Abstract
Upper thermal limits vary less than lower limits among related species of terrestrial ectotherms. This pattern may reflect weak or uniform selection on upper limits, or alternatively tight evolutionary constraints. We investigated this issue in 94 Drosophila species from diverse climates and reared in a common environment to control for plastic effects that may confound species comparisons. We found substantial variation in upper thermal limits among species, negatively correlated with annual precipitation at the central point of their distribution and also with the interaction between precipitation and maximum temperature, showing that heat resistance is an important determinant of Drosophila species distributions. Species from hot and relatively dry regions had higher resistance, whereas resistance was uncorrelated with temperature in wetter regions. Using a suite of analyses we showed that phylogenetic signal in heat resistance reflects phylogenetic inertia rather than common selection pressures. Current species distributions are therefore more likely to reflect environmental sorting of lineages rather than local adaptation. Similar to previous studies, thermal safety margins were small at low latitudes, with safety margins smallest for species occupying both humid and dry tropical environments. Thus, species from a range of environments are likely to be at risk owing to climate change. Together these findings suggest that this group of insects is unlikely to buffer global change effects through marked evolutionary changes, highlighting the importance of facilitating range shifts for maintaining biodiversity.
Keywords: niche conservatism, stress resistance, thermal adaptation, evolutionary history, space
Temperatures are expected to rise across the globe over the coming decades and centuries (1), and many studies suggest the potential for range shifts/reductions in many species (2). When assessing the likely impact of temperature changes on species survival, an implicit assumption is that the thermal environment shapes resistance to temperature extremes and thus dictates species range limits. Nevertheless, few studies have directly tested for such links between physiological upper thermal limits of ectothermic species and temperature conditions within their geographic range (3, 4). A related, little-investigated key point for predicting species range responses to climate change is whether upper thermal limits are modifiable through plastic and/or evolutionary responses (5, 6). Ideally, evolutionary and plastic responses within and across generations, including short-term hardening and acclimation, should be separated, as through a common garden approach whereby species are kept under controlled laboratory conditions (7, 8). Without controlling for plastic responses, it is not possible to distinguish adaptive evolutionary responses, and species may erroneously seem close to their upper thermal thresholds (9–12), biasing extinction risk estimates.
Furthermore, by examining the evolution of upper thermal limits (heat resistance) across a known phylogeny, it is possible to determine when trait limits related to evolutionary history have arisen, which will aid in predicting potential evolutionary response to climate change (13, 14). In the past phylogenetic analyses have mainly aimed to control for the effects of phylogeny (4, 15, 16); however, studies are now emerging that examine phylogenetic signal with the purpose of determining the role of adaptation vs. constraints (17–20).
In the present study we undertake a large and rigorous evaluation of heat resistance (estimated as critical thermal maxima) in 94 Drosophila species. We examine the link between climate conditions within species ranges and heat resistance as well as safety margins, and assess the relative contribution of phylogenetic inertia and common selection pressures in resistance variation using recently developed approaches (18, 21). Related species may exhibit similar heat resistance (phylogenetic signal) owing to either evolutionary phylogenetic constraints (phylogenetic inertia) or spatial proximity; the latter may result in species being similar owing to common selection regimes (13, 17, 18, 21). We further examined the question of which species groups are likely to be more threatened by global climate change. Both mean temperatures and temperature extremes are expected to increase under the prevailing climate change scenarios (1), but the impact of these changes on species performances and distributions is still unclear. Previous studies comparing upper thermal limits of species provided conflicting geographical differences in thermal safety margins, with some finding these to be smaller for species from tropical regions (10, 22, 23) and others finding species from temperate or dry environments to be closer to their thermal maxima (11, 16, 24).
Results
The positive association between heat resistance and average and maximal temperature was generally weak (R2 < 0.05; Table S1; nonphylogenetic analysis), whereas heat resistance was more strongly negatively associated with annual precipitation (PANN) (R2 = 0.18–0.20) (Fig. S1 and Table S1). Combining temperature and precipitation into the variable drying power of air improved this relationship only slightly (R2 = 0.21–0.22) (Table S1). Using a multiple regression approach, a stronger association with climate emerged. The best multiple predictor model included PANN and maximum temperature of the warmest month (Tmax) for females (R2 = 0.29, P < 0.01) and PANN, Tmax, and precipitation of the driest month (PDRY) (R2 = 0.32, P < 0.01) for males (Table S1). Notably, heat resistance increased as precipitation decreased, suggesting that factors related to water in the environment are more important in driving upper thermal limits than high temperature alone. For Drosophila species, in which behavioral thermal regulation is likely to play a key role in avoiding heat stress, we predict that low canopy-cover environments will select for higher heat resistance owing to less microclimatic heterogeneity and thus smaller scope for thermal refuges. Because decreased canopy cover in warm regions coincides with PANN <1,000 mm (25, 26), we divided the species into low annual precipitation (<1,000 mm) and high annual precipitation (≥1,000 mm) groups. A strong relationship between heat resistance and Tmax was detected for species occupying dry environments (<1,000 mm annual precipitation; R2 = 0.44, slope = 0.30 °C/°C, P = 0.001), whereas there was no relationship for species occupying wet environments (≥1,000 mm annual precipitation; R2 = 0.008, slope = 0.05 °C/°C, P = 0.44) (Fig. S2; species occupying environments ≥1,000 mm of annual precipitation include both tropical and subtropical species).
