Abstract
The homeodomain transcription factor Nanog plays an important role in embryonic stem cell (ESC) self-renewal and is essential for acquiring ground-state pluripotency during reprogramming. Understanding how Nanog is transcriptionally regulated is important for further dissecting mechanisms of ESC pluripotency and somatic cell reprogramming. Here, we report that Nanog is subjected to a negative autoregulatory mechanism, i.e., autorepression, in ESCs, and that such autorepression requires the coordinated action of the Nanog partner and transcriptional repressor Zfp281. Mechanistically, Zfp281 recruits the NuRD repressor complex onto the Nanog locus and maintains its integrity to mediate Nanog autorepression and, functionally, Zfp281-mediated Nanog autorepression presents a roadblock to efficient somatic cell reprogramming. Our results identify a unique transcriptional regulatory mode of Nanog gene expression and shed light into the mechanistic understanding of Nanog function in pluripotency and reprogramming.
Keywords: iPSC, Nanog autoregulation
An understanding of the molecular underpinnings of stem cell pluripotency and somatic cell reprogramming is a prerequisite for therapeutic application of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). Initial efforts in dissecting transcriptional (1) and protein interaction (2–4) networks operative in ESCs form a foundation for such mechanistic studies. The common view of the Oct4-Sox2-Nanog network suggests that these core factors activate their own expression and each other’s expression to form a positive feedback circuit (5). Although it is well recognized that a negative feedback mechanism must exist to fine-tune this core network and allow for optimal expression of these dosage-sensitive transcription factors, it remains to be determined how these core factors execute a “self-control” regulatory mechanism to prevent excessive expression in maintaining the ESC state.
Enforced expression of Nanog relieves ESCs from their leukemia inhibitory factor (LIF) requirement (6), promotes transfer of pluripotency after cell fusion (7), and ensures direct reprogramming of somatic cells to the pluripotent ground state (8). How Nanog is transcriptionally regulated and participates in the transcriptional machinery to control pluripotency and reprogramming is still poorly understood. Several modes of Nanog gene regulation have been published. First, during the early differentiation process of ESCs Nanog (and Oct4) is subjected to epigenetic regulation at its enhancer/promoter region by DNA methyltransferases (9) and histone methyltransferases (10). Second, studies have documented direct transcriptional regulation of Nanog by both positive and negative regulators (11). Third, the Nanog interactome contains many factors whose genes are also downstream targets of themselves, thus forming autoregulatory loops in the pluripotency network (3, 12). Nanog is known to regulate its own expression by positive feedback in ESCs (i.e., autoactivation) (13), which in one case was shown to be mediated by the Nanog partner and transcriptional regulator Sall4 (14). However, the fine-tuning of Nanog levels is necessary for balancing self-renewal and pluripotency of ESCs as too much Nanog favors self-renewal and impedes the execution of pluripotency under proper differentiation cues (6). Little is known about whether negative autoregulatory feedback, i.e., autorepression, exists in ESCs to control Nanog expression and how such autorepression relates to its function in pluripotency and reprogramming.
In this study, we provide molecular and biochemical data uncovering Nanog autorepression as a unique transcriptional reg-ulatory mode of Nanog expression in ESCs. We establish Zfp281 as an important Nanog regulator and cofactor that mediates Nanog autorepression through recruitment and maintenance of the NuRD repressor complex on the Nanog locus and that restricts Nanog reactivation during somatic cell reprogramming.
Results
Nanog Is Subjected to Autorepression in ESCs.
