Abstract
Objectives
Tobacco-derived carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, NNK, activates β-adrenergic receptor (β-AR) signaling through Src/focal adhesion kinases (FAK)/MAPK to modulate proliferation, migration and survival. Apigenin (4′, 5, 7-trihydroxyflavone) is reported to attenuate proliferation and migration of cancer cells. This study was designed to determine the effects of apigenin on NNK-induced pro-carcinogenesis using human pancreatic cancer cells BxPC-3 and MIA PaCa-2 that express β-AR.
Methods
Proliferation and migration were assessed by standard MTT and scratch assays. β-AR, FAK/MAPK and ERK expression and activation were assessed by Western blotting and real time PCR.
Results
NNK caused a dose- and time-dependent increase in BxPC-3 and MIA PaCa-2 cell proliferation that was inhibited by propranolol or apigenin. NNK also stimulated a time-dependent increase in FAK and ERK activation that was suppressed by propranolol or apigenin. NNK-enhanced gap closure at 24 hr was prevented by either propranolol or apigenin.
Conclusion
Apigenin suppressed the effects of NNK on pancreatic cancer cell proliferation and migration that are mediated through the β-AR and its downstream signals FAK and ERK activation. These findings suggest a therapeutic role for this natural phytochemical in attenuating the pro-carcinogenic effects of NNK on pancreatic cancer proliferation and migration.
Keywords: apigenin, propranolol, NNK, FAK, smoking, pancreatic cancer
Introduction
Smoking is a well-documented risk factor for the development of pancreatic cancer that ranks fourth for cancer-related mortality in industrialized countries and exhibits extremely high mortality rates, with five-year survival rates below 10 %.1 Among the carcinogens identified in tobacco products, the water soluble, nicotine-derived nitrosamine compounds has been implicated in pancreatic carcinogenesis.2 The nitrosylation of nicotine yielding 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) has been demonstrated in vivo in mediating pancreatic tumor formation.3 NNK functions similarly to other nitrosamine compounds in its ability to induce formation of DNA adducts and gene mutations, leading to carcinogenesis. Interestingly, NNK has been shown to bind to and activate the β-adrenergic receptor (β-AR) in human pancreatic ductal carcinoma cells.4,5 Both β1AR and β2AR are present in pancreatic tissue and its natural ligands including epinephrine and norepinephrine activates signaling pathway that include the Gs-adenylate cyclase/cAMP/PKA as well as Ras/Raf/MEK/ERK.6-8 NNK activation of β2AR triggering Src has been reported in human cells,8 suggesting a role for β-AR mediated signaling involving Src/(focal adhesion kinase) FAK and MAPK events.
FAK belongs to the Src family of non-receptor tyrosine kinases regulating cellular processes that includes growth, proliferation, and survival.9 Enhanced Src expression and activity correlating with oncogenic potential are supported with Src over-expression in vivo in rat pancreatic tumors and confirmed in human pancreatic carcinoma tissue and carcinoma cell lines that includes AsPC-1, HPAF and Capan-2.10,11 Activation of FAK by Gi- or actin-mediated tyrosine phosphorylation through adrenergic receptor stimulation as well as autophosphorylation of FAK at tyrosine residue 397 triggers signaling events in focal complex assembly, including the recruitment of and interaction with Src.6,12,13 FAK over-expression correlates with tumor size in human pancreatic ductal carcinoma tissue and cell lines.14,15 In contrast, silencing of FAK resulted in anoikis in the Panc-1, BxPC-3, and MIA PaCa-2 cancer cell lines.16 Knockdown of FAK expression by siRNA or by the flavonoids luteolin and quercetin inhibited MIA PaCa-2 cell migration.17 As an intermediary convergence kinase for proliferation, migration and survival processes, FAK is a recognized therapeutic target but remains a challenge as current available approaches are limited.14
Apigenin (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) is a naturally occurring plant flavonoid belonging to the flavone subclass of polyphenolic compounds that exhibit chemopreventive and chemotherapeutic properties.18 In human pancreatic cancer, apigenin has been shown to attenuate abnormal DNA replication through the suppression of geminin in CD18 and S2-013 cell lines.19 Moreover, apigenin inhibited the proliferation of human pancreatic cell lines AsPC-1, CD18, MIA PaCa-2 and S2-013 through the G2/M cell cycle arrest by suppressing cyclin and activated cdc2 and cdc25 expression.20 In the context of cancer cell survival, apigenin has been reported to induce caspase-dependent apoptosis through the suppression of casein kinase II expression and subsequent inhibition of NF-κB activity in MIA PaCa-2 and DanG carcinoma cells.21 Furthermore, the inhibition of phosphoinositide 3′-kinase (PI3′K) and Akt activation by apigenin in CD18 and S2-013 human pancreatic cancer cells were comparable to the PI3′K selective inhibitors LY294002 and wortmannin.22 These findings support and demonstrate the anti-carcinogenic activities of apigenin targeting of proliferative pathways in cancer cells, although the mechanisms of action is poorly understood.18,23 In addition, attenuation by apigenin on carcinogenesis mediated by compounds of smoking products have not been reported and remains unclear.
Thus, the aim of this report was to investigate the potential of apigenin in attenuating NNK activation of the β-AR signaling through Src/FAK/ERK and enhanced proliferation of pancreatic cancer cells. Human pancreatic cancer cells treated with NNK augmented cellular proliferation and FAK and ERK activation. Pre-treatment with propranolol abrogated the NNK effect on cellular proliferation and FAK/ERK activation, implicating the involvement of β1AR and β2AR activation in mediating the increased proliferation of the cells. Pre-treatment with apigenin, similarly, abrogated the NNK effect on cellular proliferation and FAK/ERK activation, suggesting that apigenin could serve as a non-toxic alternative to propranolol at the same concentration. NNK exacerbated proliferation and migration whereas pre-treatment with either propranolol or apigenin abated the NNK effect on the pancreatic cells. These findings demonstrate the potential of apigenin to attenuate NNK-induced cellular proliferation and migration that appears to proceed through the β-AR and subsequent activation of FAK and ERK.
Materials and Methods
Materials
Apigenin, NNK, AG 1478 (4-(3-chloroanilino)-6,7-dimethoxyquinazoline), wortmannin, mitomycin C, fetal bovine serum (FBS), DMSO, chloroform and methanol were purchased from Sigma (St. Louis, MO). FAK, phosphorylated Y397 FAK, phosphorylated ERK phosphorylated CREB and CREB antibodies were purchased from Cell Signaling (Danvers, MA). β1AR, ERK and β-actin antibodies were acquired from Santa Cruz Biotechnology (Santa Cruz, CA). β2AR was acquired from Abcam (Cambridge, MA). Horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG and ECL reagents were obtained from Pierce (Fisher, Pittsburg, PA). U0126 (1,4-Diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene), propranolol ((±)-1-Isopropylamino-3-(1-naphthyloxy)-2-propanol) and PD98059 (2′-amino-3′-methoxyflavone) were obtained from Calbiochem (EMD Biosciences, San Diego, CA). LY294002 (2-morpholin-4-yl-8-phenylchromen-4-one) was acquired from Cell Signaling (Danvers, MA). PP1 (4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo-d-3,4-pyrimidine), PP2 (3-(4-chlorophenyl) 1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine) and FAK Inhibitor 14 (FI14) were acquired from Tocris Bioscience (Ellisville, MO).
Cell Culture
Moderately differentiated adenocarcinoma BxPC-3 (CRL-1687) and undifferentiated carcinoma MIA PaCa-2 (CRL-1420) human pancreatic cell lines were purchased from American Type Culture Collection (Rockville, MD). Stock cultures of BxPC-3 were propagated with RPMI 1640 medium containing 2 mM L-glutamine, 2.0 g/L sodium bicarbonate, and 2.0 g/L glucose, supplemented with 10% fetal bovine serum (FBS) and PSG antibiotic mix (100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine; Invitrogen, Carlsbad, CA), CO2 at 5 % and temperature at 37 °C. Stock cultures of MIA PaCa-2 were propagated with Dulbecco’s modified Eagle’s medium (DMEM) containing 4 mM L-glutamine, 1.5 g/L sodium bicarbonate, and 4.5 g/L glucose, supplemented with 10% fetal bovine serum (FBS) and PSG antibiotic mix (100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine; Invitrogen), CO2 at 10 % and temperature at 37 °C. For experiments, these cells were plated in 100-mm tissue culture dishes, allowed to achieve confluence (5-7 days) and replenished with respective serum-free media overnight.
