Abstract
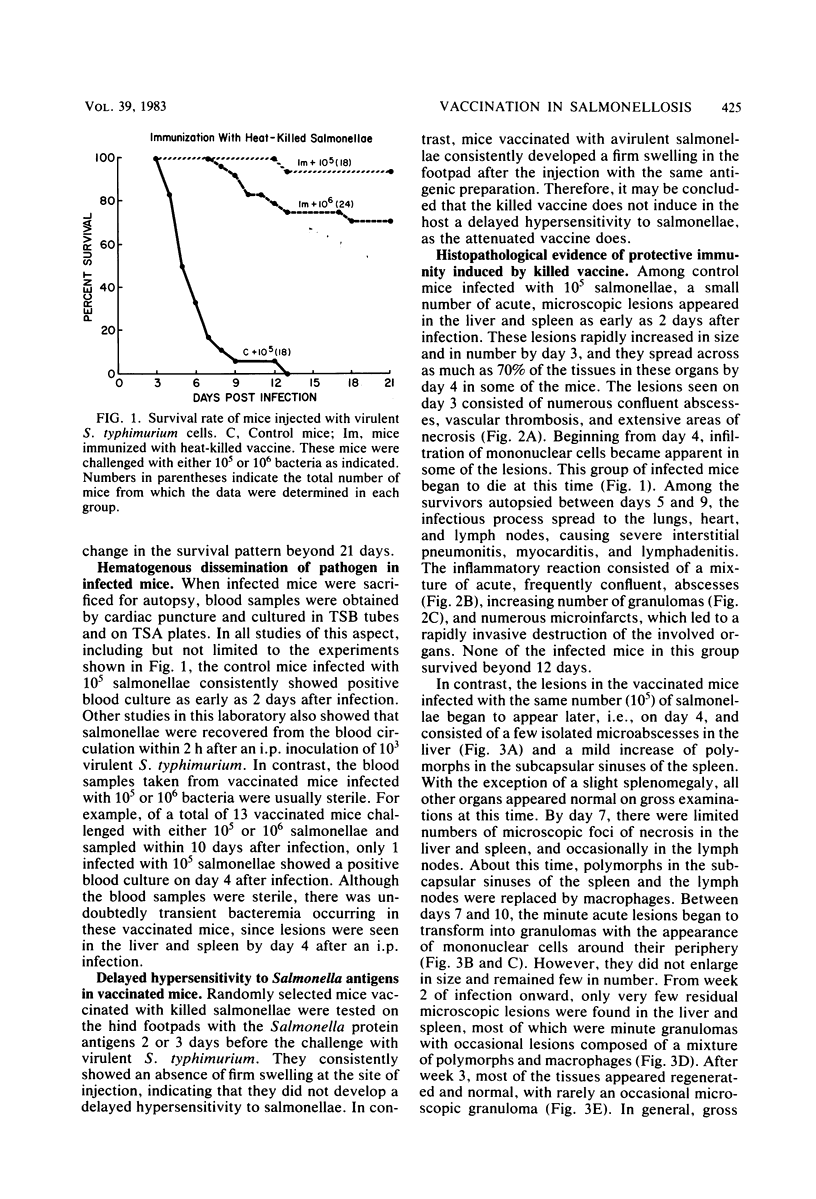
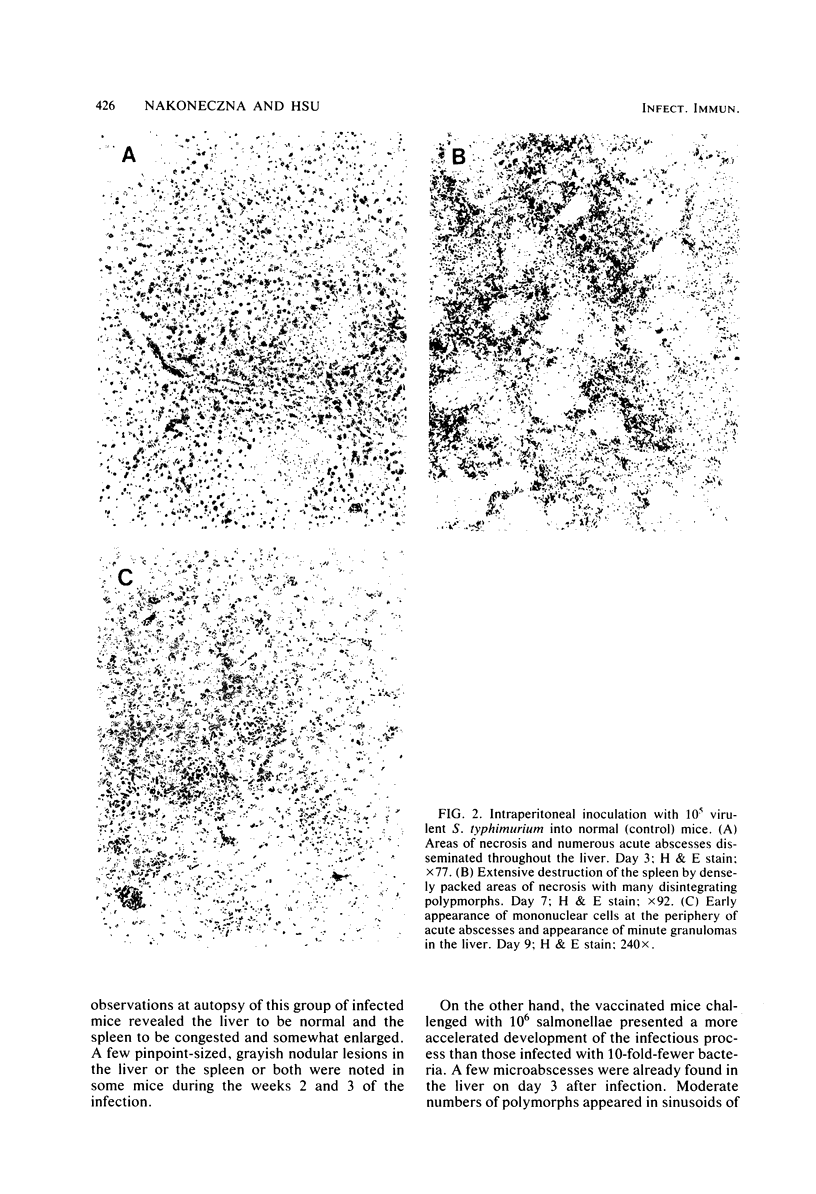
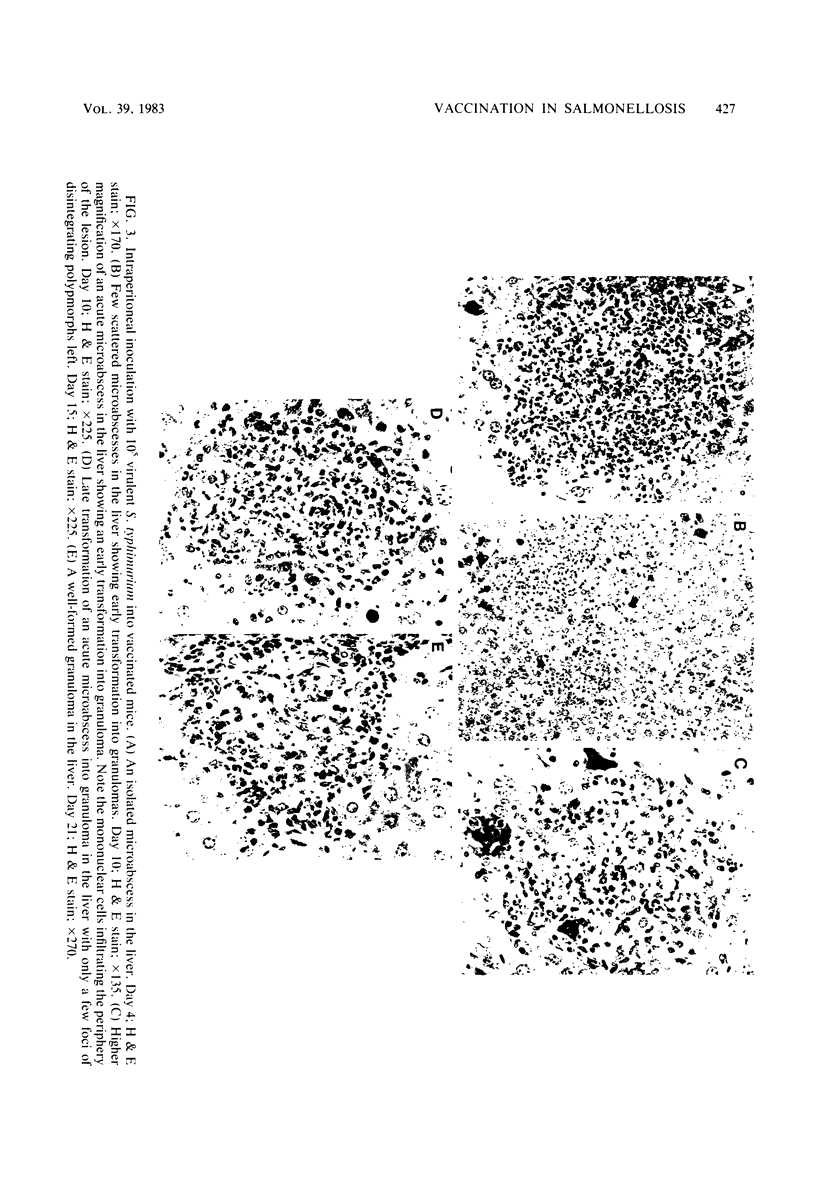
Swiss-Webster mice were vaccinated with heat-killed salmonellae and then were infected with virulent Salmonella typhimurium. Only 1 of the 18 vaccinated mice died from a challenge of 10(4) X the 50% lethal dose, and about 70% of them survived a challenge of 10(5) X the 50% lethal dose. Histopathological examinations of the lesions developed in these vaccinated mice showed that they followed the characteristic features of a primary lesion in murine salmonellosis. There was an early necrosis with infiltration of polymorphonuclear leukocytes and abscess formation within the first 6 to 7 days after infection. However, these abscesses remained small and discrete. By days 7 to 10, the lesions began to transform into granulomas, first with the appearance of peripheral mononuclear cells and then by the replacement of polymorphs. By the third week of the infection, minute and discrete granulomas were seen scattered in the spleen, liver, and lymph nodes. Beyond this stage, healing and tissue regeneration followed. Thus, the characteristics of infectious lesions developed in mice vaccinated with heat-killed salmonellae are distinctly different from those developed in mice protected by the avirulent vaccine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerman C. R., Eisenstein T. K. Correlation of the duration and magnitude of protection against Salmonella infection afforded by various vaccines with antibody titers. Infect Immun. 1980 Feb;27(2):435–443. doi: 10.1128/iai.27.2.435-443.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerdite S., Mooney C., Weiss J., Franson R., Elsbach P. Early and discrete changes in permeability of Escherichia coli and certain other gram-negative bacteria during killing by granulocytes. J Exp Med. 1974 Aug 1;140(2):396–409. doi: 10.1084/jem.140.