Abstract
A core brain network is engaged in remembering the past and envisioning the future. This network overlaps with the so-called default-mode network, the activity of which increases when demands for focused attention are low. Because of their shared brain substrates, an intriguing hypothesis is that default-mode activity, measured at rest, is related to performance in separate attention-focused recall and imagination tasks. However, we do not know how functional connectivity of the default-mode network is related to individual differences in reconstruction of the past and imagination of the future. Here, we show that functional connectivity of the default-mode network in children and adolescents is related to the quality of past remembering and marginally to future imagination. These results corroborate previous findings of a common neuronal substrate for memory and imagination and provide evidence suggesting that mental time travel is modulated by the task-independent functional architecture of the default-mode network in the developing brain. A further analysis showed that local cortical arealization also contributed to explain recall of the past and imagination of the future, underscoring the benefits of studying both functional and structural properties to understand the brain basis for complex human cognition.
Keywords: cortical area, development, fMRI, independent component analysis
It is widely accepted that reconstruction and “re-experience” are important aspects of vivid episodic memory. Reconstruction of memories based on impoverished bits of information represents an economical way of storing information and may also facilitate anticipation of the future (1). A reconstructive memory system, enables mental time travel by use of previous experiences as a basis for construction of imagined future situations (2). Thus, there is a theoretical and empirical connection between the ability to reconstruct and re-experience our own personal past and the ability to imagine new experiences (3). However, little is known about individual differences in the brain characteristics underlying the ability to form rich representations of the past and future and, especially, how these characteristics support episodic recall and imagination during development. The purpose of the present study was to delineate both functional and structural brain correlates of vividness in recall and future imagination in children and adolescents.
Brain lesion (4, 5) and functional magnetic resonance imaging (fMRI) studies (3, 6) have yielded evidence for a common core network of brain areas involved in recall of episodic memories and imagination of future scenarios. This network includes the medial temporal cortex, posterior cingulate/retrosplenial cortex/precuneus, lateral parietal (inferior parietal lobule, temporo-parietal junction), medial prefrontal, and lateral temporal cortices (3), although the role played by the hippocampus in imagination is debated (7–9). These areas overlap to a substantial degree with the default-mode network (10) and may, in a more general sense, support self-projection, whether to the future, the past, or the viewpoint of others (11). An alternative view to the self-projection hypothesis on the relationship between the memory and imagination is based on the notion of scene construction. Scene-construction is the process of mentally generating and maintaining a complex and coherent scene or event (12), according to which the hippocampus plays a critical role in imagination by binding together discrete elements of an event (4, 9, 12). Thus, the common involvement of the core network does not need to be related to future imagination but could just as well be imagination of experiences that are “not necessarily self-relevant, plausible or even possible” (12).
Properties of the default-mode network can be quantified in terms of functional connectivity, which may refer to the temporal relationships between activity in spatially remote areas within the network or between the default-mode network and other networks. Default-mode functional connectivity is often measured at “rest” (i.e., with no specific demands for focused attention). In contrast, memory and imagination performance are typically measured in attention-focused tasks, often cue-word tasks.
Importantly, brain networks identified in resting conditions show similarities with networks identified during specific cognitively demanding task sessions (13), demonstrating that the functional organization of the brain remain relatively stable across a range of psychological states. Because the default-mode network overlaps substantially with the core network implied in remembering and imagination, there is an intriguing possibility that default-network connectivity, as measured during a resting condition, is related to performance on independent attention-demanding memory and imagination tasks. However, it needs to be established how default-mode network functional connectivity is related to individual differences in recall of the past and construction of the future (14, 15). This information is important for the understanding of the brain substrates for differences in subjective quality of memories. Thus, we tested how these abilities in children and adolescents were related to resting-state functional connectivity of the default-mode network. Furthermore, individual differences in brain activation during development can hardly be understood without taking possible differences in brain structure into account. To allow integration of functional and structural aspects of the brain, volume of the hippocampus and local arealization of the cerebral cortex were included in an additional analysis. Local arealization was chosen because this measure has received much recent attention and has shown to be of great interest as a metric of cortical structure (16–19).