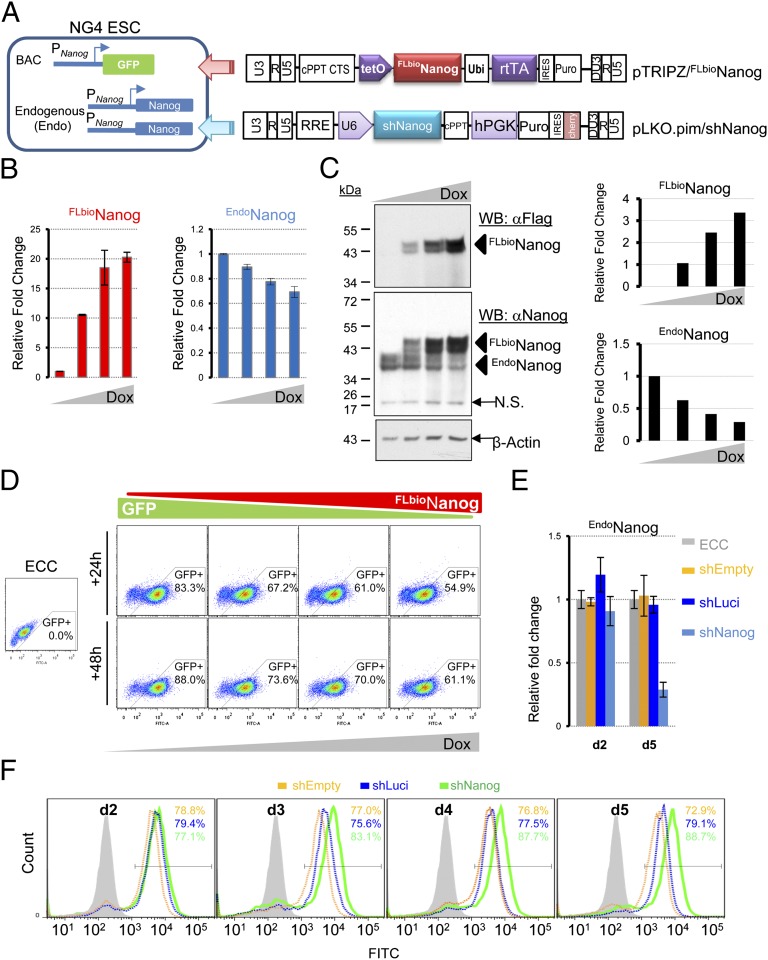
To test whether Nanog autorepression exists in ESCs, we performed both Nanog overexpression and knockdown studies in NG4 transgenic ESCs expressing the enhanced green fluorescent protein (GFP) reporter gene under the control of the endogenous Nanog promoter (PNanog) (Fig. 1A, Left) (15). First, we introduced a doxycycline (Dox)-inducible Nanog transgene bearing a Flag-biotin dual tag (FLbio) and established stable clones by puromycin selection (Fig. 1A, Right). We found that induced expression of FLbioNanog upon Dox treatment (Fig. 1B, Left and C) resulted in down-regulation of both endogenous Nanog (EndoNanog) transcripts (Fig. 1B, Right) and protein (Fig. 1C) and Nanog-GFP reporter activity (Fig. 1D) in a dose-dependent manner. We then asked whether knockdown of EndoNanog expression would enhance transgenic Nanog-GFP reporter expression. We infected NG4 cells with lentiviruses expressing a constitutive shRNA against the 3′-UTR of Nanog (shNanog) (Fig. 1A and Table S1). Nanog-GFP reporter activity was measured over a 5-d period by flow cytometry. We confirmed efficient knockdown of Nanog by RT-quantitative PCR (qPCR) (Fig. 1E), and more importantly, we found that Nanog-GFP reporter activity was up-regulated over the time course (Fig. 1F). In contrast, the control shRNAs (shEmpty and shLuci) affected neither EndoNanog expression levels (Fig. 1E) nor Nanog-GFP reporter activity (Fig. 1F). These results support the existence of Nanog autorepression as a regulatory mode of Nanog expression in ESCs.
Fig. 1.
Nanog autorepression in ESCs. (A) The strategy for inducible FLbioNanog expression or constitutive Nanog knockdown by shRNA (shNanog) in NG4 ESCs. (B) RT-qPCR analyses of ectopic (FLbioNanog) and endogenous (EndoNanog) expression of Nanog upon Dox (0, 0.625, 1.25, or 2.5 μg/mL) treatment. (C) Western blotting (WB) analyses of FLbioNanog and EndoNanog expression upon Dox treatment. Western gel images are shown on Left, and quantitation of the western signals is on Right. N.S., nonspecific signal. (D) Flow cytometry analyses of Nanog-GFP reporter activity upon Dox treatment for 24 and 48 h. The parental ESC line of NG4 cells (ECC) was used as a GFP negative control. (E) RT-qPCR analyses of EndoNanog expression upon Nanog knockdown (shNanog) in NG4 ESCs. ECC line and stable NG4 transgenic lines infected with pLKO lentivirues expressing no shRNA (shEmpty) or shRNA against luciferase (shLuci) were used as controls. (F) Flow cytometry analyses of Nanog-GFP reporter activity upon shRNA-mediated knockdown as indicated.
To confirm that Nanog autorepression is a general phenomenon in ESCs, we further examined the effects of enforced Nanog expression on EndoNanog levels in a previously published episomal overexpression system in E14T ESCs (6) (Fig. S1A). Consistent with the published study, we confirmed enhanced ESC self-renewal (Fig. S1B) and an overall increase in Nanog expression at both total transcript (Fig. S1C) and protein levels (Fig. S1D). More importantly, we found that ectopic Nanog expression led to down-regulation of EndoNanog transcript levels (Fig. S1E), supporting Nanog autorepression in ESCs. Together, these results establish a mode of Nanog transcriptional regulation, i.e., Nanog autorepression, in ESCs.
Zfp281 Is Required for Nanog Autorepression via Its Association with the NuRD Repressor Complex in ESCs.