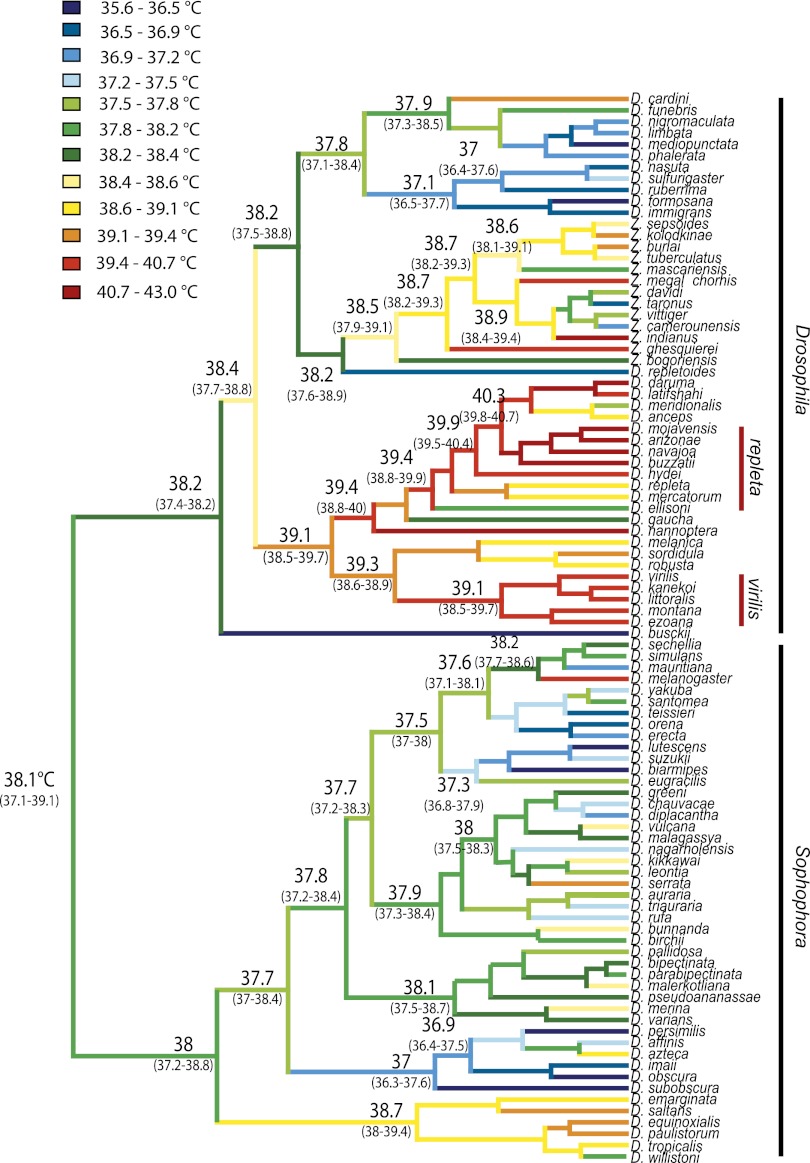
Estimates of phylogenetic signal were moderate, with both Pagel’s λ and Bloomberg’s K significantly different from both 0 and 1 (Table 1). These results suggest that heat resistance is neither evolving completely in accordance with Brownian motion nor evolving free from phylogenetic associations. A third estimate of phylogenetic signal, the SLOUCH analysis, further confirmed these results, with moderate to high levels of phylogenetic signal detected (see t1/2 estimate in Table 1). Using Moran’s I we estimated phylogenetic signal at three taxonomic levels and found only a weak association at the subgenus level, with correlations increasing between the species group and species subgroup levels, indicating that heat resistance has arisen relatively late in the phylogeny (Fig. 1 and Table 1). In comparison with the observed range of heat resistance across species, the ancestral state was low to moderate heat resistance. This further supports the more recent evolution of heat resistance within the Drosophila phylogeny (Fig. 1). Importantly, highly heat-resistant phenotypes were tightly clustered, with only a few groups of related species having evolved a critical thermal maximum (CTmax) greater than 39 °C (Fig. 1). The virilis and repleta species group were most notable, with phenotypes >1.5 °C higher than the mean heat resistance across all species (virilis
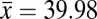 °C ± 0.09 repleta
°C ± 0.09 repleta
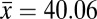 °C ± 0.19, overall
°C ± 0.19, overall 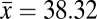 °C ± 0.15) (Fig. 1). These results suggest that high heat resistance has evolved rarely within drosophilids.
°C ± 0.15) (Fig. 1). These results suggest that high heat resistance has evolved rarely within drosophilids.
Table 1.
Phylogenetic signal in CTmax estimated using alternative methods
| Moran’s I |
|||||||
| Variable | λ | AICc | K | SLOUCH t1/2 (trait) | SubG | SppG | SubSppG |
| CTmax♀ | 0.69 | 288.94 | 0.36* | 0.58 (0.27-∞) | 0.05 | 0.33* | 0.42* |
| λ0 315.47 | |||||||
| λ1 295.77 | |||||||
| CTmax ♂ | 0.64 | 291.86 | 0.35* | 0.45 (0.22-∞) | 0.08† | 0.27* | 0.40* |
| λ0 314.40 | |||||||
| λ1 299.74 | |||||||
Estimates of phylogenetic signal for λ and K range from no phylogenetic signal with λ0 and K = 0, to high phylogenetic signal with λ1 and K ≥ 1. The appropriate λ model is chosen by comparing the AICc with λ0 or λ1. The SLOUCH phylogenetic t1/2 estimates the association between phylogeny and heat resistance, where t1/2 = 0 reflects no phylogenetic signal in the trait of interest, and an increasing t1/2 reflects a stronger relationship, with t1/2 = 1 reflecting strong phylogenetic signal. The two-unit support surface is shown in parentheses. Moran’s I characterizes the level of phylogenetic signal across three taxonomic levels (SubG, subgenus; SppG, species group; and SubSppG, subspecies group) using a phylogenetic autocorrelation method.
