Abstract
Numerous studies from the clinical and preclinical literature indicate that general anesthetic agents have toxic effects on the developing brain, but the mechanism of this toxicity is still unknown. Previous studies have focused on the effects of anesthetics on cell survival, dendrite elaboration, and synapse formation, but little attention has been paid to possible effects of anesthetics on the developing axon. Using dissociated mouse cortical neurons in culture, we found that isoflurane delays the acquisition of neuronal polarity by interfering with axon specification. The magnitude of this effect is dependent on isoflurane concentration and exposure time over clinically relevant ranges, and it is neither a precursor to nor the result of neuronal cell death. Propofol also appears to interfere with the acquisition of neuronal polarity, but the mechanism does not require activity at GABAA receptors. Rather, the delay in axon specification likely results from a slowing of the extension of pre-polarized neurites. The effect is not unique to isoflurane as propofol also appears to interfere with the acquisition of neuronal polarity. These findings demonstrate that anesthetics may interfere with brain development via effects on axon growth and specification, thus introducing a new potential target in the search for mechanisms of pediatric anesthetic neurotoxicity.
Keywords: Anesthetic, Isoflurane, Neurotoxicity, Axon, Polarity, Neuron
INTRODUCTION
The evidence from a wide range of clinical and pre-clinical studies suggests that exposure to general anesthetics (GAs) results in harmful effects on brain development. These concerns were first raised in the 1970s and 1980s by studies in which long-term, chronic exposure to low levels of halothane from gestation through the early postnatal period in rodents resulted in poorer performance in behavioral testing as well as disturbances in synaptogenesis and neurite development. 1–3 The clinical question driving this work was a concern that chronic exposure to anesthetic gases in the operating room might pose a risk to operating room personnel who are pregnant. More recently the focus of investigation has been on whether a single GA exposure or several discrete exposures at clinically relevant doses can impair brain development. In a groundbreaking study, Todorovic and coworkers showed that in early postnatal rodents a single exposure to a combination of nitrous oxide, midazolam, and isoflurane, agents commonly used together in pediatric anesthetic practice, could cause persistent deficits in performance on behavioral tests of learning and memory. 4 Furthermore, clinical studies using epidemiologic techniques have showed a correlation between early pediatric exposure to GA and subsequent learning and/or behavioral disorders. 5–8 Taken together these findings have generated a renewed interest in anesthetic toxicity in the developing brain. 9–12
The mechanism by which GAs cause clinically relevant alterations in brain development has not been fully elucidated. Numerous studies have shown that commonly used GAs can cause enhanced neuronal apoptosis in the developing brain in animal models. 4, 13–15 However, apoptosis is a normal event in brain development that serves the adaptive purpose of pruning unnecessary elements from neuronal circuits. 16, 17 Thus, it is unclear whether anesthetic-induced apoptosis is a cause of impaired brain function, a consequence of some underlying disruption of circuit formation, or perhaps even an epiphenomenon. A number of groups have begun to investigate alternate mechanisms related to circuit formation and have found disruptions in synapse formation, dendritic development, and astrocyte function. 14, 18–21 There has been relatively little study of the effects of GAs on the generation and development of axons.
The polarization of the neuron that results from the differentiation of the axon is a critical event in brain development, as neuronal function depends on the segregation of axon-specific and dendrite-specific components to their respective compartments. 22–24 Prior to polarization, the developing neuron gives rise to pre-polarized neurites that do not possess axonal or dendritic characteristics. These minor processes alternate between retraction and extension for a period of time, and subsequently one rapidly elongates and begins to acquire axonal characteristics. 25–27 To address the question of whether anesthetic toxicity in brain development might be due in part to effects on the development of axons, we asked whether exposure to GAs interferes with the acquisition of neuronal polarity in cortical neurons in dissociated culture.
METHODS
Cultures
Care of animals adhered strictly to the guidelines of the NIH, Columbia University, and the Mount Sinai School of Medicine. Dissociated neurons were prepared from embryonic day 18 C57BL/6 mouse neocortex as previously described, 28 using techniques modified from well-established hippocampal culture protocols. 29 Briefly, neocortex was dissected out into cold PBS and digested for 15 minutes at 37°C in 320 μM papain solution (Sigma-Aldrich, St. Louis, MO). Subsequently the tissue was triturated with a fire-polished Pasteur pipette into a single-cell suspension and layered on top of a 20 mg/mL albumin for a discontinuous gradient centrifugation at 800 rpm for ten minutes. Neurons were then plated at a density of 100/mm2 on cover slips coated with poly-D-lysine (Sigma-Aldrich), where they were allowed to settle for three hours in media containing 10% horse serum. They were maintained in B-27/L-glutamine supplemented Neurobasal media (Invitrogen, Carlsbad, CA) and co-cultured with a feeder layer of an immortalized astrocytic cell line (gift from JW Jacobberger lab). 30 In the case of L1 immunocytochemistry experiments, cultures were prepared from Sprague-Dawley rats using similar protocols 31 and plated on poly-L-lysine. 28 Experiments on dissociated neurons were performed at one to three days in vitro and represent at least two separate cultures.