To gain insight into the molecular mechanism of Nanog autorepression in ESCs, we focused on the Krüppel-like zinc finger transcription factor Zfp281. We reported it to be a close partner of Nanog (3) and later demonstrated it to be a transcriptional repressor to restrict Nanog expression in maintaining ESC pluripotency (16). In this study, we evaluated how knockdown of Zfp281 might affect EndoNanog and Nanog-GFP reporter expression in NG4 cells (Fig. 2A). Using a Dox-inducible Zfp281 shRNA (Table S1), we demonstrated that down-regulation of Zfp281 upon Dox induction (Fig. 2C, gray bars) led to an increase in both transgenic Nanog-GFP reporter activity (Fig. 2B) and EndoNanog transcript levels (Fig. 2C, blue bars). These results confirm that Zfp281 functions as a transcriptional repressor for Nanog expression in ESCs and suggest that Zfp281 may play a role in Nanog autorepression. To test whether Zfp281 is necessary for Nanog autorepression, we infected both Zfp281 wild-type (Zfp281+/+) and null (Zfp281−/−) ESCs (16) with lentiviruses expressing a Dox-inducible FLbioNanog transgene (Fig. 2D) and examined EndoNanog expression upon Dox treatment. We confirmed Dox-dependent up-regulation of FLbioNanog expression in both Zfp281+/+ and Zfp281−/− ESCs (Fig. 2 E and F, Left). Importantly, although we observed (as expected) down-regulation of EndoNanog transcript levels in Zfp281+/+ ESCs (Fig. 2E, Right), we found that inducible FLbioNanog overexpression in Zfp281−/− cells failed to repress Nanog promoter activity. Intriguingly, EndoNanog expression increased in a dose-dependent manner (Fig. 2F, Right). These results demonstrate that Zfp281 is required for Nanog autorepression, and that Nanog is able to activate its own promoter, either directly or indirectly, in the absence of Zfp281 (see more in Discussion).
Fig. 2.
Zfp281 is required for Nanog autorepression and the integrity of the NuRD repressor complex in ESCs. (A) The strategy for inducible knockdown of Zfp281 in NG4 cells. (B) Flow cytometry analyses of Nanog-GFP reporter activity upon shRNA-mediated knockdown of Zfp281. Nanog-GFP was analyzed 3d after puromycin selection and Dox treatment. (C) RT-qPCR analyses of EndoNanog and Zfp281 expression in the samples described in B. (D) The strategy for inducible FLbioNanog expression in both Zfp281+/+ and Zfp281−/− ESCs. (E and F) RT-qPCR analyses of FLbioNanog and EndoNanog expression upon Dox treatment in Zfp281+/+ (E) and Zfp281−/− (F) ESCs. (G) Zfp281 is associated with the NuRD repressor complex in ESCs. Total peptide numbers identified by mass spectrometry are listed. (H) Confirmation of endogenous association of Zfp281 with Nanog and the NuRD proteins by immunoprecipitation (IP) and WB analyses in Zfp281+/+ and Zfp281−/− ESCs. (I) Zfp281 is required for the integrity of the NuRD repressor complex. (J) Interaction between Mta1/2 and Hdac2 is not affected by Zfp281 depletion.
To further explore the molecular mechanism by which Zfp281 mediates Nanog autorepression, we tested whether Zfp281 may assist Nanog in recruiting certain corepressor complexes into the Nanog promoter/enhancer region for transcriptional repression. We performed affinity purification of Zfp281 protein complexes in wild-type ESCs by using an anti-Zfp281 antibody (Fig. S2) and identified Zfp281-associated proteins by mass spectrometry. Our results indicate a preferential association of Zfp281 with all the major NuRD components in ESCs (Fig. 2G). We confirmed the endogenous association of Zfp281 with Nanog and with the NuRD components Mta1/2, Hdac2, and Chd4 (Mi-2β) by performing immunoprecipitation (IP) with antibodies against Nanog, Zfp281, and NuRD proteins in both Zfp281+/+ and Zfp281−/− ESCs (Fig. 2H). Interestingly, we found that, although endogenous association of the core NuRD protein Chd4 with Mta1/2 and Hdac2 is readily detected in wild-type ESCs, it is greatly diminished in the absence of Zfp281 (Fig. 2I, Left). In contrast, the interactions between other “peripheral” NuRD proteins (e.g., Hdac2 and Mta1/2) are maintained regardless of Zfp281 expression (Fig. 2J), suggesting that Zfp281 might be an important factor to maintain the physical and functional integrity of the NuRD complex in ESCs. Taken together, our data demonstrate a critical role of Zfp281 in mediating Nanog autorepression through its interaction with the NuRD repressor complex.