*Significant at the 0.001 level.
†Significant at the 0.01.
Fig. 1.
Phylogenetic hypothesis for 94 Drosophila species. CTmax was mapped onto the phylogeny using ancestral trait reconstruction via maximum likelihood; the 95% confidence intervals are shown in parenthesis. Branches were color-coded according to their likelihood states; color groups were determined by dividing all 94 species into 12 equal-sized groups.
Strong phylogenetic signal need not reflect evolutionary constraints but could alternatively be driven by similar selection pressures in similar environments occurring in spatial proximity (phylogenetic structured adaptation) (18). Here we tested for such spatial association by examining the relationship between levels of heat resistance and species’ spatial proximity (distance in kilometers between the midpoint of each species’ distribution), with the hypothesis that this relationship should be strong if closely related species share similar adaptations because they are closely associated spatially. A significant association between spatial parameters and heat resistance was found, but this explained <1% of variation in heat resistance (females: R2 < 0.01, slope = 2.30 km/°C, P < 0.01; males: R2 < 0.01, slope = 2.45 km/°C, P < 0.01). An alternative analysis, testing qualitatively similar ideas, determines the degree to which heat resistance is structured across the phylogeny, estimating the covariance between phylogeny, heat resistance, and climatic variables as the phylogenetic t1/2 (t1/2 is equal to total tree height = 1, thus a t1/2 > 1 reflects a strong association between phylogeny and heat resistance) (Table 2, SLOUCH) (24). Strong to moderate phylogenetic signal (estimated from the relationship between phylogeny and CTmax) was detected for heat resistance (t1/2 = 0.45–0.58). With the inclusion of the predictor variables PANN and Tmax, a weaker albeit still-strong association (t1/2 = 0.38–0.46) was found between heat resistance, predictors, and phylogeny, suggesting that most phylogenetic signal in heat resistance was related to phylogenetic inertia. These results coupled with only weak associations between heat resistance and spatial proximity suggest phylogenetic signal driven by phylogenetic inertia rather than phylogenetically structured adaptation.
Table 2.
Phylogenetic association in the relationship between CTmax and Tmax and PANN
| Optimal regression |
Evolutionary regression |
||||||
| Trait and predictor | t1/2 | Intercept | Slope | Intercept | Slope | r2 | AIC |
| CTmax: ♀ | 0.58 (0.27-∞) | 38.15 ± 0.57 | — | — | — | — | 295.70 |
| Tmax | 0.51 (0.25-∞) | 35.97 ± 1.46 | 0.17 ± 0.11 | 35.98 ± 1.47 | 0.08 ± 0.05 | 0.03 | 295.53 |
| PANN | 0.55 (0.25-∞) | 44.19 ± 2.46 | −4.46 ± 1.79 | 44.47 ± 2.56 | −2.01 ± 0.81 | 0.06 | 292.35 |
| Tmax | 0.46 (0.24-∞) | 42.23 ± 2.76 | 0.19 ± 0.10 | 42.66 ± 2.76 | 0.10 ± 0.05 | 0.10 | 291.18* |
| + PANN | −4.50 ± 1.60 | −2.41 ± 0.82 | |||||
| CTmax: ♂ | 0.45 (0.22-∞) | 38.13 ± 0.43 | 300.12 | ||||
| Tmax | 0.45 (0.22-∞) | 34.89 ± 1.47 | 0.23 ± 0.10 | 34.89 ± 1.47 | 0.11 ± 0.05 | 0.05 | 297.57 |
| PANN | 0.40 (0.22-∞) | 43.81 ± 2.59 | −3.45 ± 1.56 | 44.05 ± 2.67 | −1.8 9 ± 0.84 | 0.05 | 297.92 |
| PDRY | 0.40 (0.20-∞) | 38.44 ± 0.45 | −0.01 ± 0.01 | 38.44 ± 0.45 | −0.008 ± 0.01 | 0.02 | 300.65 |
| Tmax | 0.38 (0.20-∞) | 41.11 ± 2.72 | 0.24 ± 0.09 | 41.61 ± 2.80 | 0.14 ± 0.05 | 0.12 | 293.64* |
| + PANN | −3.98 ± 1.48 | −2.38 ± 0.84 | |||||
| Tmax | 0.44 (0.20-∞) | 41.61 ± 2.76 | 0.27 ± 0.10 | 42.30 ± 2.87 | 0.16 ± 0.05 | 0.13 | 295.95 |
| + PANN | −4.72 ± 1.70 | −2.91 ± 0.98 | |||||
| + PDRY | 0.01 ± 0.01 | 0.01 ± 0.01 | |||||
The SLOUCH method estimates the degree of phylogenetic inertia in heat resistance. The phylogenetic t1/2 (in units of tree height, i.e., 1) and the two-unit support surface (in parentheses) are shown. The optimal regression describes the relationship between heat resistance and the predictor variables without the influence of ancestry (phylogeny), whereas the evolutionary regression controls for any phylogenetic association. The r2 value describes the variance in heat resistance while controlling for phylogeny.