Anesthetic exposure
Anesthetic exposures were performed in supplemented Neurobasal media immediately after neurons were allowed to adhere to coverslips. After exposure neurons were placed in fresh media conditioned by the astrocytic feeder layer. For isoflurane exposure, coverslips with adhered neurons were placed in an airtight, humidified modular chamber (Billups-Rothenberg, Del Mar, CA) connected to an agent-specific calibrated vaporizer (Datex-Ohmeda, Madison, WI) that delivered the agent mixed with 5% CO2/95% air carrier gas at 12 L/min as previously described. 32 Controls for these exposures consist of carrier gas in the absence of isoflurane. To ensure consistent delivery of the agent, gas composition was periodically measured using a 5250 RGM gas analyzer (Datex-Ohmeda). The standard exposure was 2.4% isoflurane for four hours, except as noted in experiments that tested concentration-response (Fig 1C) and exposure time-response. (Fig 1D) This concentration was chosen in pilot experiments because it produced the maximal effect on axon specification without inducing any measurable cell death. Given that the minimum alveolar concentration (MAC) of isoflurane has been measured at 1.7% in infants,33 a 2.4% isoflurane exposure is somewhat higher than is used clinically, but significant effects of lesser magnitude were also seen at concentrations of 1.8% and 1.2%. (Fig 1C)
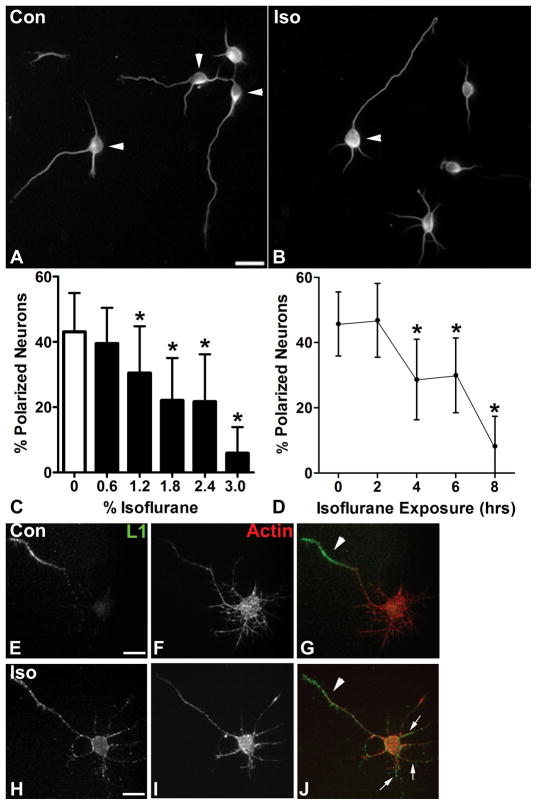
Figure 1. Isoflurane inhibits axon specification.
A: On DIV1 under control conditions dissociated cortical neurons, visualized here with immunolabeling for βIII tubulin, show a mixed population of polarized (arrowheads) and unpolarized neurons (cell bodies without arrowheads), as assessed by the Banker length criteria. B: Cultures treated for four hours with 2.4% isoflurane immediately after plating and assayed at DIV1 demonstrate a reduced percentage of polarized neurons (arrowhead). C: Quantitative analysis shows a significant reduction in the percentage of polarized neurons relative to control that is concentration-dependent over a range from 1.2% to 3% isoflurane for four hours. D: The magnitude of this effect also varies with exposure time, and there is a significant reduction in neuronal polarity with 2.4% isoflurane treatment in exposures lasting four hours or longer. E–G: At DIV2 neurons immunolabeled for the axon-specific protein L1CAM (E, green in G) and stained for F-actin to define the cell (F, red in G), show a segregation of L1CAM signal to the axon (arrowhead). H–J: By contrast, neurons treated with isoflurane at a one millimolar for six hours and labeled for L1CAM (H, green in J) and F-actin (I, red in J) show diffuse labeling for that is seen in the axon (arrowhead) and also in minor processes (arrows). Scale bar in A is 20 μm for A and B. n = 2151 cells in 210 fields for C and n = 1,523 cells in 150 fieldsfor D. Scale bar in E is 10 μm for E–J. * = p<0.05.