Zfp281 Mediates Nanog Autorepression by Directly Recruiting the NuRD Repressor Complex to the Nanog Locus.
The association of both Nanog (3, 17) and Zfp281 (Fig. 2 G and H) with the NuRD repressor complex prompted us to investigate the mechanistic action of the NuRD complex in Nanog autorepression. First, we used chromatin immunoprecipitation (ChIP) coupled with qPCR (ChIP-qPCR) to analyze whether Zfp281 is required for recruitment of NuRD proteins to the Nanog regulatory regions (Fig. 3A). Consistent with a previous report (17) and our coimmunoprecipitation (co-IP) data (Fig. 2H), we confirmed that Nanog, Mta1/2, and Hdac2 occupy the Nanog enhancer region (sites B′ and B′′) and, to a lesser extent, the promoter region (site C) (Fig. 3B, black bars), but not a remote control region (Fig. 3A, A). More importantly, we found that binding of Nanog, Mta1/2, and Hdac2 to these regulatory regions (B′, B′′, and C) is drastically diminished upon Zfp281 depletion (Fig. 3B, gray bars), an effect that is not due to down-regulation of protein levels (Fig. 3C).
Fig. 3.
Requirement of the NuRD repressor complex for Nanog autorepression. (A) Illustration of the upstream regulatory regions of the Nanog gene. The amplicons corresponding to a control region, the enhancer, and the promoter are indicated as A, B′/B′′, and C, respectively. TSS, transcription start site. (B) Relative enrichment of Nanog, Mta1/2, and Hdac2 in the genomic loci of Nanog in Zfp281+/+ and Zfp281−/− ESCs. (C) WB analyses of Nanog, Mta1/2, Hdac2, and Zfp281 in Zfp281+/+ and Zfp281−/− ESCs. (D and E) A Dox-inducible Nanog expression cell system (D) indicates that ectopic Nanog expression by Dox promotes Nanog, Zfp281, and Mta1/2 binding to the Nanog enhancer (E). ESCs without (−) or with (+) Dox (1.5 μg/mL) treatment for 48 h were harvested for ChIP-qPCR analyses. (F) The strategy for knockdown of NuRD proteins in NG4 ESCs that express inducible FLbioNanog. (G) Efficient knockdown of NuRD proteins in NG4 ESCs analyzed by RT-qPCR. Expression levels of individual genes upon knockdown were normalized to the control knockdown (shEmpty). (H) Knockdown of NuRD protein expression reduces or abrogates Nanog autorepression in ESCs. The Nanog–GFP-positive cell population in uninfected samples (no infection) or the GFP/mCherry–double-positive cell population (shRNA-transduced cells) were measured after treatment with or without Dox (2 μg/mL) for 24 h.
Next, we examined the occupancy of Nanog, Zfp281, and NuRD proteins on the Nanog enhancer upon inducible Nanog overexpression in NG4 ESCs (Fig. 3D). ChIP-qPCR analysis not only confirmed binding of endogenous Nanog, Zfp281, and Mta1/2 to the Nanog enhancer (site B′) in these cells (Fig. 3E, black bars), but also revealed enhanced binding of these factors upon Dox-induced FLbioNanog expression (Fig. 3E, gray bars). These data support the notion that Nanog autorepression is likely mediated by Zfp281 and its associated NuRD repressor complex. To address whether Nanog autorepression depends on the NuRD repressor complex, we introduced lentiviral shRNAs against NuRD complex proteins (Chd4, Gatad2b, Mta2, and Mta3) into the Dox-inducible FLbioNanog transgenic line as shown in Fig. 3D (Fig. 3F). The expression of these shRNAs in NG4 ESCs caused a reduction of corresponding gene expression by 80–90% compared with the control knockdown (shEmpty) (Fig. 3G). As expected, control cells without virus infection or with infection of empty shRNA virus (shEmpty) exhibit Nanog autorepression upon inducible FLbioNanog expression (+Dox), as measured by flow cytometry of Nanog-GFP reporter activity (Fig. 3H, compare the black bars with the gray bars in the first two columns). Importantly, down-regulation of the NuRD proteins Chd4, Gatad2b, Mta2, and Mta3 by shRNAs attenuates or abrogates such autorepression (Fig. 3H, bars in the last four columns), which indicates that the NuRD complex is necessary for Zfp281-mediated Nanog autorepression. Together, these data demonstrate that Zfp281 mediates Nanog autorepression through recruitment of the NuRD repressor complex onto the Nanog locus in ESCs.
Zfp281 Restricts Nanog Reactivation and Inhibits Somatic Cell Reprogramming.