A total of 103 children and adolescents (age, 9.1–21.9 y) were tested with a recall–imagination cue-word task. Children’s ability to imagine the future has been subject of several recent studies (20–23). Episodic remembering and the ability to envision the future emerge at the same time during development, approximately between the ages of 3 and 5 y (3, 24). Although it is known that much younger children have memories of their past (25), there is controversy regarding the degree to which these memories are truly episodic in the autonoetic, “self-knowing,” sense described by Tulving (2), or are more semantic in nature, representing “knowledge” of the past (3, 24). In any case, the ability to form episodic memories and to engage in episodic future thinking should be undisputed in the age range sampled in the present study, although compared with adults, relatively little is known about children’s autobiographical memory formation and ability to imagine the future (9). On a separate day from the completion of the recall-imagination task, participants underwent multimodal neuroimaging with resting-state fMRI and structural MRI. Based on the previous research reviewed above, we hypothesized that the degree of detail and self-awareness in the subjective reports of past and future scenarios (i.e., the level of autonoetic consciousness) would be reflected in the default-mode network functional-connectivity pattern, local cortical arealization in the memory network, and, possibly, hippocampal volume.
Results
Descriptive Results.
Partial correlations controlling for age and sex showed that autonoetic score for past and future was related (r = 0.20; P < 0.05), and none of them was related to age (past: r = 0.15, not significant; future: r = −0.08, not significant; sex partialled out), but the past–future relationship was not significantly stronger than the age relationships. Neither score was related to sex. Although the past–future correlation of 0.20 was not significantly different from the age correlation of 0.15, the former was likely not dependent on an age effect because age was partialled out. Age did not correlate significantly with local arealization when stringent criteria for multiple comparison corrections were applied (SI Text) or with hippocampal volume (r = −0.07; not significant) when sex and intracranial volume (ICV) were partialled out. Age correlated negatively with default-network functional connectivity in a restricted area including the midcingulate gyrus, only to a minor degree overlapping with the default-mode network (SI Text).
Functional Connectivity.
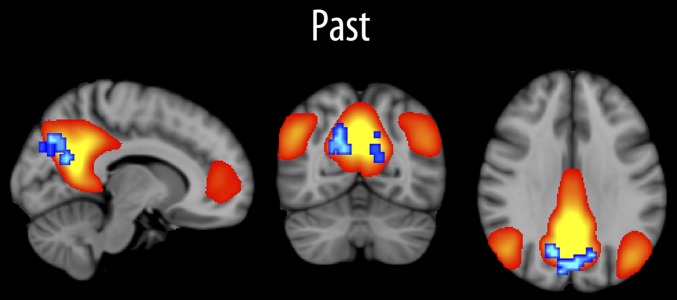
The independent component best representing the default network is shown in Fig. 1. The linear relationship between component strength and scores for past and future was tested voxel wise, with sex and age used as covariates. Higher past scores correlated significantly (P < 0.05, corrected) with reduced default-mode network functional connectivity in a bilateral cluster in the precuneus (cluster size, 152 voxels; peak z-score, 3.65; peak MNI voxel coordinates: x = 25, y = 20, z = 36). The correlation between mean connectivity in this cluster and past score was −0.46 (P < 10−5), partialling out age and sex. The inclusion of participant movement as an additional covariate did not affect the results (r = −0.45; P < 10−5), nor did inclusion of full-scale intelligence quotient (IQ) from Wechsler Abbreviated Scale of Intelligence (WASI) (r = −0.44; P < 10−4). No interaction with age was found (P = 0.86), and running the analysis in the younger and older part of the sample split by median age (16.5 y) yielded similar correlations (r = −0.50 vs. −0.48, respectively; both P values, <0.001). Because these correlations are based on already-identified significant voxels, they should not be interpreted as an estimate of the strength of the correlation in the population (26) but as a convenient way of testing the effect of including additional covariates. Follow-up analyses were also run for the three subscales of the autonoetic score, with none showing larger correlations than the total autonoetic score (r = −0.37 for experiential score; r = −0.25 for perspective; r = −0.23 for coherence).