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Modification of macrophage function. J Reticuloendothel Soc. 1968 Jun;5(3):179–202. [PubMed] [Google Scholar]
- Briles D. E., Lehmeyer J., Forman C. Phagocytosis and killing of salmonella typhimurium by peritoneal exudate cells. Infect Immun. 1981 Aug;33(2):380–388. doi: 10.1128/iai.33.2.380-388.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. Delayed hypersensitivity and arthus reactivity in relation to host resistance in salmonella-infected mice. J Immunol. 1968 Nov;101(5):830–845. [PubMed] [Google Scholar]
- Collins F. M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974 Dec;38(4):371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanier R. Immunity in experimental salmonellosis. 3. Comparative immunization with viable and heat-inactivated cells of Salmonella typhimurium. Infect Immun. 1972 May;5(5):792–797. doi: 10.1128/iai.5.5.792-797.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M., Nash P., Hino S. Degree of immunity induced by killed vaccines to experimental salmonellosis in mice. Infect Immun. 1972 Jan;5(1):83–90. doi: 10.1128/iai.5.1.83-90.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochadel J. F., Keller K. F. Protective effects of passively transferred immune T- or B-lymphocytes in mice infected with Salmonella typhimurium. J Infect Dis. 1977 May;135(5):813–823. doi: 10.1093/infdis/135.5.813. [DOI] [PubMed] [Google Scholar]