RNA Extraction
For cell culture, cells were washed with 4 ml of Versene containing 0.48 mM EDTA in phosphate-buffered saline (Invitrogen) and harvested with 1 ml of Trizol reagent (Invitrogen). Total RNA was extracted with 0.2 ml of chloroform at 12,000 × g for 15 min at 4 °C and precipitated with 0.5 ml of 2-propanol at 12,000 × g for 10 min at 4 °C. The RNA pellet was washed with 75% ethanol at 7,500 × g for 5 min at 4 °C, dissolved in 30 μL of RNA Storage Solution with 1 mM sodium citrate, pH 6.4 (Ambion, Austin, TX) and stored at −20 °C for subsequent analysis. RNA concentration was quantified on a spectrophotometer (GeneQuant Pro, Amersham Biotechnology, Piscataway, NJ) reading dual wavelengths of 260 and 280 nm.
Real Time PCR (RT-PCR)
Total RNA samples (25 ng) were reverse transcribed and cDNAs amplified using TaqMan Gold RT-PCR kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. Transcripts encoding human β1AR (accession NM_001619), β2AR (accession NM_005160) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control were quantified by real-time PCR analysis using an ABI Prism 7700 Sequence Detection System (PE Biosystems, Foster City, CA). The human primers used are as follows: β1AR sense 5′-GCG AGG TGA CCT TTG AGA AG-3′, antisense 5′-GAT CTC CTC ATA GAA TTC CAC CAA-3′ with corresponding universal probe 25 (Roche, Indianapolis, IN) and β2AR sense 5′-TAA GCA ACT TGG CCA CGA A-3′ and antisense 5′-CAG CAT GTA CCC GTG CAT AA -3′ with corresponding universal probe 60. The human GAPDH primer and probe set was acquired from Applied Biosystems (Foster City, CA). Thermal cycling conditions for reverse transcription and amplification activation were set at 50 °C for 30 minutes and 95 °C for 10 minutes, respectively. PCR denaturing was set at 95 °C for 15 seconds and annealing/extending at 60 °C for 60 seconds with a maximum 40 cycles, according manufacturer’s protocol (Brilliant II, Stratagene, La Jolla, CA).
MTT Assay
BxPC-3 and MIA PaCa-2 cells were seeded at 8000 cells/well in 96-well plates and propagated in their respective media supplemented with 10 % FBS. After 24 hrs, the cells were replenished with their respective media supplemented with 0.5 % FBS for viability maintenance. For experiments, the cells were untreated for 0, 24, 48 or 72 hrs; treated with 0, 25, 50, 100 or 200 μM of NNK for 48 hrs; and treated with a combination of 0, 5, 10, 25, 50 or 100 μM of propranolol or apigenin and/or 100 μM of NNK for 48 hrs. These cells were further incubated with 10 % 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma) for 4 hrs, aspirated and precipitated with DMSO for the formazan product. Absorbance was measured at 560 nm with a reference wavelength at 700 nm on a Bio-Rad spectrophotometer (Hercules, CA).
Protein Expression
Protein from the cells were harvested using RIPA lysis buffer (Thermoscientific, Pittsburg, PA), diluted 1:1 (vol/vol) with 2X LDS buffer containing SDS (Invitrogen) and denatured at 95 °C for 10 min in a water bath. For cell culture, confluent, serum-starved cells were washed with 1 ml of ice-cold phosphate buffered saline (PBS, Sigma), harvested in LDS loading buffer and denatured at 95 °C for 10 min in a water bath. These protein extracts were subjected to a variable 4-12% SDS-polyacrylamide gel electrophoresis (NuPAGE Novex Bis-Tris Gels, Invitrogen) for 45 min at 200 V, and transferred to a PVDF membrane (90 min at 30 V). The membrane was washed with Tris buffered saline (TBS, Sigma), blocked with 5% dried nonfat milk (Bio-Rad) and 5% BSA (Sigma) in 1% tween-TBS and probed with antibody raised against β1AR (1:1000) or β2AR (1:1000) with β-actin (1:2500) as a visual loading control. Membranes probed with antibodies against pFAK (1:1000), pERK (1:1000) or pCREB (1:1000) were accompanied with total FAK antibody (1:1000), total ERK antibody (1:1000) or total CREB (1:1000) as visual loading control. Primary antibodies were followed by secondary antibody IgG linked to horseradish peroxidase conjugate (1:2500). The blot was visualized by enhanced chemiluminescence (Amersham Biosciences) and scanned using the ChemiDoc XRS Imager (Bio-Rad).
Scratch Assay
BxPC-3 and MIA PaCa-2 cells were propagated to confluence with complete RPMI or DMEM media, respectively, in 35-mm dishes for 5-7 days. Scratch assays were performed using a 10-μL pipette tip and vacuum aspiration. Remaining adherent cells were washed with PBS containing 1 % antibiotics, replenished with serum deficient RPMI or DMEM, respectively, and allowed to stabilized overnight. The cells were treated on the following day with 0.1 % DMSO for vehicle control, 100 μM of NNK, 100 μM NNK with 50 μM of propranolol or 100 μM of NNK with 50 μM of apigenin for 24 hrs. For migration study, cells were stabilized overnight in serum-free media and treated on the following day with 100 μM of NNK, 100 μM of NNK and 150 μM of mitomycin C, 100 μM of NNK and 150 μM of mitomycin C with 50 μM of propranolol or 100 μM of NNK and 150 μM of mitomycin C with 50 μM of apigenin for 6 hrs. Gap closures of marked areas were recorded at 10X magnification using a Fisher Scientific Micromaster digital microscope (Pittsburg, PA) and Micron USB2 live-field capture software (Westover Scientific, Mill Creek, WA).
Statistical Analyses
Data are expressed as means ± SEM from triplicates for TaqMan, quadruplicates for MTT assays from three experiments and triplicates for protein densitometry from three experiments. Two-way ANOVA was performed using Sigma Plot (SPSS, Chicago, IL) and p< 0.05 was considered statistically significant.
Results
Expression of β1- and β2-Adrenergic Receptors in Pancreatic Cancer Cell Lines
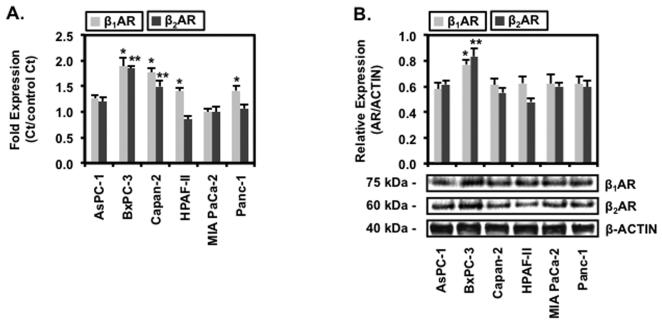
To test whether human pancreatic cell lines exhibit responsiveness to NNK treatment, six cell lines: AsPC-1, BxPC-3, Capan-2, HPAF-II, MIA PaCa-2 and Panc-1 were profiled for β1-AR and β2-AR mRNA and protein expression. BxPC-3 expressed endogenous β1AR and β2AR mRNA of 1.5 ± 0.1 fold and 1.9 ± 0.1 fold, respectively, as shown in Fig. 1A. These fold level of β-AR expression for AsPC-1, HPAF-II and Panc-1 were significantly lower, with the lowest basal expression at 0.8 ± 0.1 fold level for β1AR and 1.0 ± 0.1 fold level for β2AR in MIA PaCa-2, compared to BxPC-3. β-AR mRNA fold expressions in Capan-2 cell line were not significantly different from the BxPC-3 cell line while fold expressions were not significantly different among AsPC-1, HPAF-II, MIA PaCa-2 or Panc-1 cell lines. In addition to mRNA expression, β1AR and β2AR protein expression were significantly elevated by 1.3 ± 0.2 fold and 1.5 ± 0.1 fold, respectively, in BxPC-3 compared to AsPC-1, Capan-2, HPAF-II, MIA PaCa-2 and Panc-1 cell lines, shown in Fig. 1B. β-AR protein expression for AsPC-1, Capan-2, HPAF-II, MIA PaCa-2 and Panc-1 were not significantly different. These findings show that protein expression levels of β1AR and β2AR mirrors the mRNA expression levels in the human pancreatic cell lines and that these cell lines exhibit receptor expression that may respond to NNK.