Fig. 1.
Remembering and functional connectivity of the default-mode network. The independent component best representing the default-mode network is shown in red-yellow. The relationship between the strength of this component and past score (remembering) was tested across all voxels with age and sex as covariates, and the results are shown in blue-cyan (P < 0.05, corrected). All relationships were negative.
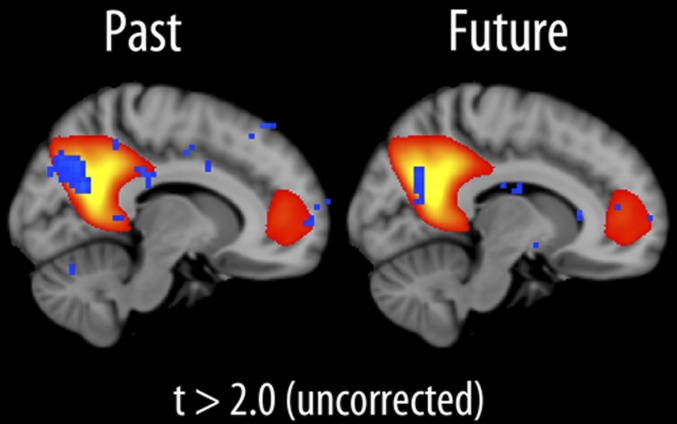
There was also a significant correlation between mean connectivity in this cluster and imagination of the future (r = −0.25; P < 0.05; age and sex partialled out). An analysis with future score as an independent variable and age and sex as covariates was therefore run across all voxels. The results revealed a cluster within the precuneus (t > 2, uncorrected; cluster size, 31 voxels; peak z-score, 3.57; peak MNI voxel coordinates: x = 32, y = 18, z = 35), overlapping with the cluster found for the past score (Fig. 2).
Fig. 2.
Overlap between remembering, imagination, and the default-mode network. The relationship between default-mode network component strength and past score is shown to the left and future score (imagination) to the right. Blue-cyan indicates P < 0.05 (uncorrected). All relationships were negative.
Post hoc exploratory analyses were also run for an independent component (IC) overlapping with a more anterior part of the default mode network (DMN), with no significant results.
Morphometry.
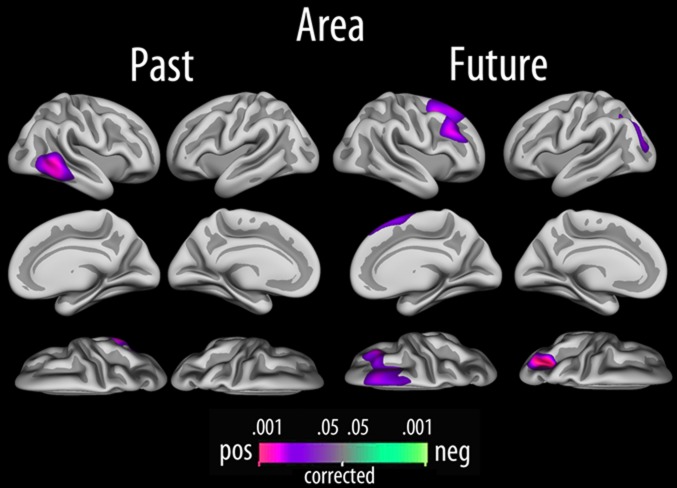
Independently of age, sex, and the interaction between them, a positive relationship between autonoetic past and surface area was found in the right lateral temporal cortex (P < 0.05, corrected), encompassing posterior parts of inferior and middle temporal gyrus (Fig. 3) (r = 0.29). Autonoetic future was positively related to surface area in a large cluster in the superior and middle frontal gyri in the right hemisphere, as well as in a cluster extending from the occipital cortex to the superior parietal cortex in the left hemisphere. The effects were not affected by including full-scale IQ as an additional covariate. Analyses on the level of subscale revealed no correlations stronger than the total scale score.