In the case of experiments involving L1 immunocytochemistry, a modified version of methods previously described for exposure in a solution of isoflurane was used. 34 A supersaturated solution of isoflurane was prepared in neurobasal media, which was diluted to 1 mM immediately prior to use. The solution was added to a glass chamber containing coverslips with adhered neurons and sealed tightly without any media-air interface. Isoflurane concentration was verified in pilot experiments using gas chromatography. A 1 mM concentration of isoflurane likely correlates with a dose that is somewhat higher than is used clinically, as 0.4 mM tissue concentrations of isoflurane are required to substantially reduce activity in neuronal networks. 35
Propofol and muscimol exposures were performed in supplemented Neurobasal media immediately after plating, and neurons were subsequently transferred to dishes with conditioned media. Because the propofol stock solution was dissolved in DMSO, the controls for these exposures contained the appropriate concentration of DMSO in the absence of propofol. Muscimol was dissolved directly in media without a carrier. The concentration of propofol that is clinically relevant is difficult to determine in this model, because propofol is highly protein bound in vivo and the culture media does not contain serum. A 3 μM concentration of propofol was chosen after pilot experiments, and this is consistent with the levels needed to activate GABAA receptors in dissociated rodent neuron culture using serum free media. 36
Cell labeling and Immunocytochemistry
For βIII tubulin immunocytochemistry, neurons adhered to coverslips were fixed for ten minutes at room temperature in a 4% paraformaldehyde phosphate-buffered saline solution (PBS, pH 7.2) and then permabilized in a 0.25% Triton-X detergent solution for one minute. After blocking for one hour at room temperature in 10% bovine serum albumin (Sigma), neurons were incubated for 24 hours at 4°C in anti-βIII tubulin antibody (1/1000, AB9354, Millipore, Billerica, MA) diluted in PBS containing 1% BSA. After washing, neurons were incubated for one hour with a fluorescent secondary antibody and mounted on slides for viewing. Immunolabeling for L1 followed a similar protocol, which differed in that the primary antibody incubation (1/2 ASCS4, Developmental Studies Hybridoma Bank, Iowa City, IA) was done at 37°C prior to fixation and that an incubation with Texas Red-conjugated phalloidin (1/75, Invitrogen) followed the secondary antibody incubation.
Microscopic Analysis
Fluorescently labeled neurons on coverslips were visualized using a Zeiss Axiophot fluorescence microscope with 40x 1.3 NA and 60x 1.25 NA objectives (Zeiss, Thornwood, NY). Neuronal polarity was assessed using the Banker criterion in which any neurite that has exceeded the length of other neurites by at least 10 μm is considered to have acquired an axonal fate. 27 Neurite length was initially measured by using Neurolucida imaging software (MicroBrightField, Colchester, VT), and subsequently judged by eye by a single investigator and periodically rechecked for accuracy. The numbers of polarized and unpolarized neurons in a single microscopic field were counted, and the data are reported as the mean percentage of polarized neurons per field, with error bars denoting the standard deviation. For experiments with two groups a student’s t-test was used to compare means, and for experiments with more than two groups an ANOVA with Dunnett’s multiple-comparison post hoc test was performed using Prism 5 software (GraphPad, La Jolla, CA). For measurements of axon or pre-polarized neurite length, tracings were made in NeuroLucida (MicroBrightField), the mean lengths were determined in NeuroLucida, and the results were analyzed as above using Prism 5.
Cell Death Assay
For the cell viability assay, neurons were cultured as described above and allowed to settle on tissue culture plates treated with poly-D-lysine, with a density of one million cells per well in a standard 12 well plate. Plates were treated with isoflurane in sealed chambers as described above. Cell viability was assessed on DIV1, 2, and 3 using the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) assay. MTT was dissolved in media at a concentration of 5 mg/mL and added in an amount equal to 10% of the culture medium volume. The plates with adhered neurons were returned to the incubator for two to four hours at which time the resulting formazen crystals were dissolved in acidified 10% Triton X-100 in isopropanol with 0.1N HCl. Absorbance was measured spectrophotometrically at a test wavelength of 570 nm and a reference wavelength of 690 nm. The data are reported as mean absorbance normalized to control levels, and statistical analysis was performed as described above.