Figure 1.
Expression of β1 and β2Adrenergic Receptors in Pancreatic Cancer Cell Lines
Human pancreatic cells AsPC-1, BxPC-3, Capan-2, HPAF-II, MIA PaCa-2 and Panc-1 were propagated to confluence and extracted for RNA and protein. (A) Total RNA extracts were used to determine mRNA expression levels of β1AR and β2AR. (B) Total protein were resolved by PAGE, transferred on PVDF membrane and probed for β1AR or β2AR specific antibody and visualized with HRP-conjugated secondary antibody and chemiluminescence. Antibody specific for β-actin was used as visual loading control. Data are presented as relative expression ± SEM based on band densitometry normalized β-actin. Statistical significance of p<0.05 are indicated by the asterisk (*) for comparison of β1AR data sets and the double asterisks (**) for comparison of β2AR data sets among the cell lines only.
Apigenin Inhibits NNK-induced Pancreatic Cellular Proliferation
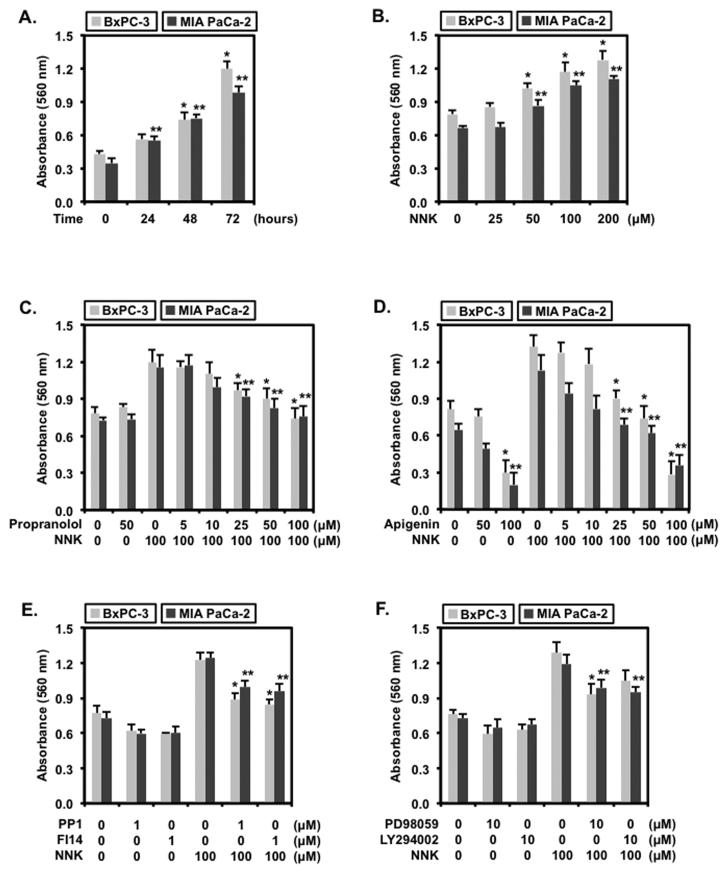
Based on the β-AR mRNA and protein expression profile for these human pancreatic cell lines, BxPC-3 exhibiting the highest basal level and MIA PaCa-2 exhibiting the lowest basal level of β-AR were used in subsequent experiments to test NNK responsiveness to high and low β-AR presence. Proliferation of BxPC-3 and MIA PaCa-2 seeded at the same density resulted in a time-dependent increase shown in Fig. 2A. Specifically, proliferation of BxPC-3 at 48 and 72 hrs increased significantly by 1.8 ± 0.2 and 2.8 ± 0.3, respectively. Similarly, proliferation of MIA PaCa-2 increased by 2.2 ± 0.3 fold at 48 hrs and 3.0 ± 0.4 fold at 72 hrs. While proliferation at 0, 24 and 48 hrs were not significantly different between BxPC-3 and MIA PaCa-2, proliferation at 72 hrs for BxPC-3 was significantly increased when compared to MIA PaCa-2. For subsequent proliferation experiments, this finding established the optimal time of 48 hr that coincides within the linear parameter of the MTT assay measurement.
Figure 2.
Apigenin Inhibits NNK-induced Pancreatic Cellular Proliferation
BxPC-3 and MIA PaCa-2 cells seeded at 8,000 cells per well in 96-well plates were (A) untreated for 0, 24, 48 or 72 hrs; (B) treated with 0, 25, 50, 100 or 200 μM of NNK for 48 hr; (C) treated with 0.1 % DMSO, 50 μM of propranolol, or 100 μM of NNK combined with either 0, 5, 10, 25, 50 or 100 μM of propranolol; (D) treated with 0.1 % DMSO, 50 μM of apigenin, 100 μM of apigenin or 100 μM of NNK combined with either 0, 5, 10, 25, 50 or 100 μM of apigenin; (E) treated with 0.1 % DMSO, 1 μM of PP1 or 1 μM of FI14 combined with or without 100 μM of NNK; or (E) treated with 0.1 % DMSO, 10 μM of PD98059 or 10 μM of LY294002 combined with or without 100 μM of NNK for 48 hr prior to MTT and absorbance measurement at 550 nm. Data are expressed as mean ± SEM from 3 separate experiments. Statistical significance of p<0.05 are indicated by the asterisk (*) for comparison of BxPC-3 data sets and the double asterisks (**) for comparison of MIA PaCa-2 data sets only.
With these basal conditions of BxPC-3 and MIA PaCa-2, treatment with varying concentrations of NNK were used to observe the modulation of cellular proliferation on these cells as shown in Fig. 2B. The findings showed that proliferation of BxPC-3 cells were significantly enhanced by 1.3 ± 0.1 fold at 50 μM, 1.5 ± 0.2 fold at 100 μM and 1.6 ± 0.2 fold at 200 μM of NNK when compared to no treatment or 25 μM of NNK treatment. Proliferation of BxPC-3 cells were not significantly different between 100 μM and 200 μM of NNK treatment, indicating that the lower concentration of 100 μM of NNK could be used to elicit an enhanced proliferative response in BxPC-3 cells. Similarly, NNK treatment of MIA PaCa-2 cells exhibited a significant dose-dependent increase in cellular proliferation of 1.3 ± 0.1 fold at 50 μM, 1.6 ± 0.2 at 100 μM and 1.7 ± 0.1 at 200 μM concentrations when compared to no treatment or 25 μM of NNK treatment. Treatment at 100 or 200 μM of NNK did not significantly enhance MIA PaCa-2 proliferation between the two concentrations, again indicating that the lower concentration of 100 μM could be used to demonstrate the enhanced proliferation of MIA PaCa-2 cells to NNK treatment.
The (RS)-1-(isopropylamino)-3-(1-naphthyloxy)-propan-2-ol (propranolol) is a non-selective β1AR and β2AR inhibitor primarily used in the treatment of hypertension and cardiovascular-related complications but has recently been tested in pancreatic cancer, particularly on NNK-mediated activation of β-AR in pancreatic ductal adenocarcinomas.24 Combined treatment with 100 μM of NNK and varying concentrations of propranolol was used to demonstrate that NNK targeted the β-AR and to determine an effective inhibitory concentration of NNK-induced BxPC-3 and MIA PaCa-2 cellular proliferation as shown in Fig. 2C. Propranolol alone at a concentration of 50 μM for 48 hrs did not exert detectable effects on the proliferation of BxPC-3 and MIA PaCa-2 cells. However, propranolol at 25, 50 and 100 μM resulted in significant inhibition of NNK-induced proliferation, respectively, by 16.7 ± 5.2 %, 25.0 ± 6.8 % and 37.7 ± 7.5 % in BxPC-3 cells and by 25.0 ± 5.0 %, 33.4 ± 5.8 % and 31.4 ± 7.5 % in MIA PaCa-2 cells. Inhibition by propranolol at 50 μM and 100 μM concentrations were not significantly different in both cell lines, indicating that the lower dose of 50 μM concentration could serve as an effective dose for subsequent experiments of this study. These findings show that propranolol treatment alone did not exhibit detectable receptor activity nor exert any effect on proliferation in either cell type. Secondly, the blockade of proliferation when treated simultaneously with 50 μM of propranolol and 100 μM of NNK suggests the possibility that propranolol competes with NNK for receptor occupation.