*Model with the best AIC.
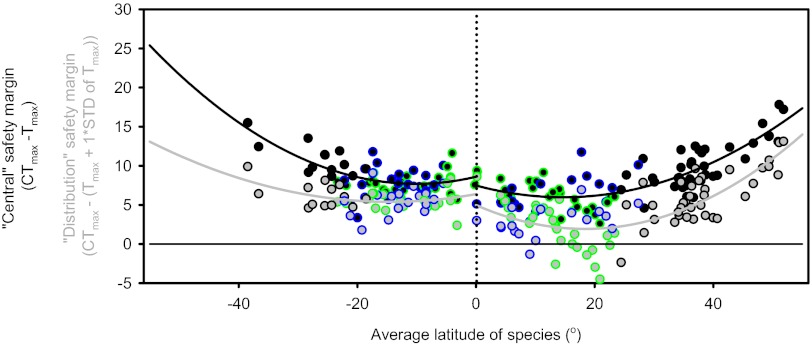
One way to predict responses to climate change is to assess how close species upper thermal limits are to the current environment (safety margin: difference between heat exposure and heat resistance) (10). Both the species-level safety margins and distributional safety margin (safety margin plus 1 SD) were lowest for species occupying latitudinal environments between 10° and 23°, particularly within the northern hemisphere (Fig. 2 and Table S2). Moreover, we also found a significant polynomial relationship between latitude and Tmax (northern hemisphere: r2 = 0.64, P < 0.01; southern hemisphere: r2 = 0.41, P < 0.01). Removal of outlier data points around the equator did not influence the strength of the polynomial relationship. Safety margins for species occupying northern latitudes were smaller than for those in the southern hemisphere (Fig. 2 and Table S2). The species closest to their safety margin (between latitudes 10° and 23°) represented species from both humid tropical and drier tropical environments (with <1,500 mm annual precipitation).
Fig. 2.
Species and distribution safety margins. The “Central” species safety margins were calculated as the difference between heat tolerance (CTmax) and the average of maximal environmental temperature (Tmax) (filled black circles). To include the species-specific populations that experience more severe heat stress we also calculated the “distribution” safety margins using the assumption that these populations experience a Tmax equivalent to average + SD of Tmax. Species present on both hemispheres are represented for the northern and southern hemisphere. The species are separated [temperate (black edge), humid tropical (green edge), and dry tropical (blue edge)] according to their average environmental habitat (see text).
Discussion
Given that drosophilids are ectothermic, it is perhaps surprising to find only a weak association between species’ heat resistance and measures of environmental temperature across their range. This observation is in line with a previous comparative study on Drosophila, which also failed to find an overall association between heat resistance and latitude (4). Studies of intraspecific variation in Drosophila are also equivocal, with some finding significant associations between measures of heat resistance and climatic variables/latitude, whereas others have not (27). Consistent with evidence that Drosophila species from desert environments tend to be more heat resistant than mesic temperate species (28), we found a positive correlation between CTmax and environmental temperature in dry environments. The association between heat resistance and precipitation was such that temperature played a larger role in shaping heat resistance in dry environments (<1,000 mm annual precipitation) and not at all in wet environments (≥1,000 mm annual precipitation). Thus, instead of temperature playing an overarching role, we found that precipitation was more important and that a combination of temperature and precipitation explained the most variation in this trait (Table S1).
Although the exact reason for this relationship is unclear, it may reflect the extent of canopy cover being linked to the amount of precipitation (25, 26). It is unlikely that small insects like drosophilids use evaporative cooling in any significant way, but humid environments are likely to give rise to a more diverse array of microclimates where flies might seek shelter when temperatures are high (i.e., by streams, under leaves) (29, 30). In drier regions it will be more challenging for the flies to find a suitable microhabitat during warm days; as a consequence they may be unable to behaviorally thermo-regulate, relying on innate heat resistance (31, 32). Thus, it would seem that warm and dry environments select for increased heat tolerance in ectotherms like Drosophila. In lizard species there is also an association between optimal body temperature and both the temperature and annual precipitation of a particular location (11, 16).
We detected moderate to strong phylogenetic signal for heat resistance. This was further supported by the ancestral reconstruction showing that highly heat resistant phenotypes have evolved rarely across the Drosophila phylogeny and within specific species groups (Fig. 1). There was little evidence for spatial associations driving common adaptations in closely related species; instead, phylogenetic signal in heat resistance was related to phylogenetic inertia (Table 2). Hence, drosophilid species have only limited evolutionary capacity to evolve and alter upper thermal limits. In line with this finding, recent studies have suggested that the ability of terrestrial insect species to evolve higher heat resistance may be limited; there is limited variance in maximal heat resistance (3), and experimental studies suggest low evolutionary potential for different estimates of resistance (33, 34). This may indicate that substantial changes at the molecular level are required to alter upper thermal limits, such as by duplications of heat shock genes (35). In contrast, there seems to be more variation among ectotherms for lower thermal limits (36). The results for both cold and heat resistance are indicative of environmental sorting, with species moving into environments where they are preadapted, rather than having selection shape resistance levels. However, the ability of flies to avoid heat stress through behavioral responses (37) was not considered here.