Because Nanog is essential for achieving ground-state pluripotency of iPSCs, we examined whether Zfp281 may play a role in somatic cell reprogramming by influencing Nanog expression. We used mouse embryonic fibroblasts (MEFs) harboring an Oct4 promoter-driven GFP reporter transgene (Oct4-GFP) for iPSC generation by following the standard iPSC generation protocol (18) with modifications (Fig. 4A). First, we evaluated relative Zfp281 and Nanog gene expression during the reprogramming process. We found that both Zfp281 and Nanog are up-regulated during reprogramming, and up-regulation of Zfp281 precedes the reactivation of Nanog gene expression (Fig. 4B). This result suggests that Zfp281 may restrict Nanog reactivation during the reprogramming process and likely also plays a similar role in fine-tuning Nanog levels in iPSCs as that in ESCs (16) to maintain pluripotency.
Fig. 4.
Loss of Zfp281 facilitates somatic cell reprogramming. (A) Summary of the procedure for iPSC generation. (B) RT-qPCR analyses of Zfp281 and Nanog expression during iPSC generation. Expression levels were normalized to those in wild-type ESCs. (C) RT-qPCR analyses of relative Nanog expression during iPSC generation upon knockdown with control scramble (SCR) or Zfp281 shRNA. Expression levels were normalized to those in wild-type ESCs. (D) Minimal reduction of total AP (+) colony numbers upon Zfp281 knockdown during reprogramming. (E) Zfp281 knockdown promotes iPSC generation. Oct4-GFP MEFs were infected with viruses expressing the four reprogramming factors (4F), alone (–) or together with three independent shRNAs against Zfp281 (1–3) and control scramble shRNA (shSCR). The same reprogramming assays were repeated independently three times (A, B, and C) with duplicates each time (1, 2). The average percentages of GFP (+) and GFP (−) colonies from three independent experiments are shown in the pie chart (Upper).
We then tested the effects of Zfp281 knockdown (KD) on Nanog reactivation during iPSC generation (Fig. 4A). We coinfected Oct4-GFP MEFs with lentiviruses constitutively expressing the reprogramming factor mixture (Oct4, Sox2, Klf4, and c-Myc; OSKM) and short hairpin RNAs (shRNAs) against Zfp281. We used three independent shRNAs that reduced Zfp281 expression by 60–80% relative to the control scramble shRNA (shSCR) (Fig. S3A). Consistent with its function in mediating Nanog autorepression, knockdown of Zfp281 resulted in up-regulation of Nanog during the reprogramming process, in particular, during the late stages (d17 and thereafter) of reprogramming (Fig. 4C). We confirmed that there is no significant change in MEF growth rates between scramble (shSCR) and Zfp281 shRNAs (Fig. S3B). Importantly, we found that, although loss of Zfp281 minimally affects the total number of AP-positive colonies (Fig. 4D and Fig. S3C), it markedly reduces the number of Oct4–GFP-negative, partially reprogrammed colonies (Fig. 4E, yellow bars/pies) and increases the percentage of overall Oct4–GFP-positive, fully pluripotent iPSC colonies (Fig. 4E, green bars/pies and Fig. S3D). Flow cytometry analysis of Oct4-GFP reporter activity further confirmed an increase in the percentage of GFP-positive cells when Zfp281 is down-regulated during reprogramming (Fig. S3E).
Together, our data demonstrate that the transcriptional repressor Zfp281 restricts Nanog reactivation during the reprogramming process and, thus, functions as a molecular barrier to the transition of intermediate cells or so-called “pre-iPSCs” (19) into ground-state, pluripotent iPSCs.
Zfp281 Depletion Promotes the PreiPSC to iPSC Transition Through Nanog Regulation.
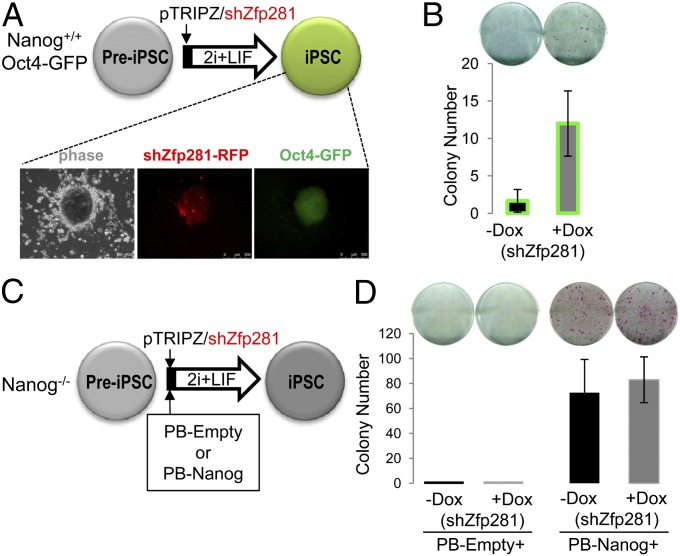
To directly address whether Zfp281 depletion can promote the preiPSC to iPSC transition as suggested above, we used a published reprogramming system that allows direct investigation of the preiPSC to iPSC transition (19). In this system, preiPSCs generated from Oct4, Klf4, and c-Myc (OKM)-transduced wild-type neural stem cells harboring an Oct4-GFP reporter transgene are maintained in normal serum/LIF culture. Only a minority of these preiPSCs will become iPSCs after switching to the 2i/LIF condition, and the reprogramming efficiency can be greatly enhanced if exogenous Nanog is provided (20). We asked whether down-regulation of Zfp281 could replace the requirement for exogenous Nanog to promote the Nanog+/+ preiPSC to iPSC transition (Fig. 5A). Indeed, we found that inducible knockdown of Zfp281 by Dox treatment (shRNA expression is positively marked by RFP, Fig. 5A, Lower) resulted in an approximately fourfold increase of both AP(+) (Fig. 5B, Upper) and Oct4-GFP(+) (Fig. 5B, Lower) iPSC colonies. We further confirmed enhanced reprogramming of preiPSCs by Zfp281 down-regulation by using two independent, retrovirally expressed constitutive shRNAs against Zfp281 (Table S1 and Fig. S4 A and B).