To determine if apigenin could elicit similar effects as propranolol, BxPC-3 and MIA PaCa-2 cells were treated with 100 μM of NNK combined with varying concentrations of apigenin for 48 hrs as shown in Fig. 2D. Treatment with 50 μM of apigenin alone did not significantly inhibit BxPC-3 proliferation but did inhibit MIA PaCa-2 proliferation by 23.4 ± 0.9 %. However, 100 μM of apigenin alone significantly inhibited both BxPC-3 and MIA PaCa-2 by 61.5 ± 6.5 % and 66.8 ± 8.8 %, respectively. Apigenin at concentrations of 25, 50 and 100 μM significantly suppressed the NNK-induced proliferation, respectively, by 30.8 ± 4.6 %, 46.2 ± 7.7 % and 76.9 ± 8.5 % in BxPC-3 cells and by 36.4 ± 4.4 %, 45.4 ± 6.3 % and 63.6 ± 7.2 % in MIA PaCa-2 cells. Inhibition of NNK-induced proliferation by apigenin at 50 μM was significantly greater than the inhibition by propranolol at the same concentration in both cell types. These findings show that the inhibitory pattern by apigenin on NNK-induced proliferation could proceed similar to that of propranolol through a competitive inhibition at the receptor level.
Since activation of β-AR has been shown to activate Src/FAK, the Src family inhibitor PP1 (4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo-d-3,4-pyrimidine) and FAK inhibitor 14 (FI14), a selective inhibitor that prevents autophosphorylation at tyrosine 397, were employed to determine if proliferation of BxPC-3 and MIA PaCa-2 could be attenuated through Src/FAK activation as shown in Fig. 2E. PP1 inhibited NNK-induced proliferation of BxPC-3 by 27.8 ± 2.9 % and MIA PaCa-2 by 20.0 ± 1.2 % whereas FI14 inhibited BxPC-3 by 31.1 ± 2.6 % and MIA PaCa-2 by 22.8 ± 1.4 %. PP1, PP2 (a selective Src inhibitor, data not shown) and FI14 resulted in a marked decrease in NNK-induced proliferation but differences between the Src and FAK inhibitors were not detected, suggesting the involvement of both Src and FAK in NNK induction of cellular proliferation.
Since Src/FAK activation can result in MEK/ERK activation, the MEK inhibitor PD98059 was used to suppress NNK-induced proliferation in the pancreatic cell lines (Fig. 2F). PD98059 inhibited BxPC-3 by 27.8 ± 2.0 % and MIA PaCa-2 by 16.9 ± 1.8 %. Similar results were obtained using the MEK1 selective inhibitor U0126 (data not shown). To determine if NNK could elicit either activation or transactivation of the EGF receptor resulting in the activation of MEK/ERK, AG1478, an EGFR kinase inhibitor, was used to suppress the NNK-induced proliferation. AG1478 exerted no effect on NNK-induced proliferation of BxPC-3 or MIA PaCa-2 cells. This inhibition by the MEK inhibitors suggests that NNK-induced maximal proliferation of the pancreatic cancer cells depends partly on the activation of MEK/ERK.
To determine the involvement of Akt signaling, the highly selective inhibitor of PI3′K LY294002 was used to suppress NNK-induced cellular proliferation (Fig. 2F). Interestingly, LY294002 inhibited NNK-induced proliferation of BxPC-3 by 18.6 ± 1.3 % and MIA PaCa-2 by 20.4 ± 1.7 %. To confirm that NNK activation of β-AR results in Akt signaling, wortmannin, a specific PI3′K inhibitor, was used to suppress NNK-induced proliferation of the pancreatic cells. Wortmannin suppressed the NNK-induced proliferation in BxPC-3 by 19.1 ± 1.7 % and MIA PaCa-2 by 18.8 ± 1.2 % (data not shown). These findings demonstrate that Akt signaling contributes to the NNK-induced proliferation of BxPC-3 and MIA PaCa-2 through survival of these cells.
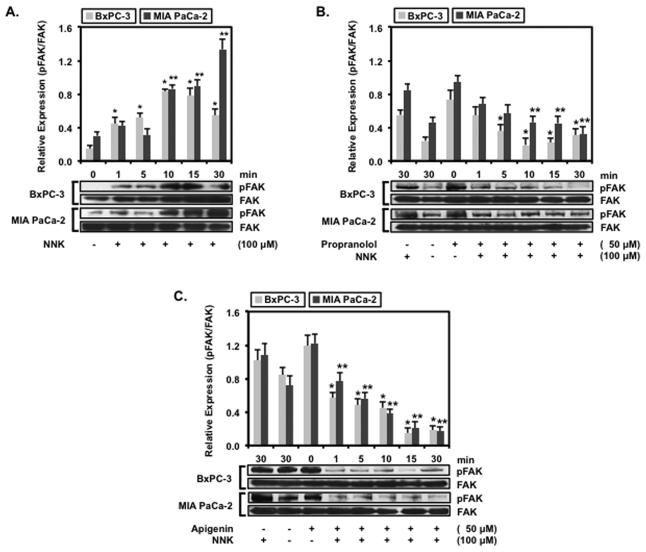
Apigenin Inhibits NNK-induced FAK Phosphorylation
Phosphorylation or autophosphorylation of FAK at tyrosine 397 in the catalytic domain has been shown to activate this kinase and promote the interaction with Src in mediating downstream signaling events.12,13 BxPC-3 and MIA PaCa-2 cells treated with 100 μM of NNK resulted in a time-dependent increase in expression of activated FAK (FAK pY397) as shown in Fig. 3A. Relative FAK pY397 expression increased significantly by 3.1 ± 0.3 fold at 10 min and 4.7 ± 0.4 fold at 15 min treatment with NNK compared to untreated BxPC-3. For MIA PaCa-2 cells, FAK pY397 expression increased significantly by 6.2 ± 0.5 at 30 min above untreated cells. This finding demonstrates that NNK could induce FAK activation in pancreatic carcinoma cells. To show that the phosphorylation of FAK resulted from β-AR activation, propranolol was used to inhibit the NNK-induced FAK activation in Fig. 3B. Relative FAK pY397 expression was abolished in BxPC-3 cells whereas FAK pY397 expression increased significantly by 2.2 ± 0.3 fold at 30 min for MIA PaCa-2 cells. Inhibition of NNK-induced FAK pY397 expression in BxPC-3 and MIA PaCa-2 cells by propranolol provides support for NNK activation of FAK through the β-AR.
Figure 3.
Apigenin Inhibits NNK-induced FAK Phosphorylation
BxPC-3 and MIA PaCa-2 and cells were propagated to confluence and then serum-starved overnight prior to treatment. The cells were (A) treated with 100 μM of NNK for 0, 1, 5, 10,15 or 30 min, (B) untreated, treated with 100 μM of NNK alone, or pre-treated with 50 μM of propranolol followed by 100 μM of NNK for 0, 1, 5, 10,15 or 30 min or (C) untreated, treated with 100 μM of NNK, or pre-treated with 50 μM of apigenin followed by 100 μM of NNK for 0, 1, 5, 10,15 or 30 min. The cells were processed for protein and probed with phospho-Y397 FAK-specific antibody. Data are presented as relative expression ± SEM based on band densitometry normalized to total FAK. Statistical significance of p<0.05 are indicated by the asterisk (*) for comparison of BxPC-3 data sets and the double asterisks (**) for comparison of MIA PaCa-2 data sets only.
