Abstract
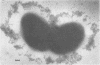
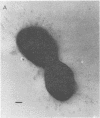
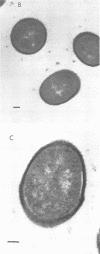
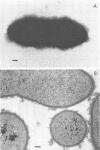
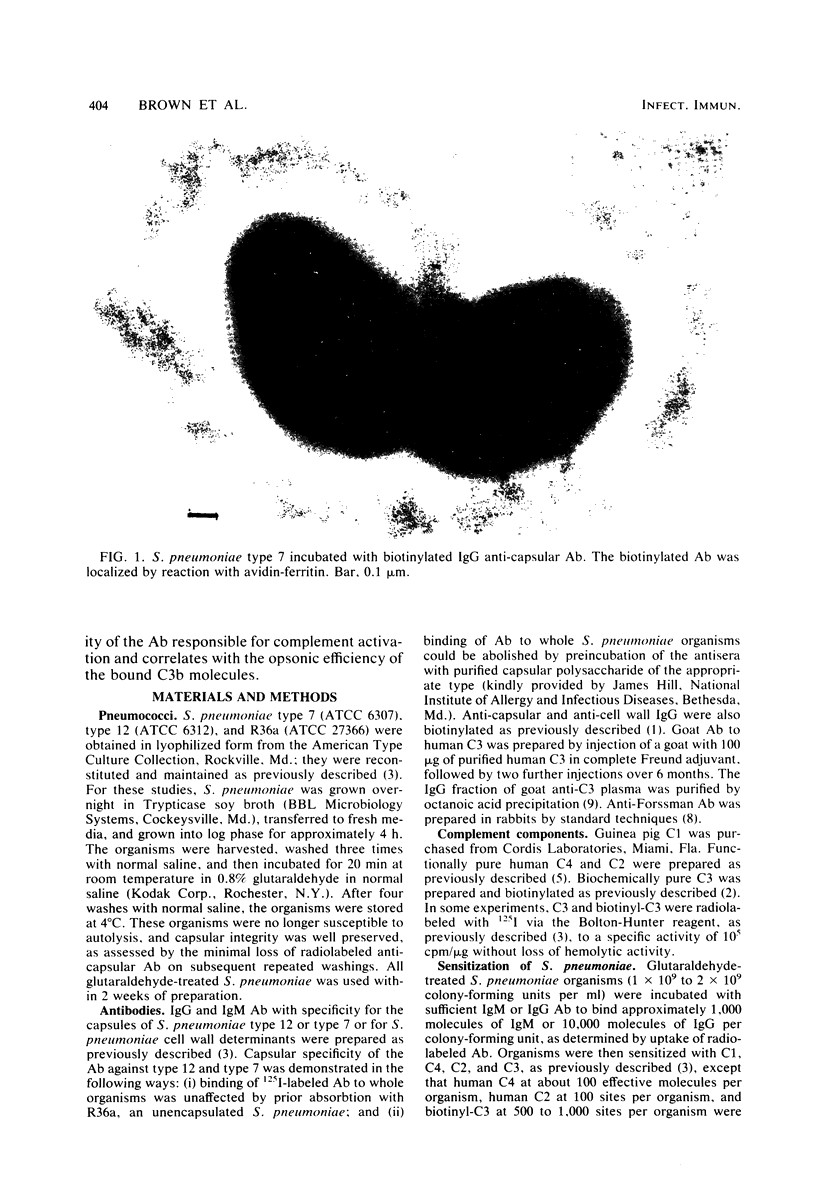
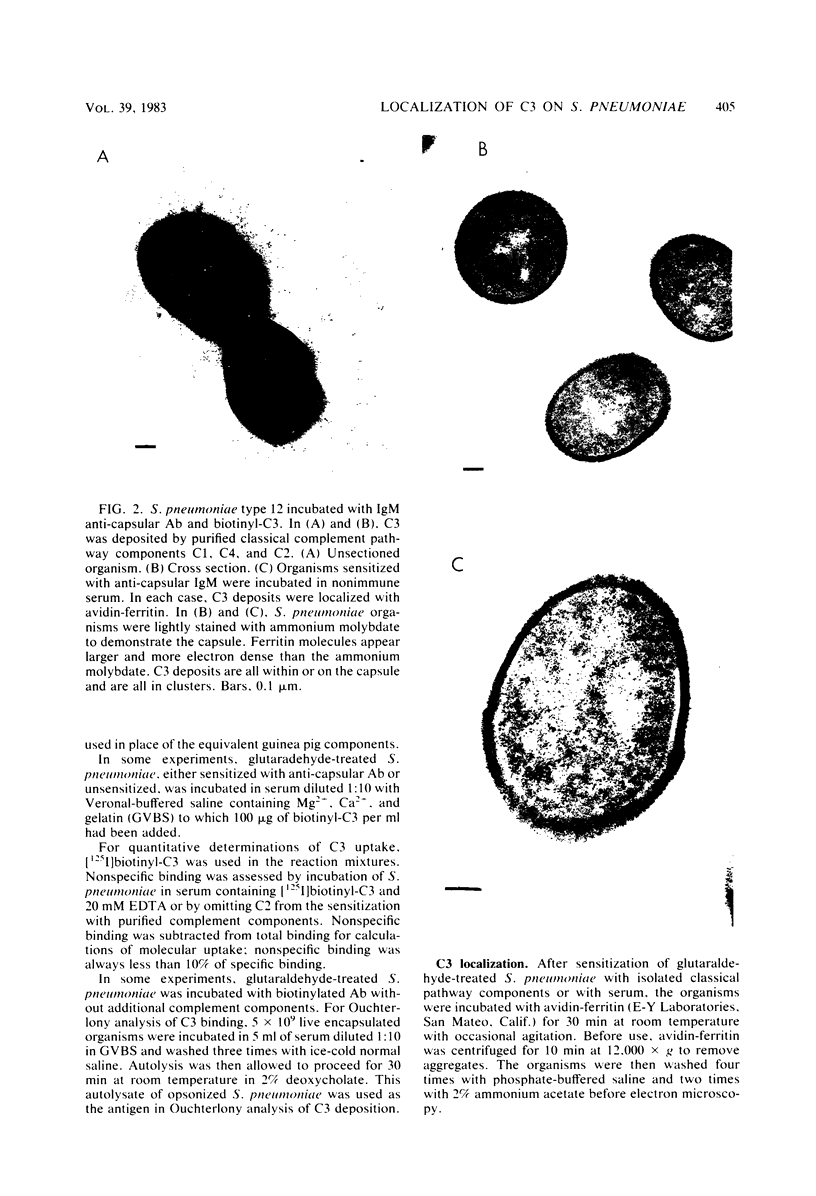
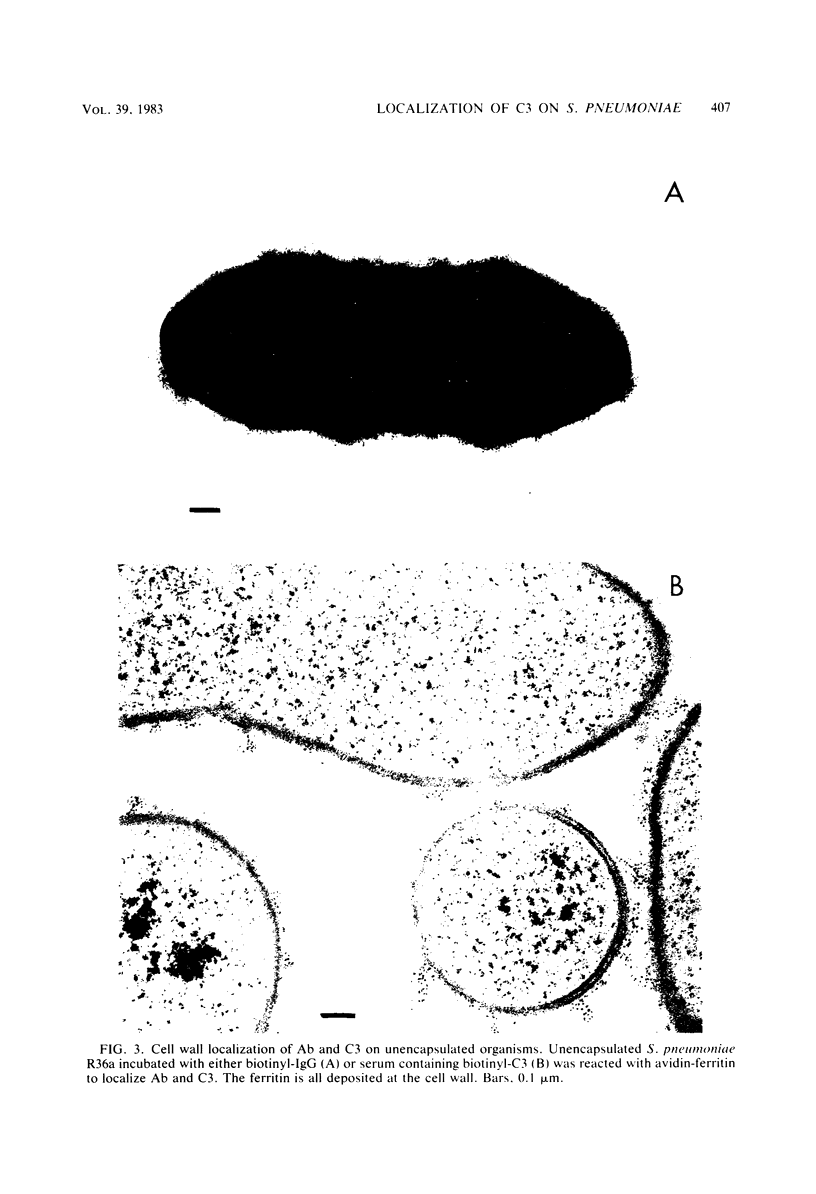
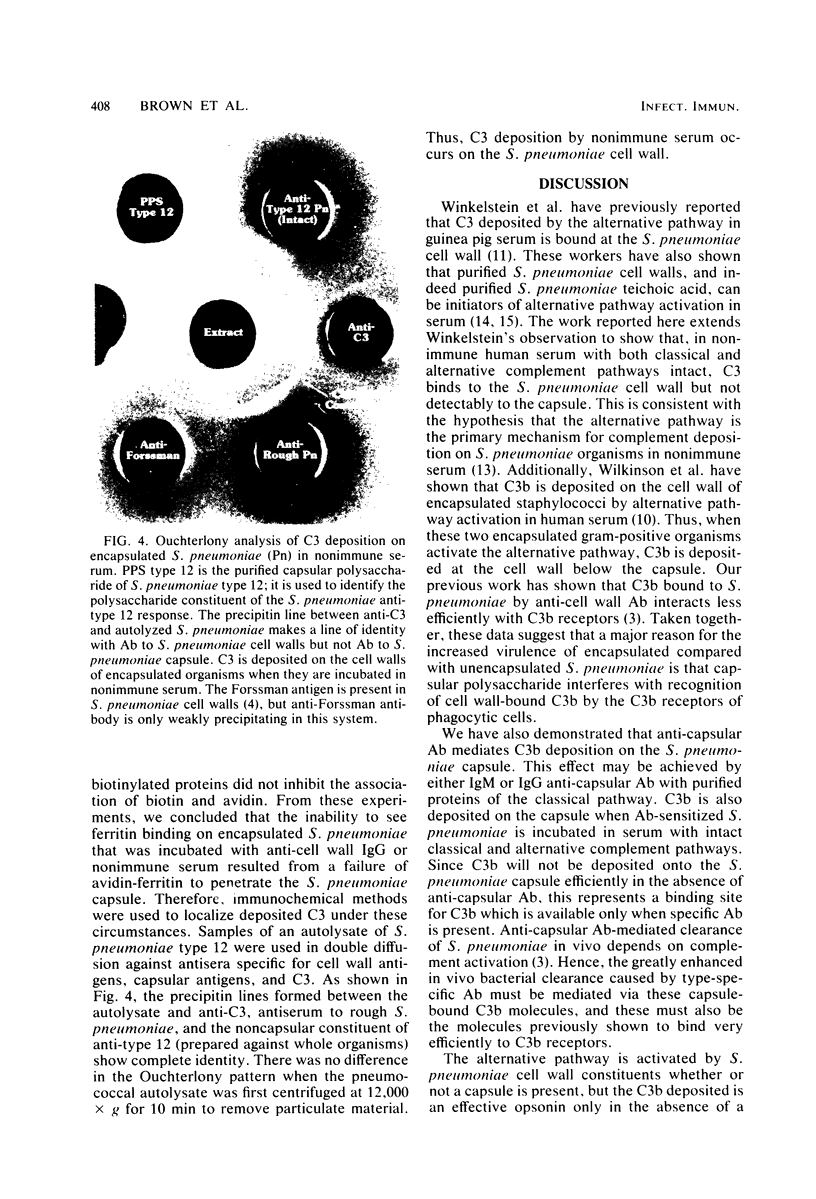
We have previously shown that complement component 3 (C3) deposited onto encapsulated Streptococcus pneumoniae by anti-capsular antibody (Ab) is a more efficient opsonin in vitro and in vivo than C3 deposited by anti-cell wall Ab (Brown et al., J. Clin. Invest. 69:85-98, 1982). In the present study, we explored the cellular location of C3b molecules that differ in opsonic efficiency by using avidin-ferritin to localize biotinylated Ab and C3 molecules on S. pneumoniae for electron microscopy. Anti-cell wall Ab and C3b molecules deposited by this Ab on unencapsulated S. pneumoniae were localized to S. pneumoniae cell walls. Anti-capsular Ab and C3b deposited by this Ab were seen in clusters on encapsulated S. pneumoniae at a distance from the cell wall. However, no avidin-ferritin staining of encapsulated S. pneumoniae was seen on incubation