 1994; Jakubowicz-Gil et al., 2005). Previously, we have shown HSP70 to form a complex with viral NS5A in vivo (Gonzalez et al., 2009). Further we recently showed that the NS5A/HSP70 complex is important for viral protein production in vivo and that disruption of this complex through a small peptide inhibitor results in a marked decrease in viral protein synthesis (Khachatoorian et al., 2012). Based on these observations, we hypothesized that the translation inhibitory effect of quercetin, as well as catechin and naringenin, may be mediated by inhibition of HSP70 expression. To test this hypothesis, huh-7.5 cells were treated with catechin, naringenin, quercetin, or DMSO (control) for 2 hours and subjected to heat shock at 42°C for 30 minutes and allowed to recover at 37°C for 6 hours. Western analysis of cellular lysates with antibody against HSP70 (specifically the HSPA1A isoform reported in our previous studies (Gonzalez et al., 2009; Khachatoorian et al., 2012)) demonstrated a marked decrease in HSP70 expression in quercetin treated cells (Figure 8C and 8D). A slight decrease in HSP70 was seen for naringenin and catechin treatments (Figure 8C and 8D) consistent with our IRES assay and viral protein production results above (Figure 8B and Figure 5, respectively).
Discussion
We have previously reported quercetin to efficiently block the NS5A-driven increase in IRES-mediated translation and HCV production (Gonzalez et al., 2009). In this study, we further analyzed the mechanisms of action of quercetin and a number of other bioflavonoids structurally related to quercetin.
Initially we tested seven bioflavonoids, including quercetin, for their cellular toxicity, and we found that genistein and luteolin were highly cytotoxic to huh-7.5 cells. Quercetin, naringenin, and silymarin displayed similar toxicity to the DMSO carrier, while catechin and epicatechin had the lowest toxicity and led to a significantly increased absorbance in MTT assays. We conclude that genistein and luteolin are not suitable as anti-HCV treatments due to their significant cytotoxicity at these concentrations.
Next, we screened the remaining five bioflavonoids for their antiviral activity. Catechin, naringenin, and quercetin significantly blocked virus production, while silymarin did not have any effect. Silymarin has previously been shown to inhibit HCV infection in tissue culture primarily through blocking viral entry (Wagoner et al., 2010). We did not see this effect. One possible reason is that we added the compounds after the 3-hour infection time, which would significantly reduce the effect of an entry blocker. Further, the concentration we used was fairly low (25μM). Others have shown approximately 25% reduction in virus when treating J6/JFH infection (the same backbone as used in this study) when treating at 80 μM (Wagoner et al., 2010).
Epicatechin, a diastereoisomer of catechin, led to increased virus production compared with DMSO, and is, therefore, not suitable as an antiviral agent. Recently it was shown that a dimer of catechin and epicatechin can block HCV pseudotype proliferation (Li et al., 2010). Our finding that catechin and epicatechin have opposite effects on cellular viral levels implies that catechin may be the element in this dimer that displayed antiviral effects. We are currently investigating the opposite effects of catechin and epicatechin on virus production as well as their shared increased absorbance seen in MTT assays. As shown in Figure 1, the di-hydro-benzopyran backbone of catechin and epicatechin possesses a di-hydroxyphenyl and a hydroxyl group on the pyran moiety. These two groups are oriented differently in three dimensional space; in catechin they are located on the same side of the backbone, while in epicatechin they point in opposite directions. We speculate that the orientation of these side chains may be responsible for the opposite effects of catechin and epicatechin on viral protein translation and are currently investigating this possibility. We have shown previously that viral protein translation is mediated in part by a complex of NS5A and HSP70 (Gonzalez et al., 2009; Khachatoorian et al., 2012). Considering our finding that catechin can effect HSP70 expression, it may be possible that the orientation of these side chains determines the effect of catechin and epicatechin on HSP70 expression potentially through their differential interactions with the HSP70 transcription factor.