Fig. 5.
Zfp281 depletion enhances reprogramming through Nanog regulation. (A) The strategy for testing the effect of Zfp281 knockdown in the preiPSC to iPSC transition. Nanog+/+ preiPSCs harboring an Oct4-GFP transgene were used for the reprogramming assay as described (19), and iPSCs generated from Zfp281 knockdown are positive for both RFP (for pTRIPZ/shZfp281) and GFP (for Oct4-GFP reporter). (B) Zfp281 knockdown promotes the Nanog+/+ preiPSC to iPSC transition. Dox treatment (shZfp281) results in a significant increase of AP (+) (Upper) and Oct4-GFP (+) (Lower) iPSC colony numbers. (C) Nanog−/− preiPSCs lacking the Oct4-GFP transgene (8) were used for the reprogramming assay together with exogenous supply of a Nanog transgene in a PiggyBac (PB) vector (PB-Nanog). The empty PB vector (PB-Empty) was used as control. (D) Zfp281 knockdown fails to reprogram Nanog−/− preiPSCs or augment Nanog-mediated reprogramming of Nanog−/− preiPSCs. A representative image of AP stained colonies (Upper) and quantitative data on the total AP (+) colony numbers (Lower) are shown. Error bars denote SDs from triplicate wells.
Next, we asked whether the effect of Zfp281 knockdown in promoting the preiPSC to iPSC transition is mediated through endogenous Nanog regulation. To this end, we used Nanog−/− preiPSCs (8) for the reprogramming assay (Fig. 5C). As reported (8), we confirmed that these Nanog−/− preiPSCs cannot transit into ground-state, pluripotent iPSCs under 2i+LIF condition unless an exogenous Nanog transgene is provided (Fig. 5D, black bars). More importantly, we found that knockdown of Zfp281 alone upon Dox induction (+Dox) is no longer effective in promoting the Nanog−/− preiPSC to iPSC transition, which is reflected by no colony formation after Dox treatment (Fig. 5D, Left, gray bar). These results suggest that the enhanced reprogramming of preiPSCs after Zfp281 down-regulation (Fig. 5B) is the direct result of endogenous Nanog up-regulation. In addition, although we observed enhanced reprogramming of Nanog−/− preiPSCs upon ectopic expression of Nanog (PB-Nanog) in the presence of Dox (i.e., down-regulation of Zfp281), no additive effect of shZfp281 and PB-Nanog relative to PB-Nanog alone was observed (Fig. 5D, Right, compare the gray bar with the black bar). These data argue strongly that the regulation of endogenous Nanog is the mechanism of Zfp281 action during reprogramming. To further reinforce this conclusion, we performed a similar reprogramming assay by using the Nanog+/+ preiPSCs in the presence of both Zfp281 knockdown and ectopic Nanog expression (Fig. S4A). In this case, we observed additive effects of the combined action of Zfp281 down-regulation (shZfp281) and ectopic Nanog expression (pMx-Nanog) in promoting the Nanog+/+ preiPSC to iPSC transition (Fig. S4C).
Finally, because Nanog overexpressing ESCs can promote reprogramming efficiency when fused with somatic cells (7), we asked whether Nanog up-regulation in Zfp281−/− ESCs could also enhance mouse ESC and human B (hB) cell heterokaryon based reprogramming (21) (Fig. S5A). Our results show that although reprogramming of hB cells is obvious in both Zfp281+/+ ESC/hB and Zfp281−/− ESC/hB heterokaryons (Fig. S5C), an enhanced human ES-specific gene expression profile indicative of improved reprogramming efficiency was observed for Zfp281−/− ESC/hB heterokaryons (Fig. S5B). These data provide additional validation of the functional implication of Zfp281 in restricting Nanog reactivation and impeding reprogramming.
