Abstract
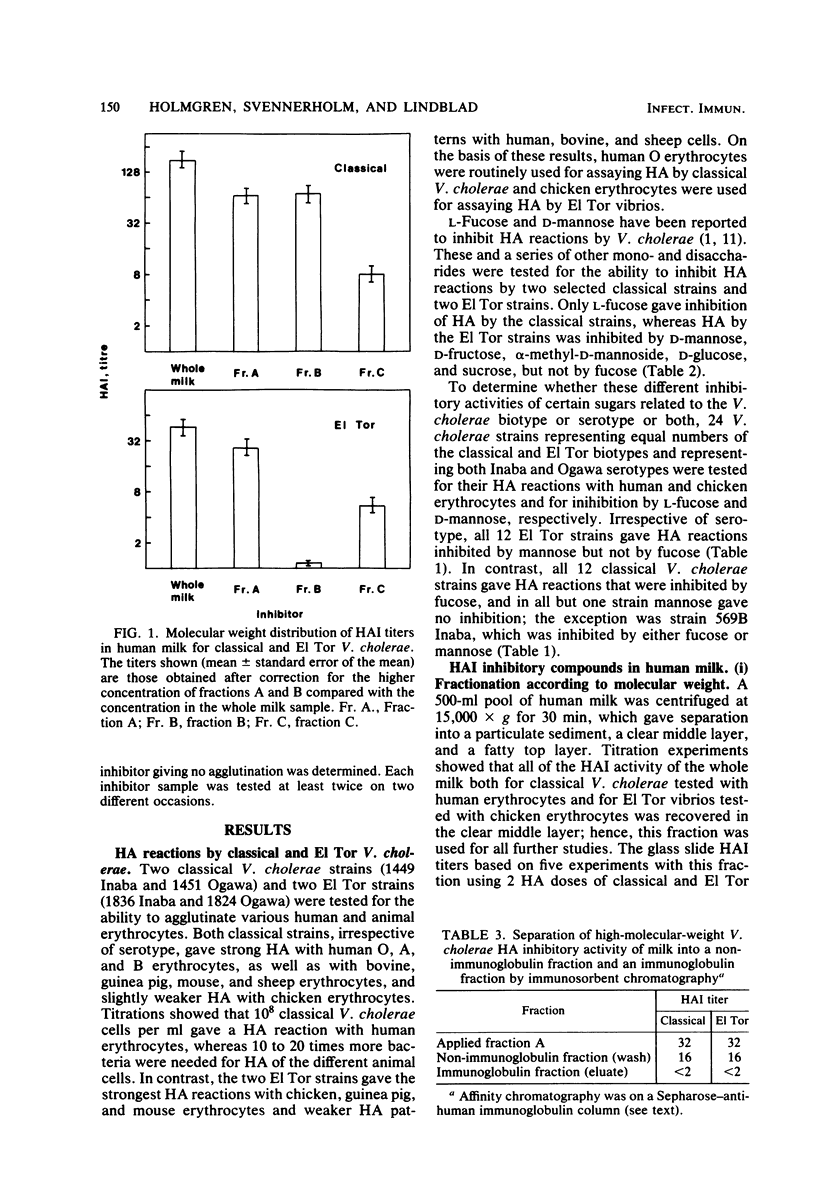
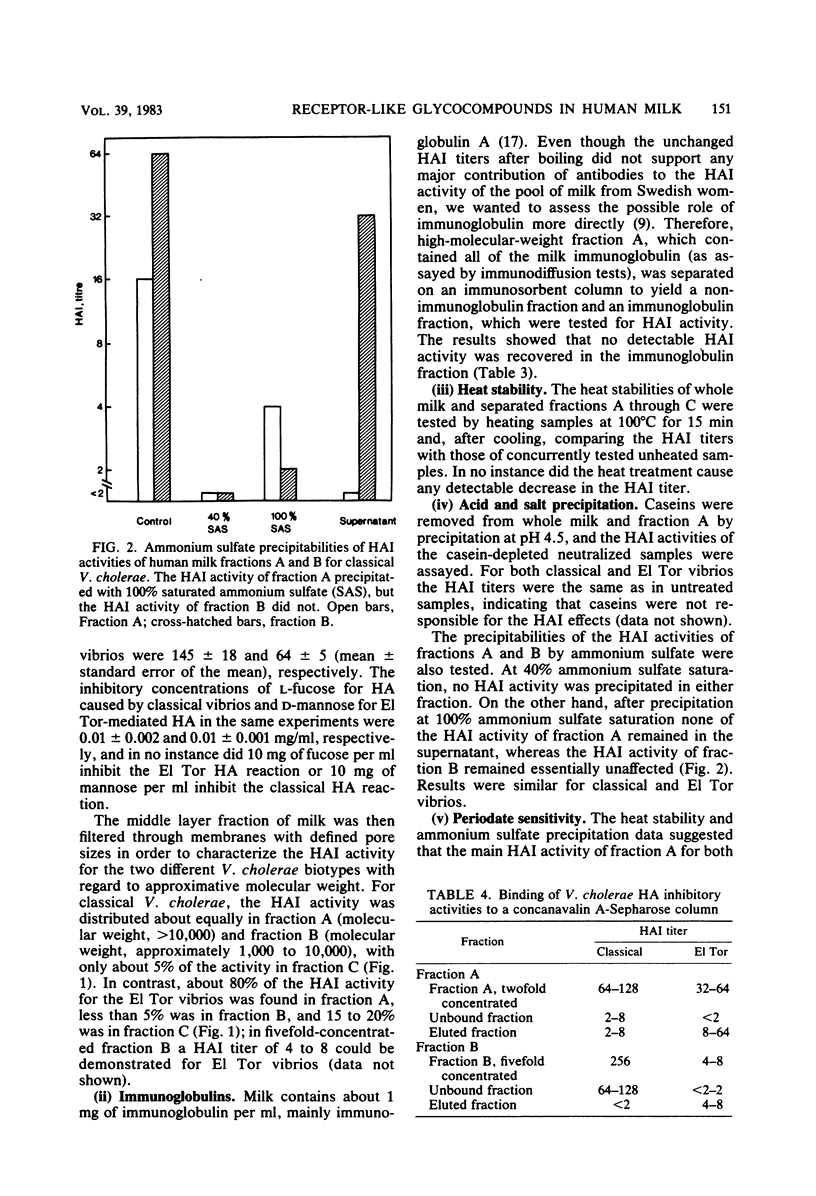
The two biotypes of Vibrio cholerae were found to have cell-associated hemagglutinins which differ with regard to binding to different species of erythrocytes and inhibition by monosaccharides. A total of 12 classical V. cholerae strains (Inaba or Ogawa) strongly agglutinated human erythrocytes in a reaction specifically inhibited by L-fucose, whereas 12 El Tor strains preferably agglutinated chicken erythrocytes, a reaction reversed by D-mannose or by higher concentrations of D-fructose, D-glucose, alpha-methyl-D-mannoside, or sucrose. Milk from Swedish women inhibited both of these adherence reactions, and the predominating inhibitory activity for each reaction resisted boiling, was destroyed by periodate treatment, and bound a concanavalin A-Sepharose column, suggesting a carbohydrate structure. Further characterization indicated that the inhibitory activity for classical V. cholerae hemagglutination was distributed about equally on glycoprotein and free oligosaccharide, but was not present on glycolipid. The El Tor inhibiting activity, on the other hand, was almost exclusively of a high-molecular-weight glycoprotein nature. These results support our previous suggestion (Holmgren et al., Infect. Immun. 33:136-141, 1981) that human milk may contain receptor-like glycocompounds which can prevent bacterial adherence by competition with receptors on target cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharjee J. W., Srivastava B. S. Mannose-sensitive haemagglutinins in adherence of Vibrio cholerae eltor to intestine. J Gen Microbiol. 1978 Aug;107(2):407–410. doi: 10.1099/00221287-107-2-407. [DOI] [PubMed] [Google Scholar]
- Chaicumpa W., Atthasisiha N. Study of intestinal immunity against V. cholerae: role of antibody to V. cholerae haemagglutinin in intestinal immunity. Southeast Asian J Trop Med Public Health. 1977 Mar;8(1):13–18. [PubMed] [Google Scholar]
- Finkelstein R. A., Hanne L. F. Purification and characterization of the soluble hemagglutinin (cholera lectin)( produced by Vibrio cholerae. Infect Immun. 1982 Jun;36(3):1199–1208. doi: 10.1128/iai.36.3.1199-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. W. Breast-feeding: second thoughts. Pediatrics. 1974 Dec;54(6):757–764. [PubMed] [Google Scholar]