We chose catechin, naringenin, and quercetin for further analysis because of their antiviral activity. All three bioflavonoids significantly block cellular viral levels in the HCVcc system. Quercetin displays a far more potent effect than catechin and naringenin. Catechin inhibits viral translation more than naringenin, and we speculate that this results in the better long-term viral attenuation by catechin compared with naringenin (Figure 4). We also used the HCV IRES bicistronic reporter assay system to show that the translation inhibitory effect of these flavonoids are NS5A dependent as there was no change in IRES-mediated translation when GFP was used (instead of NS5A) as a control. This result is consistent with our previous findings on the mechanism of NS5A/HSP70 complex-driven IRES-mediated translation of viral proteins (Gonzalez et al., 2009; Khachatoorian et al., 2012).
Intracellular infectious virion assembly is also significantly blocked by catechin and naringenin and to a lesser extent by quercetin. Naringenin has also been previously reported to block virion assembly using a different assay (Goldwasser et al., 2011). Infectious virion secretion is not significantly affected by catechin, naringenin (in agreement with a previous report (Goldwasser et al., 2011)) and quercetin.
Furthermore, we have shown that catechin, naringenin, and quercetin effect induction of HSP70 in cells that are subjected to heat shock. In particular, quercetin has a far stronger inhibitory effect on HSP70 expression. These results support our hypothesis that bioflavonoids mediate their antiviral effects at least in part by blocking heat shock protein (HSP) expression and underscore the role of HSPs in HCV life cycle.
Current HCV treatments are limited and display significant side effect and suboptimal sustained virological response (SVR). For these reasons, it is necessary to identify/develop additional antiviral therapies that could be used in place of pegylated interferon-α (PEG-IFN) and ribavirin or as adjunct therapies to increase the SVR. In this study, we have demonstrated the significant antiviral activity of catechin, quercetin and naringenin. Therefore, these bioflavonoids may be candidates for HCV therapy and may be beneficial for patients unable to receive PEG-IFN therapy. Furthermore, because of the different mechanisms of action of these bioflavonoids, combining them may allow for synergistic antiviral activity resulting in better suppression of HCV.
Materials and methods
Bioflavonoids
Quercetin (Sigma-Aldrich, 00200595-50MG), catechin (Sigma-Aldrich, C1251-5G), naringenin (Sigma-Aldrich, N5893-1G), epicatechin (Sigma-Aldrich, E4018-5MG), silymarin (Sigma-Aldrich, S0292-10G), genistein (Sigma-Aldrich, G6649-5MG), and luteolin (Sigma-Aldrich, L9283-10MG).
Cell culture
Cell lines Huh-7.5 and 293T were maintained in a humidified atmosphere containing 5% CO2 at 37°C in Dulbecco’s Modified Eagle Medium (Mediatech, 10-013-CM) supplemented with 10% fetal bovine serum (Omega Scientific, FB-01) and 2 mM L-glutamine (Invitrogen, 25030). 293T cells were purchased from ATCC (CRL-11268). Huh-7.5 cells were a kind gift from Charles Rice (The Rockefeller University, New York, NY)(Blight et al., 2002).
Cell viability
Cell viability was determined using MTT Cell Proliferation assay (ATCC, 30–1010K).
Plasmid constructs
The HCV IRES reporter plasmid and the NS5A and GFP retroviral expression vectors pMSCV-NS5A-FLAG and pMSCV-GFP, respectively, have been previously described (Gonzalez et al., 2009). An intra-genotype 2 chimeric monocistronic reporter virus virus, pNRLFC based on pJ6/JFH-C parental virus has been described previously (Arumugaswami et al., 2008). For the current study, we have used a chemically synthesized plasmid pFNX-RLuc (having similar sequences to pNRLFC) for construction of recombinant virus. The pRL-TK (Promega, E2241) plasmid expresses Renilla luciferase.
Infectious virus production
pFNX-RLuc was in vitro transcribed, and the purified RNA was electroporated into huh-7.5 cells to generate infectious viral supernatant as previously described (Arumugaswami et al., 2008).
Viral assays
All viral assays were done using the HCV reporter virus and with the same titer and multiplicity of infection as described previously (Gonzalez et al., 2009; Khachatoorian et al., 2012).
Intracellular viral protein production
Huh-7.5 cells were infected for 3 hours, and cells were harvested at the indicated time points. Luciferase activity was measured using the Renilla Luciferase Assay System (Promega, E2820).
Infectious virion secretion
The supernatants from the above cultures were concentrated 30-fold by using Amicon Ultra-0.5 mL Centrifugal Filters (Millipore, UFC510096) to remove excess bioflavonoids and used to infect naïve cells for 3 hours. Cells were harvested 72 hours later, and Renilla luciferase activity was measured.