RESULTS
In order to investigate the effects of anesthetics on neuron development, we treated developing mouse cortical neurons in dissociated culture with isoflurane at a range of clinically relevant concentrations and exposure times, and we examined the effects on neuronal morphology, using βIII tubulin labeling to identify neurons and define their structure. We chose to investigate the effects of isoflurane, because it is the most commonly used potent volatile anesthetic agent. We found that neurons treated with isoflurane immediately after plating and examined at one day in vitro (DIV1), were considerably less likely to have specified an axon from amongst the pre-polarized pool of neurites (Fig 1A and B, arrowhead indicates neurons that have polarized) as compared to carrier gas treated controls. Using the Banker criteria for neuronal polarization, 27 we quantified the percentage of polarized neurons and found that it was concentration dependent. The mean percentage of polarized neurons was significantly reduced at concentrations of 1.2% and above. (Fig 1C) We found that this effect depended on exposure time as well. At least four hours of isoflurane treatment (2.4%) was required to significantly reduce the mean percentage of polarized neurons. (Fig 1D)
We next asked whether the effects of isoflurane on neuronal polarity are limited to changes in morphology. The L1 cell adhesion molecule (L1CAM), which is highly expressed in axons of the developing nervous system, plays a key role in neuron development including axon growth, fasciculation, and guidance. 37 L1CAM is concentrated in the axon shortly after axon specification, and it is one of the earliest proteins to adopt a polarized distribution 38 (e.g. Fig 1H–J, arrowhead indicates L1 labeling concentrated in the axon). We treated neurons after plating with isoflurane at one millimolar for six hours, and examined patterns of L1 localization at DIV2. We found numerous neurons that had specified an axon by morphological criteria, but had not completely segregated L1 to the axon (Fig 1H–J, arrows indicate L1 labeling in minor processes, and arrowhead indicates L1 label segregated to the axon). This finding indicates that the effects of isoflurane on neuronal polarity have additional consequences in the axon that may persist beyond DIV1.
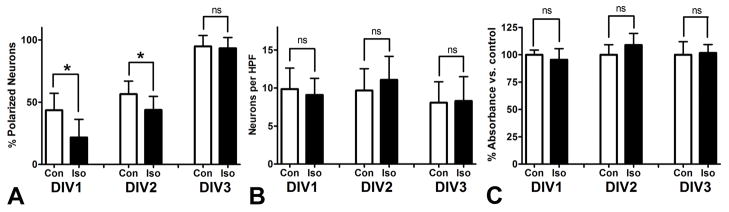
Next we sought to examine the time course of the effects of isoflurane on the acquisition of neuronal polarity. To this end, we assayed polarity on DIV1, DIV2, and DIV3 after a four hour exposure to 2.4% isoflurane. (Fig 2A) The mean percent of polarized neurons on DIV1 was 43% for controls as compared to 22% for the isoflurane treated group. This difference was reduced but still significant at DIV2, with 56% polarized in the control group and 44% in the isoflurane group. By DIV3 there was no statistically significant difference and over 90% of neurons assayed were polarized in both groups. Given the association between isoflurane and neuronal cell death,4 these results raised the question of whether the group of neurons treated with isoflurane that did not specify an axon on time were undergoing cell death or would do so shortly. To address this question, using the same exposure paradigm we examined neuron density at DIV1, DIV2, and DIV3 and found no differences between the isoflurane and control groups at any time point. (Fig 2B) Similarly, we found that an MTT assay of cell viability demonstrated no differences between isoflurane and control groups on DIV1, DIV2, or DIV3. (Fig 2C) The absence of cell death seen in other contexts may relate either to the culture model or the age of the cells, given that another study which examined the effects of isoflurane in early stage dissociated cell culture failed to demonstrate significant cell death resulting from a 3.4% isoflurane exposure for four hours. 39 Taken together our data indicate that isoflurane exposure delays the acquisition of neuronal polarity, but that this process is distinct from the apoptotic mechanism of anesthetic neurotoxicity that has been reported in some animal models.
Figure 2. Isoflurane delays the acquisition of neuronal polarity without causing cell death.
A: Neuronal polarity was assayed at DIV1, DIV2, and DIV3 after a four hour treatment with 2.4% isoflurane at the time of plating. The mean percent of polarized neurons is markedly reduced on DIV1, somewhat reduced on DIV2, and not significantly different on DIV3, when nearly all neurons have polarized. B: The number of neurons per microscopic field does not differ significantly between control and isoflurane treated cultures on DIV1, DIV2, or DIV3. C: An MTT assay for cell viability shows no significant reduction in absorbance for isoflurane treated cultures over the same time frame, confirming that cell death does not occur with this isoflurane exposure paradigm. n = 1,682 cells in 180 fields for A and B. n = 50 samples for C. * = p<0.05, ns = not significant.