Fig. 3.
Local cortical arealization. Relationships between past score, future score, and cortical area, tested vertex-wise across the cortical mantle, with sex and age included as covariates (P < 0.05; corrected).
The relationship between hippocampal volume and past and future scores was tested with partial correlations, with age, sex, and ICV partialled out. No significant relationships were revealed (past: r = 0.10, not significant; future: r = 0.03, not significant). Additional analyses were run with full-scale IQ as an additional covariate, which did not change the results. Subscale analyses showed a significant correlation with past coherence score, which did not survive corrections for multiple comparisons (r = −0.22; P < 0.05, uncorrected).
Discussion
A common network for remembering and imagination has been identified (27–29), and this network overlaps substantially with the default-mode network (3, 11, 30, 31). Functional neuroimaging studies using similar cue-word paradigms as the present study have shown activation in multiple regions within the default-mode network, including medial frontal cortex, posterior cingulate, retrosplenial cortex, and the precuneus, the medial and lateral temporal lobe, and the temporo-parietal junction (6, 11, 32–34). However, the degree to which resting-state functional connectivity of the default-mode network is related to performance on attention-focused remembering and imagination tasks on a separate occasion is important to establish. This pertains to the question of whether default-mode activity itself is related to the ability to recall past events and envision the future. All analyses were done by controlling for age and sex, ensuring that these variables were not confounding the results.
Vividness of past experiences, and, at a trend level, imagination of future episodes, was related to reduced default-mode functional connectivity in the precuneus. Remembering, imagination and default-mode activity are supported by a common set of brain regions, which support stimulus-independent thoughts, self-projection, scene-construction, and retrieval of stored information (35). In the present study, the task used to measure the character of memory and imagination was separated from the resting-state situation in time, space, and instructions. Because the cue-word task was administered on a separate day from the scanning, and the participants were not asked to engage in the task during scanning, it is unlikely that the resting-state activity directly reflected the remembered or imagined events or that the activity in itself is driven by the specific cognitive task. This means that the functional connectivity of the default-mode network, reflecting task-free individual differences in spontaneous fluctuations in resting-state activity over time, was predictive of participants’ subjective experience of memories beyond the scan session.
Furthermore, cortical structure (area) was related to the subjective quality of past memories. Normal variation in cortical morphology has not been related previously to such phenomenological or autonoetic aspects of remembering and imagination. This shows that both functional and structural human neuroimaging data can be used to get a more complete picture of how complex cognitive processes are implemented in the brain.
Functional Connectivity and Vivid Experiences of Past and Future.
Buckner and Carroll suggested that the basic common process between remembering and imagination is the simulation of an alternative perspective to the present, i.e., events must be imagined beyond the information that emerges from the immediate environment (11). Hassabis and Maguire proposed an alternative theory, based on the notion of scene-construction as the unifying element in remembering and imagination (12). Both theories agree on the importance of the default-mode network for remembering and imagination. The default-mode network is preferentially involved in internally focused tasks rather than in tasks where the attention is directed toward the external environment. These types of tasks will often include autobiographical memory retrieval and imagination of the future: flexible mental explorations or simulations that facilitate anticipation and evaluation of events that have not yet happened (11, 35). Thus, it is interesting that the subjective quality of children and adolescents’ recall of the past was related to resting-state functional connectivity in this network. Imagination of the future showed a relationship in a similar area, but this did not survive corrections. As an additional point, the relationship was independent of general cognitive function as quantified by full-scale IQ.