- Hsu H. S., Mayo D. R. Interactions between macrophages of guinea pigs and salmonellae. 3. Bactericidal action and cytophilic antibodies of macrophages of infected guinea pigs. Infect Immun. 1973 Aug;8(2):165–172. doi: 10.1128/iai.8.2.165-172.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. S., Miller K. B., Nakoneczna I. The role of delayed hypersensitivity in the enhancement of host resistance to infection. Can J Microbiol. 1980 Dec;26(12):1438–1442. doi: 10.1139/m80-239. [DOI] [PubMed] [Google Scholar]
- MITSUHASHI S., SATO I., TANAKA T. Experimental salmonellosis. Intracellular growth of Salmonella enteritidis ingested in mononuclear phagocytes of mice, and cellular basis of immunity. J Bacteriol. 1961 Jun;81:863–868. doi: 10.1128/jb.81.6.863-868.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marecki N. M., Hsu H. S., Mayo D. R. Cellular and humoral aspects of host resistance in murine salmonellosis. Br J Exp Pathol. 1975 Jun;56(3):231–243. [PMC free article] [PubMed] [Google Scholar]
- Mayo D. R., Hsu H. S., Lim F. Interactions between salmonellae and macrophages of guinea pigs. IV. Relationship between migration inhibition and antibacterial action of macrophages. Infect Immun. 1977 Oct;18(1):52–59. doi: 10.1128/iai.18.1.52-59.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrzakowski M. C., Spitznagel J. K. Bactericidal activity of fractionated granule contents from human polymorphonuclear leukocytes: antagonism of granule cationic proteins by lipopolysaccharide. Infect Immun. 1979 Aug;25(2):597–602. doi: 10.1128/iai.25.2.597-602.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. A., Wray C., Sojka W. J. The effect of T and B lymphocyte depletion on the protection of mice vaccinated with a Gal E mutant of Salmonella typhimurium. Br J Exp Pathol. 1976 Jun;57(3):354–360. [PMC free article] [PubMed] [Google Scholar]
- Nakoneczna I., Hsu H. S. The comparative histopathology of primary and secondary lesions in murine salmonellosis. Br J Exp Pathol. 1980 Feb;61(1):76–84. [PMC free article] [PubMed] [Google Scholar]