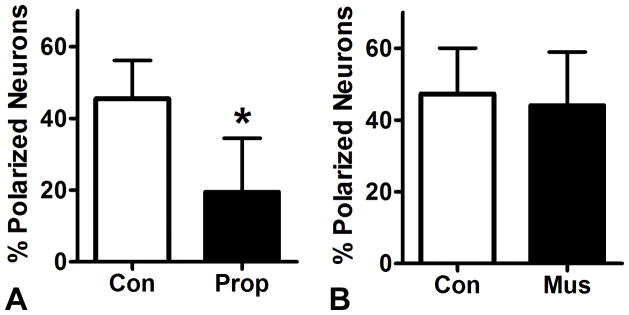
Given the clinical relevance of anesthetic effects on brain development, we asked whether propofol, the most commonly used general anesthetic alternative to the potent inhalational agents, also had effects on axon specification. We found that a four hour exposure to propofol at three micromolar reduced mean polarity at DIV1 by 26% compared to a vehicle treated control. (Fig 3A) This raised the possibility that the effects of propofol and isoflurane on the acquisition of neuronal polarity are due to activity at the GABAA receptor, which is common to both agents. 40 We found, however, that a four hour treatment with the GABAA agonist muscimol at a high dose of 10 μM did not significantly alter neuronal polarity compared to vehicle controls. (Fig 3B) This finding indicates that activity at GABAA receptors, a shared property of isoflurane and propofol, is not likely to be the molecular mechanism by which these GAs inhibit axon specification.
Figure 3. Propofol inhibits the acquisition of neuronal polarity.
A: Cortical neuron cultures were treated with 3 μM propofol for four hours immediately after plating and assayed for neuronal polarity on DIV1. Similarly to isoflurane, propofol significantly reduces the mean percentage of polarized neurons. B: The GABAAR agonist muscimol does not alter mean percentage of polarized neurons in the same exposure paradigm, suggesting that the effects of isoflurane and propofol on axon specification do not occur via activity at GABAARs. n = 666 cells in 61 fields for A and n = 527 cells in 60 fields for B. * = p<0.05
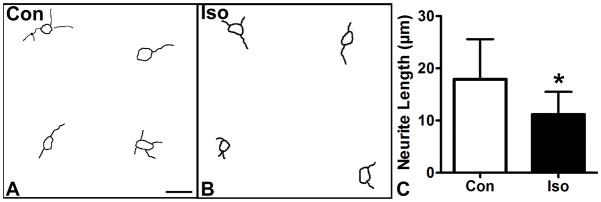
Early work in axonal specification demonstrated that pre-polarized neurites are all equally capable of assuming an axonal fate, 25 and it has been hypothesized that the neurite that grows the longest by chance during the stage prior to polarization will assume an axonal fate. 41 Isoflurane has been shown to interfere with cellular motility in mature fibroblasts and neurons. 42 To test the effects of isoflurane on neurite growth in our model system we performed NeuroLucida tracings of unpolarized neurites in cultured cortical neurons at DIV1 after our standard 2.4% isoflurane treatment after plating. We found that the mean neurite length in the isoflurane treated group was reduced to 11.2 μm from 17.9 μm in the control group. (Fig 4A–C) These data suggest that the delay neuronal polarization caused by isoflurane treatment is caused by a slowing of pre-polarized neurite growth.
Figure 4. Isoflurane reduces outgrowth of pre-polarized neurites.
A, B: Neurolucida tracings were made of unpolarized neurons from control (A) and 2.4% isoflurane treated cultures (B) at DIV1. The isoflurane treated group has similar numbers of neurites, but the lengths appear shorter on average. C: Quantitiatve analysis shows that neurite length is reduced, indicating that the delay in acquisition of neuronal polarity seen with isoflurane treatment is due to a reduction in neurite outgrowth speed. Scale bar in A is 25 μm for A and B. n = 128 neurons and 42 neurons. * = p<0.05.
DISCUSSION
Our data demonstrate that isoflurane retards the acquisition of neuronal polarity in a concentration and exposure time dependent fashion, by both morphological and molecular criteria. The delay in axon specification is neither the result of acute, ongoing cell death nor is it a cause of cell death at later time points. Interestingly, similar effects on polarity are seen with propofol, but not with a specific GABAA agonist. The mechanism by which isoflurane affects the development of neuronal polarity likely involves effects on minor process extension, as the neurites of unpolarized neurons were found to be shorter in isoflurane treated groups than in controls. These findings demonstrate that GAs such as isoflurane may interfere with brain development via mechanisms that do not involve cell death, and furthermore that developing axons may be vulnerable to effects of GAs.
Even a transient effect on the acquisition of neuronal polarity in a subset of neurons undergoing axon specification at the time of exposure could have serious consequences for brain development. Brain function depends on the appropriate generation of circuits, and many of the cues that guide axons to their appropriate targets are present for a limited time during specific developmental stages. 43 If the axon “misses” the window when the appropriate cue is present, the parent neuron may not be incorporated into key circuits. Furthermore, a delay in axon specification can also delay dendritic development. In cerebellar neurons when the acquisition of neuronal polarity is delayed by inhibiting tau expression, the growth and development of the remaining neurites is also delayed. 44 Thus, both axonal and dendritic incorporation into neuronal circuits could be altered permanently by delays in polarization.
