Abstract
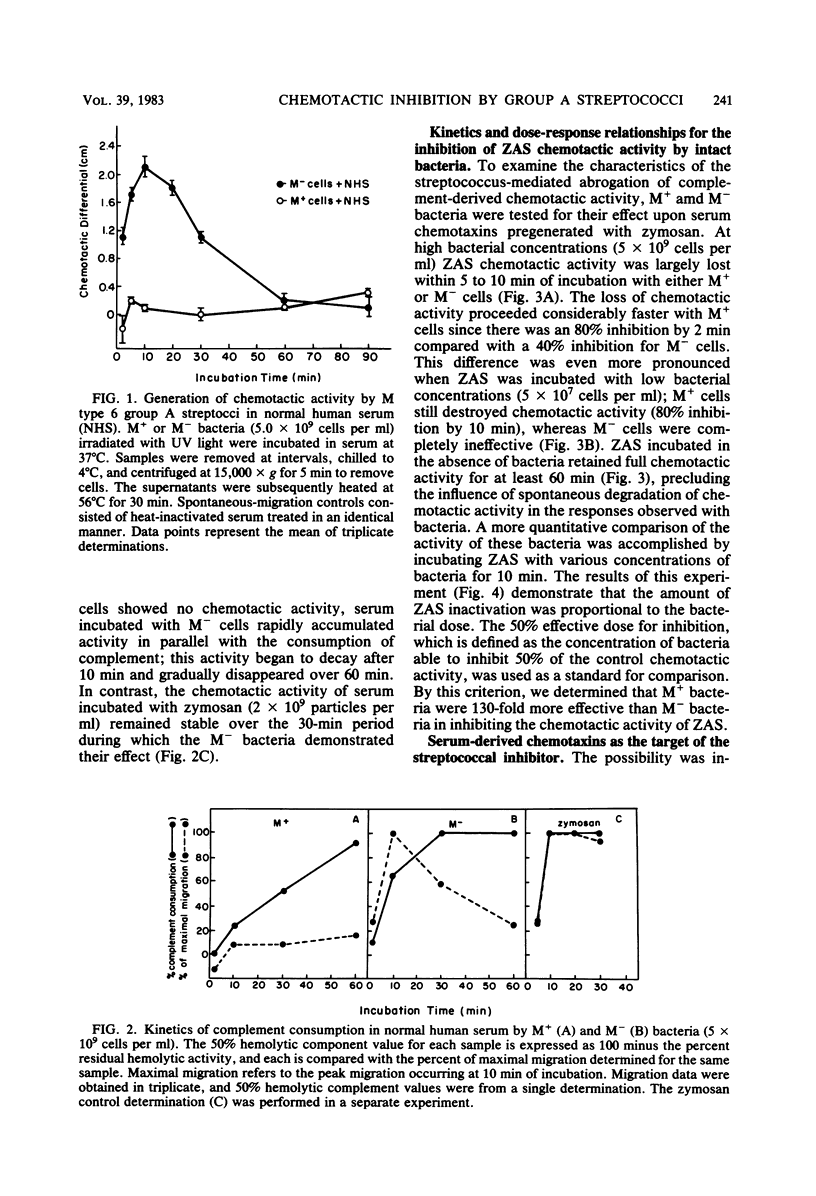
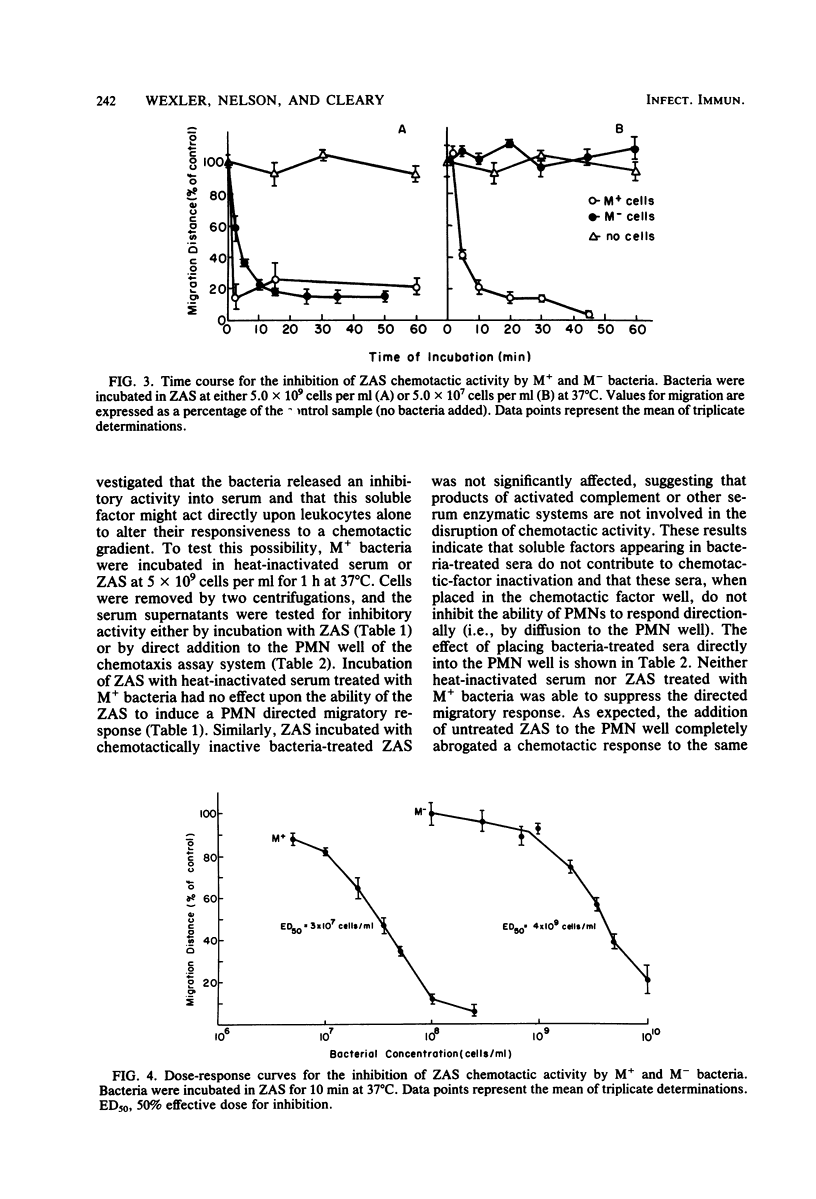
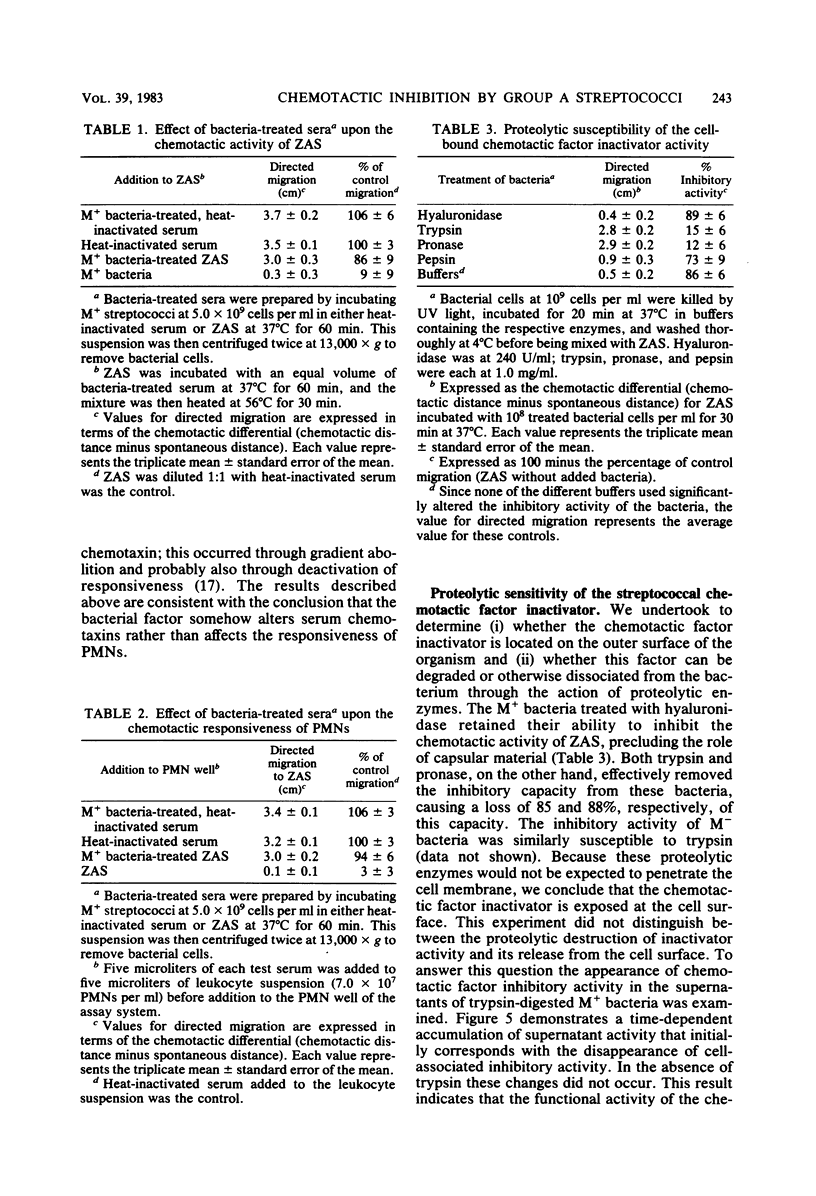
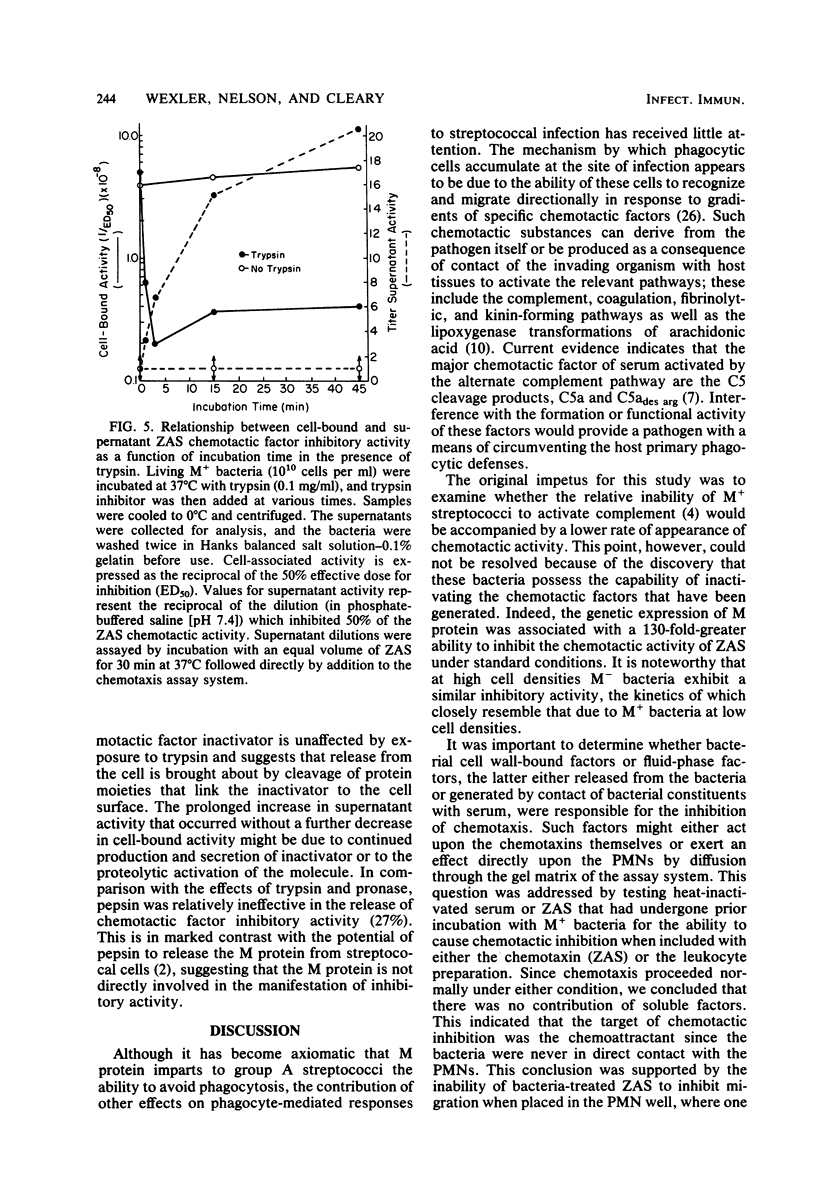
The influence of M protein on the capacity of group A streptococci to generate neutrophil chemotactic activity in normal human serum was examined. Incubation of serum with M- bacteria for up to 10 min led to the production of chemotactic activity. In contrast, incubation of serum with M+ bacteria did not elicit serum chemotactic activity over a 1-h period, even though complement was activated to completion. Further experiments revealed that both M+ and M- bacteria could inhibit the chemotactic activity of serum preexposed to zymosan. However, the M+ bacteria possessed a 130-fold-greater inhibitory capacity in this regard than the M- bacteria. This antichemotactic property was not detectable in the fluid phase of serum incubated with bacteria, thereby ruling out neutrophil-directed effects. Treatment of the bacteria with trypsin resulted in the release of the inhibitory molecule, suggesting that proteins are involved in its maintenance at the cell surface. However, the resistance of the chemotactic factor inactivator to pepsin and trypsin indicated that the protease-sensitive M protein was not involved. These results demonstrate a heretofore uncharacterized activity of group A streptococci that may contribute to virulence through modulation of the host chemotactic response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Beachey E. H., Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976 Apr 1;143(4):759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenberg J. L., Ward P. A. Chemotactic factor inactivator in normal human serum. J Clin Invest. 