Similarly, BxPC-3 and MIA PaCa-2 cells were treated apigenin to determine if this flavonoid could inhibit the NNK-induced expression of FAK pY397 shown in Fig. 3C. Apigenin suppressed relative NNK-induced FAK pY397 expression in BxPC-3 cells and significantly decreased basal FAK pY397 expression by 0.6 ± 0.1 fold at 30 min compared to untreated cells. MIA PaCa-2 cells treated with 50 μM of apigenin also exhibited significant decrease in NNK-induced FAK pY397 expression by 0.4 ± 0.1 fold at 30 min compared to untreated MIA PaCa-2 cells. The findings that apigenin inhibited NNK-induced FAK pY397 expression appears comparable to the inhibition exhibited by propranolol, suggesting that the inhibition by apigenin could possibly proceed through similar competitive mechanism as that of propranolol.
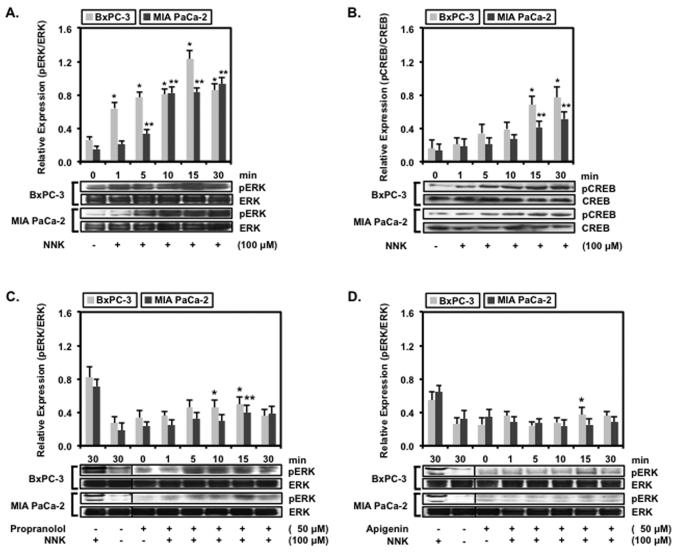
Apigenin Inhibits NNK-induced ERK activation
Downstream signaling events of FAK activation have been reported to involve the MAPK pathways.7 BxPC-3 and MIA PaCa-2 cells treated with NNK were probed for the expression of phosphorylated ERK (pERK) ERK to confirm the role of MAPK pathway involvement in FAK activation in Fig. 4A. BxPC-3 cells treated with 100 μM of NNK resulted in maximal pERK expression by 4.8 ± 0.4 fold at 15 min and decreased by 3.4 ± 0.3 fold at 30 min whereas MIA PaCa-2 treated with NNK resulted in continual increased pERK expression by 6.2 ± 0.3 fold at 30 min. The expression of pERK in response to the presence of NNK paralleled the expression of FAK pY397, implicating a possible interaction between of FAK activation and ERK activation through but not limited to the MAPK pathway in pancreatic carcinoma cells. Since the activation of β-AR has also been confirmed to signal through Gs/AC/PKA/CREB6-8, BxPC-3 and MIA PaCa-2 were assayed for the activation of CREB at serine 133 by Western blotting (Fig. 4B). Treatment with 100 μM of NNK resulted in a time-dependent increase in pCREB expression that was significant in BxPC-3 by 2.8 ± 0.7-fold at 15 min and 3.3 ± 0.8 at 30 min at and MIA PaCa-2 by 3.1 ± 0.6-fold at 15 min and 3.6 ± 0.7-fold at 30 min. While the NNK-induced increase in pCREB expression was not as robust compared to pERK expression, this finding does indicate the contribution of Gs/AC/PKA/CREB signaling to the proliferation of these pancreatic cancer cells.
Figure 4.
Apigenin Inhibits NNK-induced ERK activation
BxPC-3 and MIA PaCa-2 and cells were propagated to confluence and then serum-starved overnight prior to treatment. The cells were (A and B) treated with 100 μM of NNK for 0, 1, 5, 10,15 or 30 min, (C) pre-treated with 50 μM of propranolol followed by 100 μM of NNK for 0, 1, 5, 10,15 or 30 min or (D) pre-treated with 50 μM of apigenin followed with 100 μM of NNK for 0, 1, 5, 10,15 or 30 min. The cells were processed for protein and probed with phosphorylated ERK or phosphorylated CREB antibody followed with total ERK or total CREB antibody. Data are presented as relative expression ± SEM based on band densitometry normalized to total ERK or CREB. Statistical significance of p<0.05 are indicated by the asterisk (*) for comparison of BxPC-3 data sets and the double asterisks (**) for comparison of MIA PaCa-2 data sets only.
Based on the finding that NNK induces ERK activation, the pancreatic cells were treated with propranolol or apigenin to determine whether these inhibitors could suppress the NNK-induced pERK expression. BxPC-3 and MIA PaCa-2 cells treated with a combination of 100 μM of NNK and 50 μM of propranolol (Fig. 4C) or 50 μM of apigenin (Fig. 4D) exhibited a significant decrease in the expression of activated ERK. The findings revealed that propranolol suppressed NNK-induced pERK expression in both BxPC-3 and MIA PaCa-2 cells. Propranolol significantly inhibited NNK-induced pERK expression by 59.8 ± 4.7 % compared to NNK treatment alone at 15 min interval in BxPC-3 cells while propranolol inhibited NNK-induced pERK expression by 61.3 ± 5.9 % compared to NNK treatment alone at the 30 min interval in MIA PaCa-2 cells. Similarly, apigenin suppressed NNK-induced pERK expression in both BxPC-3 and MIA PaCa-2 cells. Inhibition of NNK-induced pERK expression by apigenin were 69.8 ± 5.3 % compared to NNK treatment alone at 15 min interval in BxPC-3 cells and 70.6 ± 6.5 % at 30 min interval compared to NNK treatment alone in MIA PaCa-2 cells. Both propranolol and apigenin were observed to inhibit NNK activation of pCREB in BxPC-3 and MIA PaCa-2 cells (data not shown). Despite the increased inhibition of NNK-induced pERK expression exerted by apigenin, the difference was not significantly compared to propranolol, suggesting that apigenin could potentially serve as a non-toxic β-blocker in the NNK-activation of β-AR.
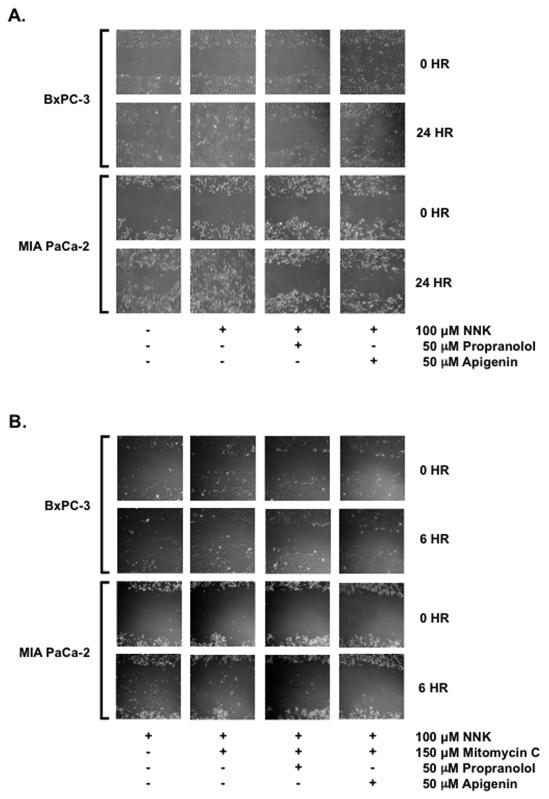
Apigenin Suppresses NNK-enhanced Proliferation/Migration
Using the scratch assay, pancreatic cells treated with 50 μM of propranolol or 50 μM of apigenin and stimulated with 100 μM of NNK for 24 hrs were observed for gap closure as a marker of cellular proliferation and migration. BxPC-3 cells untreated, propranolol-treated or apigenin-treated at 24 hrs did not proliferate and/or migrate in closing the gap whereas when treated NNK for 24 hrs, gap closure was complete (Fig. 5A). Similarly, MIA PaCa-2 cells untreated, propranolol-treated or apigenin-treated at 24 hrs gap closure was incomplete whereas NNK-treated MIA PaCa-2 cells for 24 hrs resulted in complete gap closure. To distinguish between proliferation and migration, BxPC-3 and MIA PaCa-2 cells were arrested with mitomycin C at 50 μg/mL (150 μM concentration) for 6 hrs and treated with 100 μM of NNK, with or without 50 μM of propranolol or apigenin as shown in Fig. 5B. Both propranolol and apigenin reduced migration of BxPC-3 and MIA PaCa-2 cells enhanced by NNK. These findings confirm the ability of apigenin to inhibit NNK-enhanced cellular proliferation and migration of human pancreatic cells.