Several lines of data strongly support that early phases of neuronal development and differentiation are modeled accurately by neuronal cultures, but of course it is not currently possible to draw exact parallels with human exposure in clinical settings. The acquisition of polarity of most neurons in the developing human occurs during gestation, and thus these data may be very relevant to prenatal exposures. Recent research suggests that a single exposure to isoflurane during gestation can cause lasting behavioral changes in rats, including worse performance on hippocampal dependent tasks. 45 However, postnatal neurogenesis of the hippocampal dentate granule cells plays a very important role in learning and memory. 46 In macaque monkeys up to 25% of the cells that will be added over a lifetime proliferate within the first three postnatal months, and thus there is a small, but important cohort of neurons in primates that must generate axons and make synaptic connections during early postnatal life. 47 Thus early postnatal anesthetic exposures that interfere with the incorporation of this population of cells via delays in the acquisition of neuronal polarity could have lasting effects on learning and memory.
Our finding that neurite lengths in unpolarized neurons are shorter in the isoflurane treated group suggests a possible mechanism for the delay in polarity acquisition that we observed. Prior research indicates that polarization occurs when one pre-polarized neurite outgrows the others, and furthermore that if an established axon is transected shortly after polarization then the longest remaining pre-polarized neurite is the one most likely to adopt an axonal fate. 25–27, 48 These findings suggest that there is something fundamental about neurite extension that drives the acquisition of polarity, and it is unsurprising that an insult which slows the growth of pre-polarized neurites also delays the acquisition of neuronal polarity. Our data do not give any further insights into mechanism, but an effect that is mediated via the cytoskeleton is a likely possibility. Isoflurane has been shown to have profound effects on the actin cytoskeleton, thus altering cellular structure and motility. Isoflurane interferes with the formation of actin stress fibers in immature astrocytes 21 and it depolymerizes actin in the dendrites of developing neurons in culture when applied during synaptogenesis, leading to a loss of dendritic spines. 14 Isoflurane also causes a striking loss of actin based motility both in dendritic spines and in fibroblast lamellipodia. 42 It is possible that the effects we have observed are due to alterations in the actin-based motility of neuronal growth cones that is necessary to establish polarity. 49, 50 Alternately, it is possible that isoflurane disrupts polarization through effects on signaling. Rho GTPases, which are known targets of isoflurane, 51 have been implicated in the acquisition of neuronal polarity. 49, 50 It is also possible that isoflurane might be exerting its effects via as yet undiscovered interactions with other signaling molecules involved in the acquisition of polarity.
Our finding that isoflurane and propofol delay the polarization of neurons represents a new class of putative mechanisms of GA toxicity in brain development. It may point to other effects on axon development that would be relevant at ages that better correspond to pediatric exposures, such as axon tract formation in the hippocampus, an event that occurs postnatally in primate models. 52 Also, effects of GAs on growth cone motility in either axons or dendrites could have profound effects on circuit formation. Finally, effects of GAs on neuronal polarity that involve the dynamic actin cytoskeleton or the molecules that regulate it might suggest effects on axon guidance and synaptogenesis, both of which are highly dependent on actin.
A limitation of this study, as with all in vitro work, is that it is difficult to be certain of the clinical relevance of anesthetic concentrations and exposure times that are required to produce measureable effects in dissociated cell culture. However, our finding that a four hour exposure to an isoflurane concentration as low as 0.9%, which is well below the MAC value for infants, significantly reduced neuronal polarization is sufficient to raise concerns that this type of anesthetic toxicity may be relevant to clinical practice. Further study of the effects of anesthetic exposure on the development of axons, including investigations in whole animal models, is a promising direction in pediatric anesthetic neurotoxicity research.
Acknowledgments
The authors thank Roxana Mesias for technical assistance, Aubrey Kalashian for help with manuscript preparation, and Dr. H.T. Lee for use of the anesthesia exposure system. Funding was provided by the Department of Anesthesiology at Columbia University and the National Institutes of Health grants GM008464 and NS050634.