High scores in the cue-word task are obtained for memories and imaginations that include presence of the self, sensory details, and high degree of vividness. A recent experimental fMRI study showed that connectivity in the autobiographical memory network changes more during retrieval of episodes with a high degree of self-involvement than episodes with a lower degree of self-involvement (36). The present results are coherent with such experimental findings, in that they suggest that the ability to remember and imagine with a higher degree of self-presence, or autonoetic consciousness, is related to default-network connectivity independently of the task used to elicit the memories and imaginations. Other recent studies have also demonstrated such relationships in adults, indicating, for instance, that high functional connectivity is related to memory performance (16, 37, 38) and occurrence of spontaneous thoughts (14). An interesting difference between these studies and the present developmental study is the negative relationship found. This is likely attributable to the age of the participants. The direction of the relationships between cognitive function and brain structure has been shown to change across development (39), and a similar phenomenon could be envisioned for functional connectivity. In the present data, the relationship was stable across the age range. If an inversion of the relationship is to occur, then this must happen in early adulthood. A previous study found negative age-connectivity relationships in development and speculated that this could reflect the higher number of synapses in children (40). The present finding of reduced functional connectivity in the participants with higher autonoetic scores could be related to differences in selective elimination of synapses during early development (41, 42), which, in turn, could affect specificity and efficiency of cognitive processes (43). Similar to the present results, a recent study of traumatic brain injury found a negative relation between functional connectivity and sustained attention (44). Thus, one explanation for the negative relationship between functional connectivity and subjective experience of memories observed in the present study could be rooted in individual differences in early synaptic pruning.
It is also an important feature of the present study that the measure of interest was not memory accuracy, but the subjective experience of episodes. This is likely related to metacognitive awareness and introspective abilities in the children and adolescents, but our knowledge about these abilities in children and adolescents are still scarce (9). An inevitable challenge in developmental studies is also that the connotations of the cue words may be very different for 9-y-olds and 20-y-olds. Thus, one can ask whether the observed relationships reflect the same processes at different ages. The functional relationships were seen independently of age and did not change as a function of age when directly tested. Still, it is possible that the relationships can have different interpretations at different ages. These themes need to be studied in more depth in future targeted experiments.
Past and future scores were also related to local cortical expansion in specific areas. Past was related to an area in the right lateral temporal cortex, part of the core network for remembering and imagination (3). Even though the structural and functional effects were not seen in the same region, it can be argued that they belong to a common core network. Few studies have attempted to integrate morphometric and functional results, and the relationship is likely complex, although some overlap between the functional and structural results would make a stronger case for concluding that they belong to the same network. Areal expansion is an efficient means to facilitate brain connectivity and functional development (45) and is largely determined by the final number of ontogenetic columns (46). It is suggested that cerebral function and form are linked through the organization of neural connectivity, dependent on pruning of synapses (47). Thus, there is reason to expect that arealization and functional connectivity both are related to cognitive function, and the present findings suggest that functional and structural aspects of the brain are both relevant for the understanding of the neural basis for complex cognitive processes. To understand why the effects were found in different parts of the core network, more research is needed.
Although we hypothesized a relationship also for hippocampus, this was not found. Thus, normal variation in hippocampal volume in children and adolescents did not impact the subjective quality of remembering or imagination in the present study, even though major lesions to the medial temporal lobe have been reported to affect both. A recent study, however, suggested that the contributions of the hippocampus to simulations of the future reflect encoding of the simulations into memory and that this function is not essential for constructing coherent scenarios (48). Also, a critical role for the hippocampus in imagination is disputed in a recent patient study (7) (but, again, see ref. 49) and fMRI studies (8). These results could explain the lack of correlation between hippocampal volume and past or future score in the present study. However, the ability of patients with hippocampal lesions to imagine fictitious events may be based on processes other than those supported by the hippocampus. For instance, developmental lesion studies have suggested that future-thinking in such patients may be based on world knowledge and semantic representations rather than true visualization or scene-construction supported by the hippocampus (9, 50). More research into the role of hippocampal morphology in remembering and imagination, especially during development, is needed to reconcile the different views.