- Hanne L. F., Finkelstein R. A. Characterization and distribution of the hemagglutinins produced by Vibrio cholerae. Infect Immun. 1982 Apr;36(1):209–214. doi: 10.1128/iai.36.1.209-214.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson L. A., Winberg J. Breast milk and defence against infection in the newborn. Arch Dis Child. 1972 Dec;47(256):845–848. doi: 10.1136/adc.47.256.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Fredman P., Lindblad M., Svennerholm A. M., Svennerholm L. Rabbit intestinal glycoprotein receptor for Escherichia coli heat-labile enterotoxin lacking affinity for cholera toxin. Infect Immun. 1982 Nov;38(2):424–433. doi: 10.1128/iai.38.2.424-433.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Ahrén C. Nonimmunoglobulin fraction of human milk inhibits bacterial adhesion (hemagglutination) and enterotoxin binding of Escherichia coli and Vibrio cholerae. Infect Immun. 1981 Jul;33(1):136–141. doi: 10.1128/iai.33.1.136-141.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Abrams G. D., Freter R. Adhesive properties of Vibrio cholerae: adhesion to isolated rabbit brush border membranes and hemagglutinating activity. Infect Immun. 1976 Jul;14(1):232–239. doi: 10.1128/iai.14.1.232-239.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otnaess A. B., Orstavik I. Effect of fractions of Ethiopian And Norwegian colostrum on rotavirus and Escherichia coli heat-labile enterotoxin. Infect Immun. 1981 Aug;33(2):459–466. doi: 10.1128/iai.33.2.459-466.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm L., Fredman P. A procedure for the quantitative isolation of brain gangliosides. Biochim Biophys Acta. 1980 Jan 18;617(1):97–109. doi: 10.1016/0005-2760(80)90227-1. [DOI] [PubMed] [Google Scholar]
- Tomasi T. B., Jr, Bienenstock J. Secretory immunoglobulins. Adv Immunol. 1968;9:1–96. doi: 10.1016/s0065-2776(08)60441-1. [DOI] [PubMed] [Google Scholar]
- WELLS M. A., DITTMER J. C. THE USE OF SEPHADEX FOR THE REMOVAL OF NONLIPID CONTAMINANTS FROM LIPID EXTRACTS. Biochemistry. 1963 Nov-Dec;2:1259–1263. doi: 10.1021/bi00906a015. [DOI] [PubMed] [Google Scholar]