Intracellular infectious virion assembly
Huh-7.5 cells were infected for 3 hours, and supernatants were removed at indicated time points. Cells were washed with PBS, and fresh medium was added. The cultures were subjected to three cycles of freeze-thaw to release assembled viral particles. These suspensions were cleared of cellular debris and used to infect naïve cells for 3 hours. Subsequently, cells were harvested 72 hours post infection, and luciferase activity was assayed.
Quantitative reverse-transcriptase PCR
Huh-7.5 cells were infected with the Renilla reporter virus for 3 hours. 72 hours post infection, cells were harvested, and total RNA was extracted using RNeasy Mini Kit (Qiagen, 74104). cDNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad, 1708891). Quantitative PCR was performed using the Applied Biosystems 7500 Fast Real-Time PCR System with 2x SYBR Green Master Mix (Diagenode, GMO-SG2x-A300) in 25μL reactions. The real-time PCR cycling conditions were 50°C for 2 minutes and 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds each as well as a final dissociation stage of 95°C for 15 seconds and 60°C for 1 minute. The primers for the viral genome were derived from the 5′-non-coding region and were CTGGGTCCTTTCTTGGATAA and CCTATCAGGCAGTACCACA. HCV RNA levels were normalized to the housekeeping gene actin using the primers CCAACCGCGAGAAGATGA and CCAGAGGCGTACAGGGATAG.
IRES reporter assay
293T cells were treated with 125μM bioflavonoids. Two hours later, cells were transfected with the HCV IRES reporter plasmid and either pMSCV-NS5A-FLAG or pMSCV-GFP. All transfections were done using Fugene6 (Roche, 11814443001). 48 hours post transfection, Renilla and Firefly luciferase activity were determined using Dual Luciferase Assay System (Promega, E1910).
Antibodies
NS5A (Abcam, ab20342), HSP70 (Santa Cruz Biotech, C92F3A-5), and tubulin (abcam, ab6160).
Densitometry
Western blot images were analyzed by ImageJ v1.45s software according to software instructions.
Statistical analysis
Error bars reflect the standard deviation. P values were determined by student t test.
Acknowledgments
Financial support: NIH R01DK090794, SWF.
NIH AI084090, AD.
California Center for Antiviral Drug Discovery grant, University of California Office of the President, MRPI # 143226, AD.
The authors thank David Dawson for helpful discussions, Charles Rice for providing huh-7.5 cells, and Michael Arensman for technical assistance.
List of abbreviations
- HCV
hepatitis C virus
- HCC
hepatocellular carcinoma
- PEG-IFN
pegylated interferon-α
- SVR
sustained virological response
- NCR
noncoding region
- IRES
internal ribosomal entry site
- NS
nonstructural
- NS5A
nonstructural protein 5A
- HSP
heat shock protein
- GFP
green fluorescent protein
- RLuc
Renilla luciferase
- PBS
phosphate-buffered saline
- DMSO
dimethyl sulfoxide
- HCVcc
HCV cell culture
- ORF
open reading frame
- FLuc
Firefly luciferase
Contributor Information
Ronik Khachatoorian, Email: RnKhch@ucla.edu.
Vaithilingaraja Arumugaswami, Email: VArumugaswami@mednet.ucla.edu.
Santanu Raychaudhuri, Email: SRaychau@ucla.edu.
George K. Yeh, Email: GgYeh@ucla.edu.
Eden M. Maloney, Email: EMaloney@ucla.edu.
Julie Wang, Email: JulieW1521@ucla.edu.
Asim Dasgupta, Email: Dasgupta@ucla.edu.
Samuel W. French, Email: SFrench@mednet.ucla.edu.