By computing safety margins, recent research has suggested that species from low tropical latitudes are at greater risk from climate change (10, 11, 22, 23). In the present study, when maximal temperatures were considered, species occupying low latitudinal environments had the smallest safety margins, particularly for species occupying latitudes from 10° to 23° in the northern hemisphere. Similar to Sunday et al. (22), northern hemisphere species were closer to their thermal limits than those from the southern hemisphere (Fig. 2 and Table S2). When dividing species belonging to humid tropical and dry tropical environments according to annual precipitation (≥1,500 mm annual precipitation = humid tropical), the smallest safety margins were found for species occupying both wet and dry environments, highlighting that species not from humid tropical environments will also be at risk (Fig. 2) (11, 16).
In this study we did not examine plastic effects but instead controlled for phenotypic plasticity to reveal the genetic component of heat resistance. In Drosophila the time to knockdown during a heat exposure has been shown to respond to both developmental acclimation and short-term hardening treatments (5, 38). However, a recent study found that developmental acclimation and short-term plastic responses increased upper thermal limits by less than 1 °C across both tropical and temperate Drosophila species (39). This finding suggests that plastic responses may be small and vary little across species when measured as a tolerance temperature rather than a tolerance time. A low capacity to respond plastically to temperature changes combined with a high level of phylogenetic inertia suggest that most species within the Drosophila phylogeny are unlikely to increase their upper thermal limits via plastic and/or evolutionary responses.
Our results could be influenced by inbreeding and laboratory adaptation because many species tested were represented by strains from stock centers. Inbreeding effects on CTmax tend to be minor across Drosophila species (40), whereas the impact of laboratory adaptation is less certain (41, 42). Nevertheless, in our opinion these confounding effects will be minor relative to heat resistance variation found between species.
CTmax as estimated in the present study may be confounded by effects of desiccation and starvation (assays are performed without food or moisture for ∼3 h) as discussed recently in the literature (43, 44). This might inflate estimates of CTmax particularly for highly desiccation/starvation-resistant species, but recent experiments on Drosophila melanogaster have found the impact of desiccation and starvation stress to be negligible with respect to CTmax when using heat exposures similar to the one used here (45). Furthermore, Mitchell et al. (33) have shown that static and ramping estimates of heat resistance correlate strongly across tropical and widespread Drosophila. Although a number of factors may influence the results of this study, we consider these effects to be small and unlikely to influence the conclusions of this study.
This study highlights three points. First, there is a high level of phylogenetic inertia in heat resistance. Second, in relatively drier environments there is a correlation between species resistance and Tmax, suggesting that upper thermal tolerance limits (measured under environmentally controlled conditions) can influence species range limits. Finally, drosophilid species across a range of environments, not only humid tropical species, are living in environments where maximal environmental temperature approaches tolerance limits; with limited evolutionary potential this raises concerns about the ability of species to counter climate change (3, 16). Thus, although studies of genetic variation and geographic variability in heat resistance point to some evolutionary adaptive potential (27, 33), this may not be enough under current Intergovernmental Panel on Climate Change scenarios.
Materials and Methods
Sources and Maintenance of Experimental Animals.
Drosophila stocks were obtained from five sources and maintained at 200 individuals for a minimum of two generations before testing. All flies were reared and tested in the same laboratory, under identical conditions. For a more detailed explanation of stocks and experimental methods, see ref. 20. Briefly, flies were reared at 20 °C at a 12-h/12-h light/dark cycle and maintained on an oat-based medium (Leeds); some species, however, required the addition of Opuntia cactus and banana to medium. Experimental flies were controlled for larval density and age effects and prepared for trait measurements (20). For each species we examined 10 flies per sex unless otherwise stated. Although the number of individuals measured for heat resistance was low, the variance was small for both females and males (Table S3). Heat resistance was estimated as CTmax under gradual heating. Individuals were placed into empty 5-mL glass vials and placed into a water bath set at 20 °C. The temperature was gradually increased at 0.1 °C/min, and the temperature at which a fly lost the ability to move, after tapping on the vial, was scored as CTmax.
Estimations of Climatic Variables.
The distribution of each species was collated from the taxodros Web site, which provides global positioning system coordinates of published Drosophila collections (http://www.taxodros.uzh.ch/). Duplicate records in terms of species, latitude, and longitude (with one-decimal precision) were removed. Climatic variables from the WorldClim data set (www.worldclim.org) (46) were extracted to these coordinates. Five temperature and precipitation variables thought to be related to heat resistance were chosen: (i) annual mean temperature and (ii) absolute maximal temperature of the warmest month (Tmax) are the most likely candidates for shaping the evolution of heat resistance, whereas (iii) absolute minimum temperature of the coldest month (Tmin) was chosen because there may be a tradeoff between cold and heat resistance. Finally, precipitation related variables (iv) annual precipitation (PANN) and (v) precipitation of the driest month (PDRY) have also been linked to upper thermal limits (16). Average values were calculated from the extracted climatic data for each species. We included a sixth variable that combined precipitation and temperature into the metric, drying power of air (dpa), in which the water saturation deficit between animal and environment is estimated using temperature and precipitation variables (20). All geographic information system (GIS) operations were performed in ArcGIS 10 (ESRI).
To examine whether spatial sampling bias existed in our climatic averages, we created an approximate 100 × 100-km grid (in Behrmann equal area projection). For all occurrence records within the same grid cell, we calculated the median of the climatic values. The grid cell medians were then used to calculate average climatic data for each species. A regression of the species average climatic values using the median values against the species average climatic values, as opposed to using all observations, demonstrated a high association (r2 > 0.95), suggesting minor sampling bias, and thus analyses are based on climate data from all observations.