Figure 5.
Apigenin Suppresses NNK-enhanced Proliferation/Migration
BxPC-3 and MIA PaCa-2 cells were propagated to confluence in 35-mm dishes for 5-7 days. At confluence, scratch assays were performed using a 10-μL pipette tip and vacuum aspiration. Remaining adherent cells were washed, replenished with serum deficient media and allowed to stabilize overnight. (A) Cells were treated on the second day with 0.1 % DMSO vehicle control, 100 μM of NNK, 100 μM NNK with 50 μM of propranolol or 100 μM of NNK with 50 μM of apigenin for 24 hrs. Gap closures in marked areas were recorded at 10X magnification. (B) Cells were treated on the second day with 100 μM of NNK, 100 μM NNK and 150 μM of mitomycin C, 100 μM NNK and 150 μM of mitomycin C with without 50 μM of propranolol or 100 μM NNK and 150 μM of mitomycin C with 50 μM of apigenin for 6 hrs. Cell migration were observed in marked areas at 10X magnification.
Discussion
Overall, the results demonstrate that NNK activates FAK, ERK and CREB, presumably through the binding and activation of β-AR, in pancreatic cancer cells and that the activation of FAK, ERK and CREB correlates with cellular proliferation and migration. NNK has been shown to serve as a high affinity agonist for the β-adrenergic receptors in lung cells25 and NNK activation of β1AR and β2AR has been reported in pancreatic ductal cells.4,5 Despite increased β1AR and β2AR expression in BxPC-3 compared to MIA PaCa-2, both these pancreatic adenocarcinoma cells responded to NNK treatment that resulted in augmented cellular proliferation and migration. Activation of FAK, as a marker of proliferation and migration, has only been reported as a consequence of β-AR activation in lung A549 cells.7 In the BxPC-3 and MIA PaCa-2 cells, however, NNK activation of β-AR resulted at least in part to the downstream activation of FAK. In addition, activation of β-AR followed by FAK phosphorylation correlated with downstream ERK activation, occurring within 30 minutes of NNK stimulation to the pancreatic cells. These findings are consistent with reports that activation of Src/FAK could proceed to signal through MAPK pathway resulting in ERK activation.7,8 Furthermore, these results suggest that NNK-enhanced proliferation and migration of pancreatic cells result in part to the signaling of β-AR through Src/FAK and ERK.
Using the non-specific β-AR blocker propranolol, the findings show that NNK-enhanced proliferation and migration of the BxPC-3 and MIA PaCa-2 could be attenuated, demonstrating that the β-AR are involved in mediating the signal. Propranolol inhibition of NNK-induced β-AR activation is further supported with the attenuation of the correlative FAK phosphorylation and ERK activation in these pancreatic cells. Indeed, inhibition of NNK-induced proliferation and β-AR activation by propranolol has also been demonstrated in human lung adenocarcinoma cell line NCI-H322.26 Whereas propranolol was shown to modulate the activation of Src by β-AR activation in small cell lung cancer and non-small cell lung cancer cells.27 In human gastric AGS cells that express nicotinic acetylcholine receptor and β-AR, propranolol inhibited the NNK-induced β-AR activation of p38MAPK and ERK activation.8 Furthermore, propranolol attenuated NNK targeted β-AR activation as well as EGFR activation and ERK activation in normal immortalized human pancreatic ductal HPDE6-c7 epithelial cells.4 It is important to note that HPDE6-c7 cells responded to NNK concentration in the picomolar to nanomolar range whereas BxPC-3 and MIA PaCa-2 responded in the micromolar range. It is unclear as to why these pancreatic cancer cells only elicit ERK activation in the micromolar range. In pancreatic ductal adenocarcinoma cells, propranolol inhibited NNK targeted alpha7 nicotinic acetylcholine receptors activation with associated CREB and ERK activation in.6,8 Blockade of NNK activation was achieved with a maximal dose of 1 μM whereas inhibition was observed at 50 μM range for BxPC-3 and MIA PaCa-2. Nevertheless, these studies appear consistent with the finding that propranolol is capable of attenuating NNK-induced β-AR activation and associated FAK phosphorylation and ERK activation in pancreatic adenocarcinoma cells.
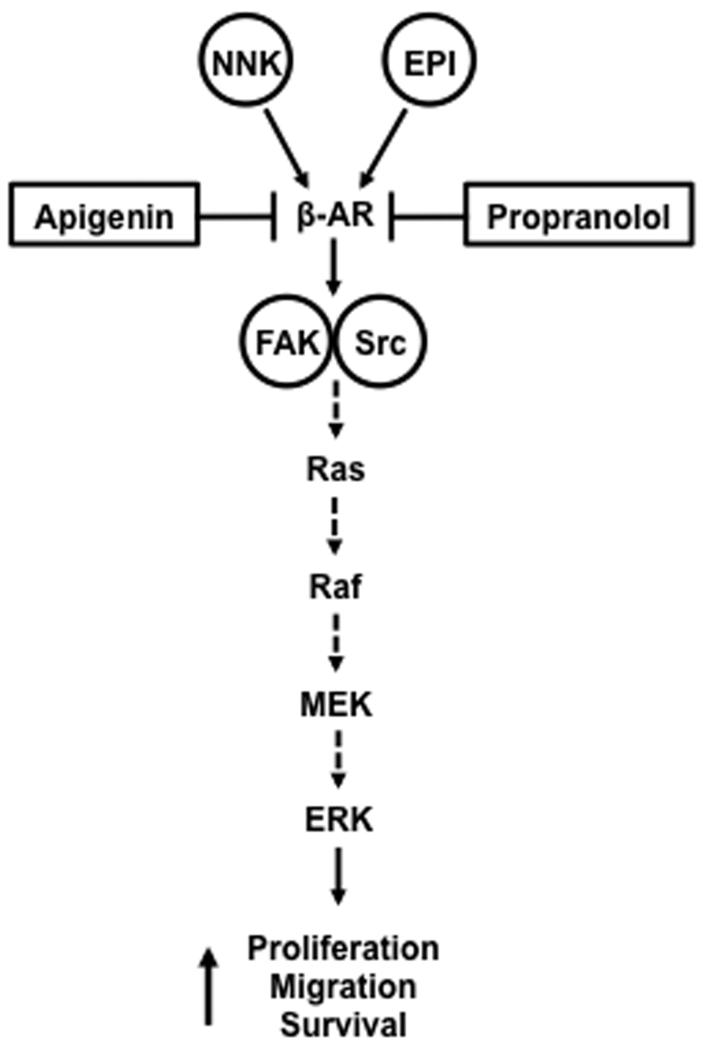
Since the flavonoids have been shown to possess anti-carcinogenic activities,18,28 apigenin was compared to propranolol as a potential non-toxic inhibitor of NNK-mediated activation of β-AR and proliferation and migration of pancreatic cells. The flavonoids are a class of polyphenolic compounds exhibiting potent anti-oxidant activity29,30 as well as anti-tumor activity for a number of malignancies.31-34 While two flavonoids genistein and apigenin have been identified in exhibiting efficacy in pancreatic cancer models,32,35 apigenin, however, exerted a greater potent growth inhibition in some cancer cell lines, including pancreatic cancer.36-39 Similar to propranolol, apigenin inhibited NNK-induced β-AR activation and proliferation and migration of BxPC-3 and MIA PaCa-2 cells as well as attenuated FAK phosphorylation and ERK activation. Interestingly, the extent of apigenin inhibition paralleled the inhibition by propranolol at the same concentration. These results were consistent with reports demonstrating the inhibition of migration and proliferation by apigenin in carcinoma cells.40,41 Furthermore, apigenin has been demonstrated to inhibit migration and invasion of human A2780 ovarian cancer cells and prostate PC3-M cancer cells through a Src/FAK signaling system.42,43 Despite studies demonstrating the therapeutic efficacy of targeting Src in attenuating tumor metastases and angiogenesis in pancreatic carcinoma and endocrine tumor cells44-46, the targeting of FAK by a non-toxic flavonoid has not yet been shown in pancreatic cancer cells, although the potential of FAK as viable candidate for therapeutic target has been suggested.47,48 These studies confirm the role of Src/FAK signaling in mediating cancer cell migration and proliferation. Our finding shows that NNK binding to the β-AR activates FAK with the subsequent downstream activation of ERK as depicted in the speculative scenario involving FAK/Src activation of the MAPK pathway resulting in ERK activation (Fig. 6). Both propranolol and apigenin appears to inhibit NNK activation of β-AR at the receptor level. The findings that apigenin attenuates NNK-induced cellular proliferation and migration through FAK and ERK activation, presumably by β-AR activation, suggest this non-toxic flavonoid as a viable therapeutic candidate for pancreatic cancer.