with biotinyl-anti-cell wall Ab, biotinylated C3 fixed by anti-cell wall Ab, or nonimmune serum containing biotinyl-C3. In each case, uptake of the biotinylated component was proven by radioactivity measurements, since biotinylated Ab and C3 were also radiolabeled with 125I. When avidin-ferritin did not bind to biotinylated components. Ouchterlony analysis indicated that C3 was bound to cell wall components on the encapsulated organisms. Thus, we conclude that, for encapsulated S. pneumoniae, opsonically efficient C3b molecules, deposited by anti-capsular Ab, are located on the S. pneumoniae capsule, whereas the opsonically inefficient C3b molecules deposited by anti-cell wall Ab or nonimmune serum are located on the cell wall. A major reason for the increased virulence of encapsulated compared to unencapsulated S. pneumoniae is that, in the absence of anti-capsular Ab, the S. pneumoniae capsule interferes with the recognition of cell wall-bound C3b molecules by phagocytic cell receptors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer E. A., Skutelsky E., Wilchek M. The avidin-biotin complex in affinity cytochemistry. Methods Enzymol. 1979;62:308–315. doi: 10.1016/0076-6879(79)62235-8. [DOI] [PubMed] [Google Scholar]
- Berger M., Gaither T. A., Cole R. M., Chused T. M., Hammer C. H., Frank M. M. Biotinylation of human C3. Mol Immunol. 1982 Jul;19(7):857–864. doi: 10.1016/0161-5890(82)90351-0. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Hosea S. W., Hammer C. H., Burch C. G., Frank M. M. A quantitative analysis of the interactions of antipneumococcal antibody and complement in experimental pneumococcal bacteremia. J Clin Invest. 1982 Jan;69(1):85–98. doi: 10.1172/JCI110444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M. The Forssman antigen of pneumococcus. Jpn J Exp Med. 1967 Dec;37(6):581–592. [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Hosea S. W., Burch C. G., Brown E. J., Berg R. A., Frank M. M. Impaired immune response of splenectomised patients to polyvalent pneumococcal vaccine. Lancet. 1981 Apr 11;1(8224):804–807. doi: 10.1016/s0140-6736(81)92681-7. [DOI] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Wilkinson B. J., Sisson S. P., Kim Y., Peterson P. K. Localization of the third component of complement on the cell wall of encapsulated Staphylococcus aureus M: implications for the mechanism of resistance to phagocytosis. Infect Immun. 