Conclusion.
In conclusion, resting-state functional connectivity of the default-mode network was related to recall of past episodes and, at a trend level, imagination of future events in children and adolescents. This highlights a functional link between characteristics of the default-mode network measured at rest and separate attention-focused cognitive tasks. In addition, both functional and structural brain measures were related to individual differences in the subjective quality of memory recall, suggesting that using a multimodal approach may be beneficial for understanding the neural basis for individual differences in complex human cognitive processes.
Materials and Methods
Sample.
See SI Materials and Methods for details. A total of 103 (female, n = 52; age, 9.1–21.9 y; mean, 16.4; SD, 3.4) right-handed, healthy children, fluent in Norwegian, without self- or parent-reported history of neurological or psychiatric disorders, chronic illness, premature birth, learning disabilities, or use of medicines known to affect nervous system functioning and with normal or corrected-to-normal hearing and vision, were recruited. A total of 93 had usable blood oxygenation level–dependent (BOLD) scans.
Remembering–Imagination Cue-Word Task.
See SI Materials and Methods for details. The task was modeled on a much-used cue-word paradigm for probing past and future events (3). In response to cue words presented on a computer screen, the participants were asked to, within 2 y into the past and future, to retrieve specific episodes from the past or to imagine episodes that they thought might actually happen to them in the future (a time frame of no longer than a day), without recasting memories as future scenarios. Three neutral-positive cue words for the past and three for the future, easy to relate to and open to many possible scenarios, were chosen. Each past and future episode was immediately rated by the participants on an 11-item questionnaire partly based on the Memory Experiences Questionnaire (51), measuring the autonoetic or phenomenological experience of the episodes on a 5-point Likert scale. This yielded one total autonoetic score for past and one for future. Cronbach’s α for past was 0.67, 0.70, and 0.59 for the three cue words and 0.69, 0.73, and 0.70 for future.
MRI Acquisition and Analysis.
Imaging data were collected using a 12-channel head coil on a 1.5-Tesla Siemens Avanto scanner (Siemens Medical Solutions). Morphometry was as follows: two 3D T1-weighted [magnetization prepared rapid acquisition gradient echo (MP-RAGE)] scans; relaxation time (TR)/echo time (TE)/inversion time (TI)/flip angle (FA), 2,400 ms/3.61 ms/1,000 ms/8°; matrix, 192 × 192; field of view, 240; scan time, 7 min and 42 s; 160 sagittal slices; voxel sizes, 1.25 × 1.25 × 1.20 mm. Only scans deemed to have no or minimal movement artifacts were included. Resting (r)BOLD was as follows: T2*-weighted single-shot gradient echo planar imaging (EPI) scan; TR/TE/FA, 3,000 ms/70 ms/90°; voxel size, 3.4375 × 3.4375 × 4 mm; 28 axial slices; field of view, 64; scan time, 5 min. Participants were instructed to not sleep.
Local cortical arealization, hippocampal volume, and ICV were estimated by use of FreeSurfer 5.1 (http://surfer.nmr.mgh.harvard.edu/fswiki). Resting-state fMRI analysis was carried out using Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC) (52) implemented in the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL) (www.fmrib.ox.ac.uk/fsl). Dual regression (53, 54) was used for voxel-wise analysis of functional connectivity. Given our a priori hypothesis of posterior default network (precuneus, posterior cingulate) involvement in pro- and retrospective memory processes, only the independent component reflecting this was selected for initial analysis. One additional component overlapping with a more anterior part of the DMN was included in a post hoc exploratory analysis. See SI Materials and Methods for details.