Footnotes
Conflict of Interest: None
References
- 1.Levin ED, Uemura E, Bowman RE. Neurobehavioral toxicology of halothane in rats. Neurotoxicol Teratol. 1991;13:461–70. doi: 10.1016/0892-0362(91)90096-f. [DOI] [PubMed] [Google Scholar]
- 2.Quimby KL, Aschkenase LJ, Bowman RE, et al. Enduring learning deficits and cerebral synaptic malformation from exposure to 10 parts of halothane per million. Science. 1974;185:625–7. doi: 10.1126/science.185.4151.625. [DOI] [PubMed] [Google Scholar]
- 3.Uemura E, Ireland WP, Levin ED, et al. Effects of halothane on the development of rat brain: a golgi study of dendritic growth. Exp Neurol. 1985;89:503–19. doi: 10.1016/0014-4886(85)90002-0. [DOI] [PubMed] [Google Scholar]
- 4.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimaggio C, Sun L, Li G. Early Childhood Exposure to Anesthesia and Risk of Developmental and Behavioral Disorders in a Sibling Birth Cohort. Anesth Analg. 2011;113(5):1143–51. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiMaggio C, Sun LS, Kakavouli A, et al. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–91. doi: 10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flick RP, Katusic SK, Colligan RC, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–61. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Istaphanous GK, Loepke AW. General anesthetics and the developing brain. Curr Opin Anaesthesiol. 2009;22:368–73. doi: 10.1097/aco.0b013e3283294c9e. [DOI] [PubMed] [Google Scholar]
- 10.Jevtovic-Todorovic V. Anesthesia and the developing brain: are we getting closer to understanding the truth? Curr Opin Anaesthesiol. 2011;24:395–9. doi: 10.1097/ACO.0b013e3283487247. [DOI] [PubMed] [Google Scholar]
- 11.Patel P, Sun L. Update on neonatal anesthetic neurotoxicity: insight into molecular mechanisms and relevance to humans. Anesthesiology. 2009;110:703–8. doi: 10.1097/ALN.0b013e31819c42a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stratmann G. Review article: Neurotoxicity of anesthetic drugs in the developing brain. Anesth Analg. 2011;113:1170–9. doi: 10.1213/ANE.0b013e318232066c. [DOI] [PubMed] [Google Scholar]
- 13.Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–41. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemkuil BP, Head BP, Pearn ML, et al. Isoflurane neurotoxicity is mediated by p75NTR-RhoA activation and actin depolymerization. Anesthesiology. 2011;114:49–57. doi: 10.1097/ALN.0b013e318201dcb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wise-Faberowski L, Zhang H, Ing R, et al. Isoflurane-induced neuronal degeneration: an evaluation in organotypic hippocampal slice cultures. Anesth Analg. 2005;101:651–7. doi: 10.1213/01.ane.0000167382.79889.7c. table of contents. [DOI] [PubMed] [Google Scholar]
- 16.Cowan WM, Fawcett JW, O’Leary DD, et al. Regressive events in neurogenesis. Science. 1984;225:1258–65. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- 17.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 18.Briner A, De Roo M, Dayer A, et al. Volatile anesthetics rapidly increase dendritic spine density in the rat medial prefrontal cortex during synaptogenesis. Anesthesiology. 2010;112:546–56. doi: 10.1097/ALN.0b013e3181cd7942. [DOI] [PubMed] [Google Scholar]
- 19.Briner A, Nikonenko I, De Roo M, et al. Developmental Stage-dependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology. 2011;115:282–93. doi: 10.1097/ALN.0b013e318221fbbd. [DOI] [PubMed] [Google Scholar]
- 20.De Roo M, Klauser P, Briner A, et al. Anesthetics rapidly promote synaptogenesis during a critical period of brain development. PLoS One. 2009;4:e7043. doi: 10.1371/journal.pone.0007043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lunardi N, Hucklenbruch C, Latham JR, et al. Isoflurane impairs immature astroglia development in vitro: the role of actin cytoskeleton. J Neuropathol Exp Neurol. 2011;70:281–91. doi: 10.1097/NEN.0b013e31821284e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winckler B, Mellman I. Neuronal polarity: controlling the sorting and diffusion of membrane components. Neuron. 1999;23:637–40. doi: 10.1016/s0896-6273(01)80021-0. [DOI] [PubMed] [Google Scholar]
- 23.Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- 24.Tahirovic S, Bradke F. Neuronal polarity. Cold Spring Harb Perspect Biol. 2009;1(3):a001644. doi: 10.1101/cshperspect.a001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dotti CG, Banker GA. Experimentally induced alteration in the polarity of developing neurons. Nature. 1987;330:254–6. doi: 10.1038/330254a0. [DOI] [PubMed] [Google Scholar]
- 26.Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–68. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goslin K, Banker G. Experimental observations on the development of polarity by hippocampal neurons in culture. J Cell Biol. 1989;108:1507–16. doi: 10.1083/jcb.108.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma L, Song L, Radoi GE, et al. Transcriptional regulation of the mouse gene encoding the alpha-4 subunit of the GABAA receptor. J Biol Chem. 2004;279:40451–61. doi: 10.1074/jbc.M406827200. [DOI] [PubMed] [Google Scholar]