Figure 6.
Speculative Scenario of NNK-activated β-adrenergic Signaling Suppressed by Apigenin NNK binds to the β-adrenergic receptor (β-AR) that subsequently activates focal adhesion kinase (FAK) and Src to elicit the activation of the mitogenic activated protein kinase (MAPK) pathway. MAPK pathway involves activation of Ras/Raf/MEK resulting in the activation of ERK. Both the synthetic drug propranolol and the phytochemical apigenin appears to inhibit NNK activation of β-AR at the receptor level.
Acknowledgement
The authors would like to thank the Hirshberg Foundation for Pancreatic Cancer Research for their continual support of this laboratory and Dr. Adrian Lunn for his consult.
This work was supported in part by NIH grant R01CA122042 (G.E.) and P01AT003960 (UCLA Center of Excellence in Pancreatic Diseases).
Abbreviations
- Apigenin
5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
- β1AR
β1-adrenergic receptor
- β2AR
β2-adrenergic receptor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- FAK
focal adhesion kinase
- MAPK
mitogen-activated protein kinase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- PI3′K
phosphoinositide 3′-kinase
- Propranolol
(RS)-1-(isopropylamino)-3-(1-naphthyloxy)-propan-2-ol.
Footnotes
Conflicts of Interest and Sources of Funding: No conflicts of interest are declared for all authors of this work.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hung Pham, Department of Medicine, Veterans Affair Greater Los Angeles Healthcare System, Los Angeles, CA 90073.
Monica Chen, Department of Surgery, Hirshberg Translational Pancreatic Cancer Research Laboratory, UCLA Center of Excellence in Pancreatic Diseases, David Geffen School of Medicine, University of California – Los Angeles, Los Angeles, CA 90095.
Hiroki Takahashi, Department of Surgery, Hirshberg Translational Pancreatic Cancer Research Laboratory, UCLA Center of Excellence in Pancreatic Diseases, David Geffen School of Medicine, University of California – Los Angeles, Los Angeles, CA 90095.
Jonathan King, Department of Surgery, Hirshberg Translational Pancreatic Cancer Research Laboratory, UCLA Center of Excellence in Pancreatic Diseases, David Geffen School of Medicine, University of California – Los Angeles, Los Angeles, CA 90095.
Howard A. Reber, Department of Surgery, Hirshberg Translational Pancreatic Cancer Research Laboratory, UCLA Center of Excellence in Pancreatic Diseases, David Geffen School of Medicine, University of California – Los Angeles, Los Angeles, CA 90095.
Oscar Joe Hines, Department of Surgery, Hirshberg Translational Pancreatic Cancer Research Laboratory, UCLA Center of Excellence in Pancreatic Diseases, David Geffen School of Medicine, University of California – Los Angeles, Los Angeles, CA 90095.
Stephen Pandol, Department of Medicine, Veterans Affair Greater Los Angeles Healthcare System, Los Angeles, CA 90073.
Guido Eibl, Department of Surgery, Hirshberg Translational Pancreatic Cancer Research Laboratory, UCLA Center of Excellence in Pancreatic Diseases, David Geffen School of Medicine, University of California – Los Angeles, Los Angeles, CA 90095.
References
- 1.Hart AR, Kennedy H, Harvey I. Pancreatic cancer: a review of evidence on causation. Clin. Gastroenterol. Hepatol. 2008;6:275–282. doi: 10.1016/j.cgh.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 2.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 3.Rivenson A, Hoffmann D, Prokopczyk B, et al. Induction of lung and exocrine pancreas tumors in F344 rats by tobacco-specific and Areca-derived N-nitrosamines. Cancer Res. 1988;48:6912–6917. [PubMed] [Google Scholar]
- 4.Askari MD, Tsao MS, Schuller HM. The tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone stimulates proliferation of immortalized human pancreatic duct epithelia through beta-adrenergic transactivation of EGF receptors. J. Cancer Res. Clin. Oncol. 2005;131:639–648. doi: 10.1007/s00432-005-0002-7. [DOI] [PubMed] [Google Scholar]
- 5.Weddle DL, Tithoff P, Williams M, et al. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22(3):473–479. doi: 10.1093/carcin/22.3.473. [DOI] [PubMed] [Google Scholar]
- 6.Bétuing S, Daviaud D, Valet P, et al. Alpha2-Adrenoceptor stimulation promotes actin polymerization and focal adhesion in 3T3F442A and BFC-1beta preadipocytes. Endocrinology. 1996;137(12):5220–5229. doi: 10.1210/endo.137.12.8940338. [DOI] [PubMed] [Google Scholar]
- 7.Ahn S, Maudsley S, Luttrell LM, et al. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J. Biol Chem. 1999;274(3):1185–8. doi: 10.1074/jbc.274.3.1185. [DOI] [PubMed] [Google Scholar]
- 8.Jin Z, Xin M, Deng X. Survival function of protein kinase C{iota} as a novel nitrosamine 4- (methylnitrosamino)-1-(3-pyridyl)-1-butanone-activated bad kinase. J. Biol Chem. 2005;280(16):16045–16052. doi: 10.1074/jbc.M413488200. [DOI] [PubMed] [Google Scholar]
- 9.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22(4):337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 10.Visser CJ, Rijksen G, Woutersen RA, et al. Increased immunoreactivity and protein tyrosine kinase activity of the protooncogene pp60c-Src in preneoplastic lesions in rat pancreas. Lab. Invest. 1996;74:2–11. [PubMed] [Google Scholar]
- 11.Lutz MP, Esser IB, Flossmann-Kast BB, et al. Overexpression and activation of the tyrosine kinase Src in human pancreatic carcinoma. Biochem. Biophys. Res. Commun. 1998;243:503–508. doi: 10.1006/bbrc.1997.8043. [DOI] [PubMed] [Google Scholar]
- 12.Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim. Biophys. Acta. 2004;1692(2-3):77–102. doi: 10.1016/j.bbamcr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Hauck CR, Hunter T, Schlaepfer DD. The v-Src SH3 domain facilitates a cell adhesion-independent association with focal adhesion kinase. J. Biol. Chem. 2001;276:17653–17662. doi: 10.1074/jbc.M009329200. [DOI] [PubMed] [Google Scholar]
- 14.McLean GW, Carragher NO, Avizienyte E, et al. The role of focal-adhesion kinase in cancer - a new therapeutic opportunity. Nat. Rev. Cancer. 2005;5(7):505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 15.Furuyama K, Doi R, Mori T, et al. Clinical significance of focal adhesion kinase in resectable pancreatic cancer. World J. Surg. 2006;30(2):219–226. doi: 10.1007/s00268-005-0165-z. [DOI] [PubMed] [Google Scholar]
- 16.Duxbury MS, Ito H, Zinner MJ, et al. Focal adhesion kinase gene silencing promotes anoikis and suppresses metastasis of human pancreatic adenocarcinoma cells. Surgery. 2004;135(5):555–562. doi: 10.1016/j.surg.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Huang YT, Lee LT, Lee PP, et al. Targeting of focal adhesion kinase by flavonoids and small-interfering RNAs reduces tumor cell migration ability. Anticancer Res. 2005;25(3B):2017–2025. [PubMed] [Google Scholar]