1979 Dec;26(3):1159–1163. doi: 10.1128/iai.26.3.1159-1163.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein J. A., Abramovitz A. S., Tomasz A. Activation of C3 via the alternative complement pathway results in fixation of C3b to the pneumococcal cell wall. J Immunol. 1980 May;124(5):2502–2506. [PubMed] [Google Scholar]
- Winkelstein J. A., Bocchini J. A., Jr, Schiffman G. The role of the capsular polysaccharide in the activation of the alternative pathway by the pneumococcus. J Immunol. 1976 Feb;116(2):367–370. [PubMed] [Google Scholar]
- Winkelstein J. A., Shin H. S., Wood W. B., Jr Heat labile opsonins to Pneumococcus. 3. The participation of immunoglobulin and of the alternate pathway of C3 activation. J Immunol. 1972 Jun;108(6):1681–1689. [PubMed] [Google Scholar]
- Winkelstein J. A., Tomasz A. Activation of the alternative complement pathway by pneumococcal cell wall teichoic acid. J Immunol. 1978 Jan;120(1):174–178. [PubMed] [Google Scholar]
- Winkelstein J. A., Tomasz A. Activation of the alternative pathway by pneumococcal cell walls. J Immunol. 1977 Feb;118(2):451–454. [PubMed] [Google Scholar]