References
- Arumugaswami V, Remenyi R, Kanagavel V, Sue EY, Ngoc Ho T, Liu C, Fontanes V, Dasgupta A, Sun R. High-resolution functional profiling of hepatitis C virus genome. PLoS Pathog. 2008;4:e1000182. doi: 10.1371/journal.ppat.1000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blight KJ, McKeating JA, Rice CM. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol. 2002;76:13001–13014. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesek S, Manns MP. Hepatitis in 2010: the dawn of a new era in HCV therapy. Nat Rev Gastroenterol Hepatol. 2011;8:69–71. doi: 10.1038/nrgastro.2010.219. [DOI] [PubMed] [Google Scholar]
- El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72–78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- Elia G, Santoro MG. Regulation of heat shock protein synthesis by quercetin in human erythroleukaemia cells. Biochem J. 1994;300 (Pt 1):201–209. doi: 10.1042/bj3000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambarin-Gelwan M, Jacobson IM. Optimal dose of peginterferon and ribavirin for treatment of chronic hepatitis C. J Viral Hepat. 2008;15:623–633. doi: 10.1111/j.1365-2893.2008.01018.x. [DOI] [PubMed] [Google Scholar]
- Goldwasser J, Cohen PY, Lin W, Kitsberg D, Balaguer P, Polyak SJ, Chung RT, Yarmush ML, Nahmias Y. Naringenin inhibits the assembly and long-term production of infectious hepatitis C virus particles through a PPAR-mediated mechanism. J Hepatol. 2011;55:963–971. doi: 10.1016/j.jhep.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez O, Fontanes V, Raychaudhuri S, Loo R, Loo J, Arumugaswami V, Sun R, Dasgupta A, French SW. The heat shock protein inhibitor Quercetin attenuates hepatitis C virus production. Hepatology. 2009;50:1756–1764. doi: 10.1002/hep.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Yan W, Coito C, Li Y, Gale M, Jr, Katze MG. The regulation of hepatitis C virus (HCV) internal ribosome-entry site-mediated translation by HCV replicons and nonstructural proteins. J Gen Virol. 2003;84:535–543. doi: 10.1099/vir.0.18658-0. [DOI] [PubMed] [Google Scholar]
- Hughes M, Griffin S, Harris M. Domain III of NS5A contributes to both RNA replication and assembly of hepatitis C virus particles. J Gen Virol. 2009;90:1329–1334. doi: 10.1099/vir.0.009332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowicz-Gil J, Pawlikowska-Pawlega B, Piersiak T, Pawelec J, Gawron A. Quercetin suppresses heat shock-induced nuclear translocation of Hsp72. Folia Histochem Cytobiol. 2005;43:123–128. [PubMed] [Google Scholar]
- Khachatoorian R, Arumugaswami V, Ruchala P, Raychaudhuri S, Maloney EM, Miao E, Dasgupta A, French SW. A cell-permeable hairpin peptide inhibits hepatitis C viral nonstructural protein 5A-mediated translation and virus production. Hepatology. 2012;55:1662–1672. doi: 10.1002/hep.25533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kodama EN, Inoue Y, Tani H, Matsuura Y, Zhang J, Tanaka T, Hattori T. Procyanidin B1 purified from Cinnamomi cortex suppresses hepatitis C virus replication. Antivir Chem Chemother. 2010;20:239–248. doi: 10.3851/IMP1597. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Patel K. Future therapy of hepatitis C. Hepatology. 2002;36:S245–252. doi: 10.1053/jhep.2002.36795. [DOI] [PubMed] [Google Scholar]
- Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- Pacheco A, Martinez-Salas E. Insights into the biology of IRES elements through riboproteomic approaches. J Biomed Biotechnol. 2010;2010:458927. doi: 10.1155/2010/458927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- Tellinghuisen TL, Foss KL, Treadaway JC, Rice CM. Identification of residues required for RNA replication in domains II and III of the hepatitis C virus NS5A protein. J Virol. 2008;82:1073–1083. doi: 10.1128/JVI.00328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagoner J, Negash A, Kane OJ, Martinez LE, Nahmias Y, Bourne N, Owen DM, Grove J, Brimacombe C, McKeating JA, Pecheur EI, Graf TN, Oberlies NH, Lohmann V, Cao F, Tavis JE, Polyak SJ. Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology. 2010;51:1912–1921. doi: 10.1002/hep.23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Sarnow P, Siddiqui A. Translation of human hepatitis C virus RNA in cultured cells is mediated by an internal ribosome-binding mechanism. J Virol. 1993;67:3338–3344. doi: 10.1128/jvi.67.6.3338-3344.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington K, Jarvis B. Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs. 2001;15:465–489. doi: 10.2165/00063030-200115070-00005. [DOI] [PubMed] [Google Scholar]