1973 May;52(5):1200–1206. doi: 10.1172/JCI107287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisno A. L. Alternate complement pathway activation by group A streptococci: role of M-protein. Infect Immun. 1979 Dec;26(3):1172–1176. doi: 10.1128/iai.26.3.1172-1176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozna J. P., Senior R. M., Kreutzer D. L., Ward P. A. Chemotactic factor inactivators of human granulocytes. J Clin Invest. 1977 Dec;60(6):1280–1288. doi: 10.1172/JCI108887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth D. E., Rowe J. G., Hugli T. E. A modified method for chemotaxis under agarose. J Immunol Methods. 1979;25(4):337–353. doi: 10.1016/0022-1759(79)90026-7. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Guckian J. C., Christensen W. D., Fine D. P. Evidence for quantitative variability of bacterial opsonic requirements. Infect Immun. 1978 Mar;19(3):822–826. doi: 10.1128/iai.19.3.822-826.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. J., Wannamaker L. W. The serum opacity reaction of Streptococcus pyogenes: general properties of the streptococcal factor and of the reaction in aged serum. J Hyg (Lond) 1968 Mar;66(1):37–47. doi: 10.1017/s0022172400040924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks-Weis J., Kim Y., Cleary P. P. Restricted deposition of C3 on M+ group A streptococci: correlation with resistance to phagocytosis. J Immunol. 1982 Apr;128(4):1897–1902. [PubMed] [Google Scholar]