- 18.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise. Int. J. Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- 19.Salabat MR, Melstrom LG, Strouch MJ, et al. Geminin is overexpressed in human pancreatic cancer and downregulated by the bioflavanoid apigenin in pancreatic cancer cell lines. Mol. Carcinog. 2008;47:835–844. doi: 10.1002/mc.20441. [DOI] [PubMed] [Google Scholar]
- 20.Ujiki MB, Ding XZ, Salabat MR, et al. Apigenin inhibits pancreatic cancer cell proliferation through G2/M cell cycle arrest. Mol. Cancer. 2006;5:76–83. doi: 10.1186/1476-4598-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamacher R, Saur D, Fritsch R, et al. Casein kinase II inhibition induces apoptosis in pancreatic cancer cells. Oncol. Rep. 2007;18(3):695–701. [PubMed] [Google Scholar]
- 22.Melstrom LG, Salabat MR, Ding XZ, et al. Apigenin inhibits the GLUT-1 glucose transporter and the phosphoinositide 3-kinase/Akt pathway in human pancreatic cancer cells. Pancreas. 2008;37(4):426–431. doi: 10.1097/MPA.0b013e3181735ccb. [DOI] [PubMed] [Google Scholar]
- 23.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 24.Al-Wadei HA, Al-Wadei MH, Schuller HM. Prevention of pancreatic cancer by the beta-blocker propranolol. Anticancer Drugs. 2009;20(6):477–482. doi: 10.1097/CAD.0b013e32832bd1e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuller HM, Tithof PK, Williams M, et al. The tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is a beta-adrenergic agonist and stimulates DNA synthesis in lung adenocarcinoma via beta-adrenergic receptor-mediated release of arachidonic acid. Cancer Res. 1999;59(18):4510–4515. [PubMed] [Google Scholar]
- 26.Schuller HM, Cole B. Regulation of cell proliferation by beta-adrenergic receptors in a human lung adenocarcinoma cell line. Carcinogenesis. 1989;10(9):1753–1755. doi: 10.1093/carcin/10.9.1753. [DOI] [PubMed] [Google Scholar]
- 27.Shin VY, Jin HC, Ng EK, et al. Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induce cyclooxygenase-2 activity in human gastric cancer cells: Involvement of nicotinic acetylcholine receptor (nAChR) and beta-adrenergic receptor signaling pathways. Toxicol. Appl. Pharmacol. 2008;233(2):254–261. doi: 10.1016/j.taap.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Jin Z, Xin M, Deng X. Survival function of protein kinase C(iota) as a novel nitrosamine 4- (methylnitrosamino)-1-(3-pyridyl)-1-butanone-activated bad kinase. J. Biol Chem. 2005;280(16):16045–16052. doi: 10.1074/jbc.M413488200. [DOI] [PubMed] [Google Scholar]
- 29.Chen D, Dou QP. Tea polyphenols and their roles in cancer prevention and chemotherapy. Int. J. Mol. Sci. 2008;9(7):1196–1206. doi: 10.3390/ijms9071196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bentrem D, Fox JE, Pearce ST, et al. Distinct molecular conformations of the estrogen receptor alpha complex exploited by environmental estrogens. Cancer Res. 2003;63:7490–7496. [PubMed] [Google Scholar]
- 31.Fotsis T, Pepper M, Adlercreutz H, et al. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc. Natl. Acad. Sci USA. 1993;90:2690–2694. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Ahmed F, Ali S, et al. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65:6934–6942. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- 33.Buchler P, Reber HA, Buchler MW, et al. Antiangiogenic activity of genistein in pancreatic carcinoma cells is mediated by the inhibition of hypoxia-inducible factor-1 and the down-regulation of VEGF gene expression. Cancer. 2004;100:201–210. doi: 10.1002/cncr.11873. [DOI] [PubMed] [Google Scholar]
- 34.Fotsis T, Pepper MS, Aktas E, et al. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916–2921. [PubMed] [Google Scholar]
- 35.Li W, Fan J, Bertino JR. Selective sensitization of retinoblastoma protein-decicient sarcoma cells to doxorubicin by flavopiridol-mediated inhibition of cyclin-dependent kinase 2 kinase activity. Cancer Res. 2001;61:2579–2582. [PubMed] [Google Scholar]
- 36.Buchler P, Gukovskaya AS, Mouria M, et al. Prevention of metastatic pancreatic cancer growth in vivo by induction of apoptosis with genistein, a naturally occurring isoflavonoid. Pancreas. 2003;26:264–273. doi: 10.1097/00006676-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Van Dross R, Xue Y, Knudson A, et al. The chemo-preventive bioflavonoid apigenin modulates signal transduction pathways in keratinocyte and colon carcinoma cell lines. J. Nutr. 2003;133:3800S–3804. doi: 10.1093/jn/133.11.3800S. [DOI] [PubMed] [Google Scholar]
- 38.Torkin R, Lavoie JF, Kaplan DR, et al. Induction of caspase-dependent, p53-mediated apoptosis by apigenin in human neuroblastoma. Mol. Cancer Ther. 2005;4:1–11. [PubMed] [Google Scholar]
- 39.Liu LZ, Fang J, Zhou Q, et al. Apigenin inhibits expression of vascular endothelial growth factor and angiogenesis in human lung cancer cells: Implication of chemoprevention of lung cancer. Mol. Pharmacol. 2005;68:635–643. doi: 10.1124/mol.105.011254. [DOI] [PubMed] [Google Scholar]
- 40.Caltagirone S, Rossi C, Poggi A, et al. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int. J. Cancer. 2000;87:595–600. doi: 10.1002/1097-0215(20000815)87:4<595::aid-ijc21>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Czyz J, Madeja Z, Irmer U, et al. Flavonoid apigenin inhibits motility and invasiveness of carcinoma cells in vitro. Int. J. Cancer. 2005;114(1):12–18. doi: 10.1002/ijc.20620. [DOI] [PubMed] [Google Scholar]
- 42.Fang J, Zhou Q, Liu LZ, et al. Apigenin inhibits tumor angiogenesis through decreasing HIF-1alpha and VEGF expression. Carcinogenesis. 2007;28:858–864. doi: 10.1093/carcin/bgl205. [DOI] [PubMed] [Google Scholar]
- 43.Hu XW, Meng D, Fang J. Apigenin inhibited migration and invasion of human ovarian cancer A2780 cells through focal adhesion kinase. Carcinogenesis. 2008;29:2369–2376. doi: 10.1093/carcin/bgn244. [DOI] [PubMed] [Google Scholar]
- 44.Franzen CA, Amargo E, Todorović V, et al. The chemopreventive bioflavonoid apigenin inhibits prostate cancer cell motility through the focal adhesion kinase/Src signaling mechanism. Cancer Prev. Res. 2009;2(9):830–841. doi: 10.1158/1940-6207.CAPR-09-0066. [DOI] [PubMed] [Google Scholar]
- 45.Ischenko I, Guba M, Yezhelyev M, et al. Effect of Src kinase inhibition on metastasis and tumor angiogenesis in human pancreatic cancer. Angiogenesis. 2007;10(3):167–182. doi: 10.1007/s10456-007-9071-3. [DOI] [PubMed] [Google Scholar]
- 46.Nagaraj NS, Smith JJ, Revetta F, Washington MK, Merchant NB. Targeted inhibition of SRC kinase signaling attenuates pancreatic tumorigenesis. Mol. Cancer Ther. 2010;9(8):2322–2332. doi: 10.1158/1535-7163.MCT-09-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Florio A, Capurso G, Milione M, et al. Src family kinase activity regulates adhesion, spreading and migration of pancreatic endocrine tumour cells. Endocr. Relat. Cancer. 2007;14(1):111–124. doi: 10.1677/erc.1.01318. [DOI] [PubMed] [Google Scholar]
- 48.Provenzano PP, Keely PJ. The role of focal adhesion kinase in tumor initiation and progression. Cell Adh. Migr. 2009;3(4):347–350. doi: 10.4161/cam.3.4.9458. [DOI] [PMC free article] [PubMed] [Google Scholar]