- Ornellas E. P., Roantree R. J., Steward J. P. The specificity and importance of humoral antibody in the protection of mice against intraperitoneal challenge with complement-sensitive and complement-resistant Salmonella. J Infect Dis. 1970 Feb;121(2):113–123. doi: 10.1093/infdis/121.2.113. [DOI] [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Bactericidal activity of specific and azurophil granules from human neutrophils: studies with outer-membrane mutants of Salmonella typhimurium LT-2. Infect Immun. 1978 Jan;19(1):131–137. doi: 10.1128/iai.19.1.131-137.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes M. W., Hsu H. S. Effect of kanamycin on the fate of Salmonella enteritidis within cultured macrophages of guinea pigs. J Reticuloendothel Soc. 1974 Jan;15(1):1–12. [PubMed] [Google Scholar]
- Valtonen M. V., Häyry P. O antigen as virulence factor in mouse typhoid: effect of B-cell suppression. Infect Immun. 1978 Jan;19(1):26–28. doi: 10.1128/iai.19.1.26-28.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Cell-mediated resistance induced with immunogenic preparations of Salmonella typhimurium. Infect Immun. 1971 Oct;4(4):381–387. doi: 10.1128/iai.4.4.381-387.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells P. S., Hsu H. S. Interactions Between Macrophages of Guinea Pigs and Salmonellae II. Phagocytosis of Salmonella typhimurium by Macrophages of Normal Guinea Pigs. Infect Immun. 1970 Aug;2(2):145–149. doi: 10.1128/iai.2.2.145-149.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwet T. L., Thompson J., Furth R. Effect of glucocorticosteroids on the phagocytosis and intracellular killing by peritoneal macrophages. Infect Immun. 1975 Oct;12(4):699–705. doi: 10.1128/iai.12.4.699-705.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]