To assess current proximity of a species to stressful environmental temperatures, we estimated safety margins for the center point of a species distribution (central safety margin), calculated as the difference in heat resistance (CTmax) from the average of the absolute maximal temperature of the environment (Tmax). To obtain the proximity of the “threatened” populations (distribution safety margins) we used the assumption that such populations experience higher heat stress, and we therefore estimated safety margins by comparing CTmax of the species to the average + 1 SD of maximal environmental temperature for this species. Thus, the estimated distribution of safety margins takes into account that, because averages of Tmax were generated from many observations for some species, local populations will experience Tmax considerably higher than the average for this species. To investigate safety margins across species groups sharing similar environments, we divided species relative to the average latitude, temperature, and precipitation of species. Species having a latitude >23° were deemed temperate, whereas tropical species (<23° latitude) were split into dry tropical (PANN <1,500 mm) or humid/wet tropical (PANN ≥1,500 mm).
Construction of Phylogeny.
We combined previously published phylogenies to generate a Drosophila phylogeny incorporating all 94 species, with the basis of the phylogeny taken from ref. 47; see ref. 20 for details.
Mapping Traits and Ecological Variables onto the Phylogeny.
Phylogenetic signal was estimated using Pagel’s λ, Bloomberg’s K, and Moran’s I (48–50). Pagel’s λ was estimated for the residuals with a comparison of the Akaike information criteria (AIC) to determine the best model, with λ0 (H0 = no phylogenetic signal) and λ1 (Ha = phylogenetic signal). A value of K not significantly different from 0 indicates the absence of phylogenetic signal, whereas K = 1 indicates that the trait is evolving under a Brownian motion model of evolution with significant phylogenetic signal. These and other methods, unless otherwise stated, were implemented in R using the ape and picante packages (51–53). Moran’s I computes the phylogenetic autocorrelation of the data at different taxonomic levels and was estimated using the ape package (48). Here we used the taxonomic divisions from the taxodros Web site (www.taxodros.uzh.ch/), dividing species into three levels: subgenus, species group, and species subgroup (20).
Trait Analyses.
Nonphylogenetic least-squares regression was applied to determine the association between heat resistance and ecological variables using the Poptools add-in for Microsoft Excel (54). Initially, the relationship between heat resistance and climatic variables was estimated singularly with the lm() function in R. To examine a multiple predictor model, variables with the lowest AIC were then added sequentially, the initial model having the climatic variable with the lowest AIC. Climatic variables with the lowest AIC were also the least autocorrelated. Consequently, of the seven climatic variables considered, the multiple predictor models included PANN, TMAX, and PDRY. Because dpa was calculated from both precipitation and temperature, this variable was not included in the multiple predictor model. Ancestral trait reconstruction was used to examine how heat resistance has evolved across the phylogeny, based on maximum likelihood methods on continuous characters with the ace function in the ape package (51). Phylogenetic analyses are only presented on environmental variables explaining the largest proportion of variation in resistance.
Phylogenetic Signal: Adaptation vs. Inertia.
The relationship between spatial patterns and heat resistance was estimated by regressing distance matrices of heat resistance onto spatial relatedness. Distance matrices were calculated for heat resistance using the dist() function in R (based on Euclidean distances). Spatial distance matrices were generated by initially calculating the longitude and latitude midpoint of a species distribution, and using the fossil package a distance matrix in kilometers was calculated between each species midpoint (55).
The SLOUCH v1.1 program models the evolution of traits according to an Ornstein-Uhlenbeck process (21). The speed at which phylogenetic covariances decay with phylogenetic distance, the phylogenetic t1/2, provides an estimate of the level of phylogenetic signal within a dataset. Increasing values of t1/2 suggest an increasing association between the trait and phylogeny, with t1/2 = 0 no association and t1/2 > 1 a high phylogenetic signal (this value depends on the height of a phylogenetic tree; here the total tree height =1). Initially regressions are fitted with only the trait values to estimate phylogenetic signal within the data. The inclusion of the predictor variables (Tmax and PANN) allows for estimation of phylogenetic inertia. This method also permits inclusion of multiple predictors such as Tmax × PANN, and the analysis also provides a phylogenetically corrected r2 value (21). For a more detailed description see ref. 20.
Supplementary Material
Acknowledgments
We thank two anonymous reviewers for their comments to the manuscript; Carla Sgro and Kim van der Linde for phylogeny files; Jean R. David, Anneli Hoikkala, Fabian Norry, and Masayoshi Watada from the Ehime Drosophila Stock Center for flies; Brody Sandel for help with geospatial modeling; Thomas Hansen for useful discussions; and Torsten Kristensen, Janneke Wit, Pernille Sarup, Anny Bang, and Doth Andersen for technical assistance. Financial support was provided by the Danish Natural Sciences Research Council (J.O. and V.L.) and the Australian Research Council and the Science and Industry Endowment Fund (A.A.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207553109/-/DCSupplemental.