- Kreutzer D. L., Claypool W. D., Jones M. L., Ward P. A. Isolation by hydrophobic chromatography of the chemotactic factor inactivators from human serum. Clin Immunol Immunopathol. 1979 Feb;12(2):162–176. doi: 10.1016/0090-1229(79)90005-9. [DOI] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- Laxalt K. A., Kozel T. R. Chemotaxigenesis and activation of the alternative complement pathway by encapsulated and non-encapsulated Cryptococcus neoformans. Infect Immun. 1979 Nov;26(2):435–440. doi: 10.1128/iai.26.2.435-440.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., McCormack R. T., Fiegel V. D., Simmons R. L. Chemotactic deactivation of human neutrophils: evidence for nonspecific and specific components. Infect Immun. 1978 Nov;22(2):441–444. doi: 10.1128/iai.22.2.441-444.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Peterson P. K., Schmeling D., Cleary P. P., Wilkinson B. J., Kim Y., Quie P. G. Inhibition of alternative complement pathway opsonization by group A streptococcal M protein. J Infect Dis. 1979 May;139(5):575–585. doi: 10.1093/infdis/139.5.575. [DOI] [PubMed] [Google Scholar]
- Ray T. L., Wuepper K. D. Activation of the alternative (properdin) pathway of complement by Candida albicans and related species. J Invest Dermatol. 1976 Dec;67(6):700–703. doi: 10.1111/1523-1747.ep12598581. [DOI] [PubMed] [Google Scholar]