Statistical Analyses.
General linear models (GLMs) were used for cortical surface area analyses, with results tested using Z Monte Carlo simulations with 10,000 iterations and a cluster-forming threshold of P < 0.05 (55, 56). Connectivity analyses were performed voxel-wise on the IC-specific connectivity values by GLMs and with nonparametric permutation testing with 5,000 permutations and an initial cluster-forming threshold of t < 2 (57, 58) of the results. Results were deemed significant at P < 0.05 (corrected). Age and sex were included as covariates.
Supplementary Material
Acknowledgments
We thank those who participated in the research. This work was supported by Norwegian Research Council Grants 177404/W50, 186092/V50, and 204935/F20 (to K.B.W.); 204966 (to L.T.W.); and 189507/V40 and 199537 (to A.M.F.); and European Research Council Grant 283634 (to A.M.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210627109/-/DCSupplemental.
References
- 1.Schacter DL, Addis DR. Constructive memory: The ghosts of past and future. Nature. 2007;445:27. doi: 10.1038/445027a. [DOI] [PubMed] [Google Scholar]
- 2.Tulving E. Elements of Episodic Memory. Oxford: Clarendon Press; 1983. [Google Scholar]
- 3.Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: The prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- 4.Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci USA. 2007;104:1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwan D, Carson N, Addis DR, Rosenbaum RS. Deficits in past remembering extend to future imagining in a case of developmental amnesia. Neuropsychologia. 2010;48:3179–3186. doi: 10.1016/j.neuropsychologia.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Squire LR, et al. Role of the hippocampus in remembering the past and imagining the future. Proc Natl Acad Sci USA. 2010;107:19044–19048. doi: 10.1073/pnas.1014391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nyberg L, Kim AS, Habib R, Levine B, Tulving E. Consciousness of subjective time in the brain. Proc Natl Acad Sci USA. 2010;107:22356–22359. doi: 10.1073/pnas.1016823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper JM, Vargha-Khadem F, Gadian DG, Maguire EA. The effect of hippocampal damage in children on recalling the past and imagining new experiences. Neuropsychologia. 2011;49:1843–1850. doi: 10.1016/j.neuropsychologia.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cogn Sci. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Smith SM, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network’s role in spontaneous cognition. J Neurophysiol. 2010;104:322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, et al. Intrinsic interhemispheric hippocampal functional connectivity predicts individual differences in memory performance ability. Hippocampus. 2010;20:345–351. doi: 10.1002/hipo.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CH, et al. Genetic influences on cortical regionalization in the human brain. Neuron. 2011;72:537–544. doi: 10.1016/j.neuron.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panizzon MS, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlaggar BL. Mapping genetic influences on cortical regionalization. Neuron. 2011;72:499–501. doi: 10.1016/j.neuron.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CH, et al. Hierarchical genetic organization of human cortical surface area. Science. 2012;335:1634–1636. doi: 10.1126/science.1215330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suddendorf T. Linking yesterday and tomorrow: Preschoolers’ ability to report temporally displaced events. Br J Dev Psychol. 2010;28:491–498. doi: 10.1348/026151009x479169. [DOI] [PubMed] [Google Scholar]
- 21.Grant JB, Suddendorf T. Young children’s ability to distinguish past and future changes in physical and mental states. Br J Dev Psychol. 2010;28:853–870. doi: 10.1348/026151009x482930. [DOI] [PubMed] [Google Scholar]
- 22.Russell J, Alexis D, Clayton N. Episodic future thinking in 3- to 5-year-old children: The ability to think of what will be needed from a different point of view. Cognition. 2010;114:56–71. doi: 10.1016/j.cognition.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Perner J, Kloo D, Rohwer M. Retro- and prospection for mental time travel: Emergence of episodic remembering and mental rotation in 5- to 8-year old children. Conscious Cogn. 2010;19:802–815. doi: 10.1016/j.concog.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atance CM, O'Neill DK. The emergence of episodic future thinking in humans. Learn Motiv. 2005;36:126–144. [Google Scholar]