References
- 1.Intergovernmental Panel on Climate Change . Climate Change 2007: Synthesis Report. Geneva: Intergovernmental Panel on Climate Change; 2007. p. 52. [Google Scholar]
- 2.Thomas CD, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- 3.Addo-Bediako A, Chown SL, Gaston KJ. Thermal tolerance, climatic variability and latitude. Proc R Soc B Biol Sci. 2000;267:739–745. doi: 10.1098/rspb.2000.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura MT. Cold and heat tolerance of drosophilid flies with reference to their latitudinal distributions. Oecologia. 2004;140:442–449. doi: 10.1007/s00442-004-1605-4. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann AA, Sorensen JG, Loeschcke V. Adaptation of Drosophila to temperature extremes: Bringing together quantitative and molecular approaches. J Therm Biol. 2003;28:175–216. [Google Scholar]
- 6.Fischer K, et al. Environmental effects on temperature stress resistance in the tropical butterfly Bicyclus anynana. PLoS ONE. 2010;5:e15284. doi: 10.1371/journal.pone.0015284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenkins NL, Hoffmann AA. Genetic and maternal variation for heat resistance in Drosophila from the field. Genetics. 1994;137:783–789. doi: 10.1093/genetics/137.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garland T, Adolph SC. Physiological differentiation of vertebrate populations. Annu Rev Ecol Syst. 1991;22:193–228. [Google Scholar]
- 9.Stillman JH, Somero GN. A comparative analysis of the upper thermal tolerance limits of eastern Pacific porcelain crabs, genus Petrolisthes: Influences of latitude, vertical zonation, acclimation, and phylogeny. Physiol Biochem Zool. 2000;73:200–208. doi: 10.1086/316738. [DOI] [PubMed] [Google Scholar]
- 10.Deutsch CA, et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA. 2008;105:6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huey RB, et al. Why tropical forest lizards are vulnerable to climate warming. Proc R Soc B Biol Sci. 2009;276:1939–1948. doi: 10.1098/rspb.2008.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duarte H, et al. Can amphibians take the heat? Vulnerability to climate warming in subtropical and temperate larval amphibian communities. Glob Change Biol. 2012;18:412–421. [Google Scholar]
- 13.Kellermann V, van Heerwaarden B, Sgrò CM, Hoffmann AA. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science. 2009;325:1244–1246. doi: 10.1126/science.1175443. [DOI] [PubMed] [Google Scholar]
- 14.Wiens JJ, et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett. 2010;13:1310–1324. doi: 10.1111/j.1461-0248.2010.01515.x. [DOI] [PubMed] [Google Scholar]
- 15.Nyamukondiwa C, Terblanche JS, Marshall KE, Sinclair BJ. Basal cold but not heat tolerance constrains plasticity among Drosophila species (Diptera: Drosophilidae) J Evol Biol. 2011;24:1927–1938. doi: 10.1111/j.1420-9101.2011.02324.x. [DOI] [PubMed] [Google Scholar]
- 16.Clusella-Trullas S, Blackburn TM, Chown SL. Climatic predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. Am Nat. 2011;177:738–751. doi: 10.1086/660021. [DOI] [PubMed] [Google Scholar]
- 17.Cooper N, Freckleton RP, Jetz W. Phylogenetic conservatism of environmental niches in mammals. Proc R Soc B Biol Sci. 2011;278:2384–2391. doi: 10.1098/rspb.2010.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freckleton RP, Jetz W. Space versus phylogeny: Disentangling phylogenetic and spatial signals in comparative data. Proc R Soc B Biol Sci. 2009;276:21–30. doi: 10.1098/rspb.2008.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willis CG, Ruhfel B, Primack RB, Miller-Rushing AJ, Davis CC. Phylogenetic patterns of species loss in Thoreau’s woods are driven by climate change. Proc Natl Acad Sci USA. 2008;105:17029–17033. doi: 10.1073/pnas.0806446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellermann V, et al. Phylogenetic constraints in key functional traits behind species’ climate niches: Patterns of desiccation and cold resistance across 95 Drosophila species. Evolution. doi: 10.1111/j.1558-5646.2012.01685.x. 10.1111/j.1558-5646.2012.01685.x. [DOI] [PubMed] [Google Scholar]
- 21.Hansen TF, Pienaar J, Orzack SH. A comparative method for studying adaptation to a randomly evolving environment. Evolution. 2008;62:1965–1977. doi: 10.1111/j.1558-5646.2008.00412.x. [DOI] [PubMed] [Google Scholar]
- 22.Sunday JM, Bates AE, Dulvy NK. Global analysis of thermal tolerance and latitude in ectotherms. Proc R Soc B Biol Sci. 2011;278:1823–1830. doi: 10.1098/rspb.2010.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamond SE, et al. Who likes it hot? A global analysis of the climatic, ecological, and evolutionary determinants of warming tolerance in ants. Glob Change Biol. 2012;18:448–456. [Google Scholar]
- 24.Bonebrake TC, Mastrandrea MD. Tolerance adaptation and precipitation changes complicate latitudinal patterns of climate change impacts. Proc Natl Acad Sci USA. 2010;107:12581–12586. doi: 10.1073/pnas.0911841107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greve M, Lykke AM, Blach-Overgaard A, Svenning JC. Environmental and anthropogenic determinants of vegetation distribution across Africa. Glob Ecol Biogeogr. 2011;20:661–674. [Google Scholar]
- 26.Hutley LB, et al. A sub-continental scale living laboratory: Spatial patterns of savanna vegetation over a rainfall gradient in northern Australia. Agric For Meteorol. 2011;151:1417–1428. [Google Scholar]