Discussion
In this study, we demonstrate that Nanog is subjected to Zfp281-mediated autoregulation of its own promoter by a negative feedback loop, which we dub Nanog autorepression, and that Zfp281 mediates autorepression by directly recruiting the NuRD repressor complex to the Nanog locus and restricts Nanog reactivation during reprogramming. Together with our previous study (16), we have thus established a dual role of Zfp281 for both an important pluripotency factor to fine-tune Nanog expression in maintaining the pluripotent state of ESCs and a transcriptional repressor to restrict Nanog activation and impede somatic cell reprogramming. These data offer insights into the regulatory mechanisms underlying optimal ESC state and efficient reprogramming.
Although our results establish Zfp281 as the key transcription regulator mediating Nanog autorepression in ESCs, we note that Zfp281 can directly regulate other pluripotency and developmentally regulated genes as reported (16). Therefore, Nanog derepression is one of many possible regulatory consequences of Zfp281 depletion. Thus, not surprisingly, we found that although down-regulation of Nanog alone in Zfp281−/− ESCs rescues the expression of endodermal markers Gata6 and Sox17 at a late stage (day 10) of EB differentiation (Fig. S6C), it fails to rescue other markers such as Oct4 and Cdx2 (Fig. S6C) or the EB size/morphology (Fig. S6 A and B). We also note that Nanog is under negative regulation by other factors including Tcf3 (22). However, the regulatory mechanism is likely different as the binding loci in the Nanog regulatory region for Zfp281 (16) and Tcf3 (22) are different and no physical association between the two factors has been detected. In addition, we recognize the importance of positive feedback loops controlled by other stem cell factors such as Oct4-Sox2 heterodimers (23) and Sall4 for Nanog gene activation (14). These observations suggest that Nanog is subjected to multilayered, tight transcriptional control. Intriguingly, we observed a switch from negative to positive feedback regulation of EndoNanog by enforced FLbioNanog expression in the absence of Zfp281 (Fig. 2 D and F). Because of the concomitant reduction in Nanog binding to its own regulatory regions upon Zfp281 depletion (Fig. 3B), we speculate that the activation function of ectopic Nanog observed in Zfp281−/− ESCs is more likely resulted from transactivation by other pluripotency factors that act on the endogenous Nanog locus. For example, Oct4, Esrrb, and Zfp143 are known to form heterodimers and directly transactivate Nanog promoter activity (23–25), and Tbx3 was shown to predominantly stimulate Nanog expression in maintaining pluripotency (26). We confirmed Dox-dependent up-regulation of Oct4, Esrrb, Zfp143, Tbx3, and Rex1 in Zfp281−/− ESCs but only slightly up-regulated or unchanged levels of these genes in Zfp281+/+ ESCs (Fig. S7 A and B), which may partly explain the Nanog activation in Zfp281−/− ESCs (Fig. 2F).
The Nanog autorepression defined in this study is unique among the core pluripotency network. In fact, down-regulation of other stem cell factor(s) does not lead to the same up-regulation of the corresponding gene promoter activity. For example, down-regulation of Oct4 by siRNA or shRNA leads only to the decrease of Oct4 promoter activity measured by Oct4-GFP reporter expression, which forms the basis for several genome-wide RNAi screening studies for important self-renewal regulators (27–29). Counterintuitively but reassuringly, we found that Nanog siRNA used in a genome-wide RNAi study in NG4 cells resulted in up-regulation of Nanog-GFP reporter activity (Fig. S8), consistent with our observation using Nanog shRNA in NG4 cells (Fig. 1 E and F).
Our study demonstrates that Zfp281 recruits a repressive chromatin remodeling complex, NuRD, to target the Nanog promoter/enhancer regions (Figs. 2 and 3). The NuRD complex contains histone deacetylase (HDAC) activity, whose inhibition by valproic acid, an HDAC inhibitor, greatly improves reprogramming efficiency (30). Our biochemical purification and coIP data confirmed association of Zfp281 with NuRD proteins (Fig. 2 G and H), and ChIP data indicate that binding of NuRD proteins Mta1/2 and Hdac2 to the Nanog promoter/enhancer is dramatically reduced upon Zfp281 depletion (Fig. 3 A–C) and increased upon ectopic Nanog expression (Fig. 3 D and E). Together with up-regulation of Nanog in Hdac1/2 knockout ESCs (31) and knockdown of NuRD proteins abrogating Nanog autorepression (Fig. 3 F–H), we provide strong evidence that Zfp281 mediates Nanog autorepression through recruitment of the NuRD repressor complex to the Nanog locus. Recent findings have implicated NuRD in choreographing multiple epigenetic events for stem cell pluripotency (see review; ref. 32). However, how NuRD is recruited to the ESC genome remains an open question. It is tempting to ask whether Zfp281 could serve as a sequence-specific transcription factor for global NuRD recruitment to target genes in ESCs. This issue requires delineation of the genomic targets of Zfp281 in ESCs by ChIP-seq.