- 25.Bauer PJ, Hertsgaard LA, Dow GA. After 8 months have passed: Long-term recall of events by 1- to 2-year-old children. Memory. 1994;2:353–382. doi: 10.1080/09658219408258955. [DOI] [PubMed] [Google Scholar]
- 26.Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly High Correlations in fMRI Studies of Emotion, Personality, and Social Cognition. Perspect Psychol Sci. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- 27.Suddendorf T, Addis DR, Corballis MC. Mental time travel and the shaping of the human mind. Philos Trans R Soc Lond B Biol Sci. 2009;364:1317–1324. doi: 10.1098/rstb.2008.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: Concepts, data, and applications. Ann N Y Acad Sci. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- 29.Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 30.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 32.D’Argembeau A, Xue G, Lu ZL, Van der Linden M, Bechara A. Neural correlates of envisioning emotional events in the near and far future. Neuroimage. 2008;40:398–407. doi: 10.1016/j.neuroimage.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okuda J, et al. Thinking of the future and past: The roles of the frontal pole and the medial temporal lobes. Neuroimage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- 34.Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proc Natl Acad Sci USA. 2007;104:642–647. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 36.Muscatell KA, Addis DR, Kensinger EA. Self-involvement modulates the effective connectivity of the autobiographical memory network. Soc Cogn Affect Neurosci. 2010;5:68–76. doi: 10.1093/scan/nsp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens WD, Buckner RL, Schacter DL. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cereb Cortex. 2010;20:1997–2006. doi: 10.1093/cercor/bhp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw P, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 40.Jolles DD, van Buchem MA, Crone EA, Rombouts SA. A comprehensive study of whole-brain functional connectivity in children and young adults. Cereb Cortex. 2011;21:385–391. doi: 10.1093/cercor/bhq104. [DOI] [PubMed] [Google Scholar]
- 41.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 42.Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Dev. 1987;58:601–622. [PubMed] [Google Scholar]
- 44.Bonnelle V, et al. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci. 2011;31:13442–13451. doi: 10.1523/JNEUROSCI.1163-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murre JM, Sturdy DP. The connectivity of the brain: Multi-level quantitative analysis. Biol Cybern. 1995;73:529–545. doi: 10.1007/BF00199545. [DOI] [PubMed] [Google Scholar]
- 46.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 47.White T, Su S, Schmidt M, Kao CY, Sapiro G. The development of gyrification in childhood and adolescence. Brain Cogn. 2010;72:36–45. doi: 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin VC, Schacter DL, Corballis MC, Addis DR. A role for the hippocampus in encoding simulations of future events. Proc Natl Acad Sci USA. 2011;108:13858–13863. doi: 10.1073/pnas.1105816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maguire EA, Hassabis D. Role of the hippocampus in imagination and future thinking. Proc Natl Acad Sci USA. 2011;108:E39. doi: 10.1073/pnas.1018876108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hurley NC, Maguire EA, Vargha-Khadem F. Patient HC with developmental amnesia can construct future scenarios. Neuropsychologia. 2011;49:3620–3628. doi: 10.1016/j.neuropsychologia.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutin AR, Robins RW. Phenomenology of autobiographical memories: The memory experiences questionnaire. Memory. 2007;15:390–411. doi: 10.1080/09658210701256654. [DOI] [PubMed] [Google Scholar]
- 52.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beckmann CF, Mackay CE, Filippini N, Smith SM. Group comparison of resting-state FMRI data using multu-subject ICA and dual regression. Neuroimage. 2009;47(Suppl 1):S39–S41. [Google Scholar]
- 54.Filippini N, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hagler DJ, Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayasaka S, Nichols TE. Validating cluster size inference: Random field and permutation methods. Neuroimage. 2003;20:2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.