- 29.Banker G, Goslin K. Culturing nerve cells. Cambridge: MIT Press; 1998. [Google Scholar]
- 30.Frisa PS, Goodman MN, Smith GM, et al. Immortalization of immature and mature mouse astrocytes with SV40 T antigen. J Neurosci Res. 1994;39:47–56. doi: 10.1002/jnr.490390107. [DOI] [PubMed] [Google Scholar]
- 31.Mintz CD, Carcea I, McNickle DG, et al. ERM proteins regulate growth cone responses to Sema3A. J Comp Neurol. 2008;510:351–66. doi: 10.1002/cne.21799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HT, Kim M, Jan M, et al. Anti-inflammatory and antinecrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol. 2006;291:F67–78. doi: 10.1152/ajprenal.00412.2005. [DOI] [PubMed] [Google Scholar]
- 33.Murray DJ, Mehta MP, Forbes RB. The additive contribution of nitrous oxide to isoflurane MAC in infants and children. Anesthesiology. 1991;75:186–90. doi: 10.1097/00000542-199108000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Sutachan JJ, Montoya-Gacharna J, et al. Isoflurane inhibits cyclic adenosine monophosphate response element-binding protein phosphorylation and calmodulin translocation to the nucleus of SH-SY5Y cells. Anesth Analg. 2009;109:1127–34. doi: 10.1213/ANE.0b013e3181b5a1b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker K, Eder M, Ranft A, et al. Low dose isoflurane exerts opposing effects on neuronal network excitability in neocortex and hippocampus. PLoS One. 2012;7:e39346. doi: 10.1371/journal.pone.0039346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hales TG, Lambert JJ. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br J Pharmacol. 1991;104:619–28. doi: 10.1111/j.1476-5381.1991.tb12479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burden-Gulley SM, Pendergast M, Lemmon V. The role of cell adhesion molecule L1 in axonal extension, growth cone motility, and signal transduction. Cell Tissue Res. 1997;290:415–22. doi: 10.1007/s004410050948. [DOI] [PubMed] [Google Scholar]
- 38.van den Pol AN, Kim WT. NILE/L1 and NCAM-polysialic acid expression on growing axons of isolated neurons. J Comp Neurol. 1993;332:237–57. doi: 10.1002/cne.903320208. [DOI] [PubMed] [Google Scholar]
- 39.Sall JW, Stratmann G, Leong J, et al. Isoflurane inhibits growth but does not cause cell death in hippocampal neural precursor cells grown in culture. Anesthesiology. 2009;110:826–33. doi: 10.1097/ALN.0b013e31819b62e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cell Mol Life Sci. 1999;55:1278–303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craig AM, Jareb M, Banker G. Neuronal polarity. Curr Opin Neurobiol. 1992;2:602–6. doi: 10.1016/0959-4388(92)90025-g. [DOI] [PubMed] [Google Scholar]
- 42.Kaech S, Brinkhaus H, Matus A. Volatile anesthetics block actin-based motility in dendritic spines. Proc Natl Acad Sci U S A. 1999;96:10433–7. doi: 10.1073/pnas.96.18.10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol. 2011;3(6):a001727. doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caceres A, Kosik KS. Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature. 1990;343:461–3. doi: 10.1038/343461a0. [DOI] [PubMed] [Google Scholar]
- 45.Palanisamy A, Baxter MG, Keel PK, et al. Rats exposed to isoflurane in utero during early gestation are behaviorally abnormal as adults. Anesthesiology. 2011;114:521–8. doi: 10.1097/ALN.0b013e318209aa71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–50. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jabes A, Lavenex PB, Amaral DG, et al. Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus. Eur J Neurosci. 2010;31:273–85. doi: 10.1111/j.1460-9568.2009.07061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goslin K, Banker G. Rapid changes in the distribution of GAP-43 correlate with the expression of neuronal polarity during normal development and under experimental conditions. J Cell Biol. 1990;110:1319–31. doi: 10.1083/jcb.110.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–4. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- 50.Witte H, Bradke F. The role of the cytoskeleton during neuronal polarization. Curr Opin Neurobiol. 2008;18:479–87. doi: 10.1016/j.conb.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 51.Tas PW, Gambaryan S, Roewer N. Volatile anesthetics affect the morphology of rat glioma C6 cells via RhoA, ERK, and Akt activation. J Cell Biochem. 2007;102:368–76. doi: 10.1002/jcb.21294. [DOI] [PubMed] [Google Scholar]
- 52.Jabes A, Lavenex PB, Amaral DG, et al. Postnatal development of the hippocampal formation: a stereological study in macaque monkeys. J Comp Neurol. 2011;519:1051–70. doi: 10.1002/cne.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]