- Repo H. Leukocyte migration agarose test for the assessment of human neutrophil chemotaxis. I. Effects of environmental factors on neutrophil migration under agarose. Scand J Immunol. 1977;6(3):203–209. doi: 10.1111/j.1365-3083.1977.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Schmeling D. J., Peterson P. K., Barr I. M., Kim Y., Quie P. G. Chemotaxigenesis by encapsulated Staphylococcus aureus M. Infect Immun. 1980 Feb;27(2):700–703. doi: 10.1128/iai.27.2.700-703.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylewska S., Kłosińska-Kita E., Malinowski W. Chemotactic activity of polymorphonuclear leukocytes to Streptococcus pyogenes. Zentralbl Bakteriol Orig A. 1978 Sep;241(3):294–300. [PubMed] [Google Scholar]
- Widdowson J. P., Maxted W. R., Grant D. L., Pinney A. M. The relationship between M-antigen and opacity factor in group A streptococci. J Gen Microbiol. 1971 Jan;65(1):69–80. doi: 10.1099/00221287-65-1-69. [DOI] [PubMed] [Google Scholar]
- Widdowson J. P., Maxted W. R., Pinney A. M. An M-associated protein antigen (MAP) of group A streptococci. J Hyg (Lond) 1971 Dec;69(4):553–564. doi: 10.1017/s0022172400021823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C., Lackie J. M. The adhesion, migration and chemotaxis of leucocytes in inflammation. Curr Top Pathol. 1979;68:47–88. doi: 10.1007/978-3-642-67311-5_3. [DOI] [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. A functional differentiation of human neutrophil granules: generation of C5a by a specific (secondary) granule product and inactivation of C5a by azurophil (primary) granule products. J Immunol. 1977 Sep;119(3):1068–1076. [PubMed] [Google Scholar]