- 27.Sgrò CM, et al. A comprehensive assessment of geographic variation in heat tolerance and hardening capacity in populations of Drosophila melanogaster from eastern Australia. J Evol Biol. 2010;23:2484–2493. doi: 10.1111/j.1420-9101.2010.02110.x. [DOI] [PubMed] [Google Scholar]
- 28.Stratman R, Markow TA. Resistance to thermal stress in desert Drosophila. Funct Ecol. 1998;12:965–970. [Google Scholar]
- 29.Willmer PG. Microclimate and the environmental physiology of insects. Adv Insect Physiol. 1982;16:1–57. [Google Scholar]
- 30.Pincebourde S, Woods HA. Climate uncertainty on leaf surfaces: The biophysics of leaf microsclimates and their consequences for leaf-dwelling organisms. Funct Ecol. 2012;26:844–853. [Google Scholar]
- 31.Parsons PA. Habitat selection and evolutionary strategies in Drosophila: An invited address. Behav Genet. 1978;8:511–526. doi: 10.1007/BF01067480. [DOI] [PubMed] [Google Scholar]
- 32.Huey RB, Pascual M. Partial thermoregulatory compensation by a rapidly evolving invasive species along a latitudinal cline. Ecology. 2009;90:1715–1720. doi: 10.1890/09-0097.1. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell KA, Hoffmann AA. Thermal ramping rate influences evolutionary potential and species differences for upper thermal limits in Drosophila. Funct Ecol. 2010;24:694–700. [Google Scholar]
- 34.Gilchrist GW, Huey RB. The direct response of Drosophila melanogaster to selection on knockdown temperature. Heredity (Edinb) 1999;83:15–29. doi: 10.1038/sj.hdy.6885330. [DOI] [PubMed] [Google Scholar]
- 35.Bettencourt BR, Feder ME. Hsp70 duplication in the Drosophila melanogaster species group: How and when did two become five? Mol Biol Evol. 2001;18:1272–1282. doi: 10.1093/oxfordjournals.molbev.a003912. [DOI] [PubMed] [Google Scholar]
- 36.Chown SL, Addo-Bediako A, Gaston KJ. Physiological variation in insects: Large-scale patterns and their implications. Comp Biochem Physiol B Biochem Mol Biol. 2002;131:587–602. doi: 10.1016/s1096-4959(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 37.Marais E, Chown SL. Beneficial acclimation and the Bogert effect. Ecol Lett. 2008;11:1027–1036. doi: 10.1111/j.1461-0248.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- 38.Levins R. Thermal acclimation and heat resistance in Drosophila species. Am Nat. 1969;103:483. [Google Scholar]
- 39.Overgaard J, Kristensen TN, Mitchell KA, Hoffmann AA. Thermal tolerance in widespread and tropical Drosophila species: Does phenotypic plasticity increase with latitude? Am Nat. 2011;178(Suppl 1):S80–S96. doi: 10.1086/661780. [DOI] [PubMed] [Google Scholar]
- 40.Kristensen TN, et al. No inbreeding depression for low temperature developmental acclimation across multiple Drosophila species. Evolution. 2011;65:3195–3201. doi: 10.1111/j.1558-5646.2011.01359.x. [DOI] [PubMed] [Google Scholar]
- 41.Griffiths JA, Schiffer M, Hoffmann AA. Clinal variation and laboratory adaptation in the rainforest species Drosophila birchii for stress resistance, wing size, wing shape and development time. J Evol Biol. 2005;18:213–222. doi: 10.1111/j.1420-9101.2004.00782.x. [DOI] [PubMed] [Google Scholar]
- 42.Cavicchi S, Guerra D, Latorre V, Huey RB. Chromosomal analysis of heat shock tolerance in Drosophila melanogaster evolving at different temperatures in the laboratory. Evolution. 1995;49:676–684. doi: 10.1111/j.1558-5646.1995.tb02304.x. [DOI] [PubMed] [Google Scholar]
- 43.Terblanche JS, et al. Ecologically relevant measures of tolerance to potentially lethal temperatures. J Exp Biol. 2011;214:3713–3725. doi: 10.1242/jeb.061283. [DOI] [PubMed] [Google Scholar]
- 44.Rezende EL, Tejedo M, Santos M. Estimating the adaptive potential of critical thermal limits: Methodological problems and evolutionary implications. Funct Ecol. 2011;25:111–121. [Google Scholar]
- 45.Overgaard J, Kristensen TN, Sørensen JG. Validity of thermal ramping assays used to assess thermal tolerance in arthropods. PLoS ONE. 2012;7:e32758. doi: 10.1371/journal.pone.0032758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hijmans RJ, et al. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 47.van der Linde K, Houle D, Spicer GS, Steppan SJ. A supermatrix-based molecular phylogeny of the family Drosophilidae. Genet Res (Camb) 2010;92:25–38. doi: 10.1017/S001667231000008X. [DOI] [PubMed] [Google Scholar]
- 48.Gittleman JL, Kot M. Adaptation statistics and a null model for estimating phylogenetic effects. Syst Zool. 1990;39:227–241. [Google Scholar]
- 49.Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
- 50.Blomberg SP, Garland T, Jr, Ives AR. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution. 2003;57:717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 51.Paradis E, Claude J, Strimmer K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 52.Kembel SW, et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010;26:1463–1464. doi: 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
- 53.Team RDC. The R environment for statistical computing and graphics. Version 2.6.2. 2011. http://www.r-project.org. Accessed March 1, 2010.
- 54.Hood GM. PopTools version 3.2.3. 2010 http://www.poptools.org. Accessed March 1, 2010. [Google Scholar]
- 55.Vavrek MJ. fossil: Palaeoecological and palaeogeographical analysis tools. Palaeontol Electronica. 2011;14:1T. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.