Understanding how the core pluripotency and reprogramming factors, Nanog and Oct4 in particular, are transcriptionally regulated and function in orchestrating the genetic and epigenetic events that maintain stem cell pluripotency and promote somatic cell reprogramming is subject of intensive studies. We present a detailed mechanistic study demonstrating that Nanog is subjected to a unique transcriptional regulatory mode, i.e., autorepression, which is mediated by one of its transcriptional coregulators, Zfp281 (16). At the molecular level, Nanog autorepression is mediated by Zfp281 and its associated NuRD repressor complex. At the functional level, Nanog autorepression mediated by Zfp281 presents a roadblock to efficient reprogramming.
Methods
ESC Culture and Colony Formation Assays.
All mouse ESCs used in this study were grown under standard ESC conditions as described (3). The colony formation assay for ESC self-renewal was performed as described (16).
Nuclear Extract Preparation, Coimmunoprecipitation, Western Blot Analysis, and Affinity Purification of Protein Complexes.
All of these procedures have been described in our previous study (2).
Additional Details.
The remaining experimental details can be found in SI Methods.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health Grant 1R01-GM095942-01A1, New York State Department of Health Grant NYSTEM#C026420, and a seed fund from the Black Family Stem Cell Institute (to J.W.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208533109/-/DCSupplemental.
References
- 1.Orkin SH, et al. The transcriptional network controlling pluripotency in ES cells. Cold Spring Harb Symp Quant Biol. 2008;73:195–202. doi: 10.1101/sqb.2008.72.001. [DOI] [PubMed] [Google Scholar]
- 2.Ding J, Xu H, Faiola F, Ma’ayan A, Wang J. Oct4 links multiple epigenetic pathways to the pluripotency network. Cell Res. 2012;22:155–167. doi: 10.1038/cr.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, et al. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 4.Wang J. 2012. Deciphering protein complexes and protein interaction networks for stem cell pluripotency. New Frontiers of Network Analysis in Systems Biology, eds Maayan A, MacArthur BD (Springer Science+Business Media, Dordrecht, The Netherlands), Vol 6, pp 97–118.
- 5.Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers I, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 7.Silva J, Chambers I, Pollard S, Smith A. Nanog promotes transfer of pluripotency after cell fusion. Nature. 2006;441:997–1001. doi: 10.1038/nature04914. [DOI] [PubMed] [Google Scholar]
- 8.Silva J, et al. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li JY, et al. Synergistic function of DNA methyltransferases Dnmt3a and Dnmt3b in the methylation of Oct4 and Nanog. Mol Cell Biol. 2007;27:8748–8759. doi: 10.1128/MCB.01380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman N, et al. G9a-mediated irreversible epigenetic inactivation of Oct-3/4 during early embryogenesis. Nat Cell Biol. 2006;8:188–194. doi: 10.1038/ncb1353. [DOI] [PubMed] [Google Scholar]
- 11.Boer B, et al. Regulation of the Nanog gene by both positive and negative cis-regulatory elements in embryonal carcinoma cells and embryonic stem cells. Mol Reprod Dev. 2009;76:173–182. doi: 10.1002/mrd.20943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Orkin SH. A protein roadmap to pluripotency and faithful reprogramming. Cells Tissues Organs. 2008;188:23–30. doi: 10.1159/000113532. [DOI] [PubMed] [Google Scholar]
- 13.Boyer LA, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Q, et al. Sall4 interacts with Nanog and co-occupies Nanog genomic sites in embryonic stem cells. J Biol Chem. 2006;281:24090–24094. doi: 10.1074/jbc.C600122200. [DOI] [PubMed] [Google Scholar]
- 15.Schaniel C, et al. Smarcc1/Baf155 couples self-renewal gene repression with changes in chromatin structure in mouse embryonic stem cells. Stem Cells. 2009;27:2979–2991. doi: 10.1002/stem.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fidalgo M, et al. 2011. Zfp281 functions as a transcriptional repressor for pluripotency of mouse embryonic stem cells. Stem Cells 29:1705–1716.
- 17.Liang J, et al. Nanog and Oct4 associate with unique transcriptional repression complexes in embryonic stem cells. Nat Cell Biol. 2008;10:731–739. doi: 10.1038/ncb1736. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 19.Silva J, et al. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theunissen TW, et al. Nanog overcomes reprogramming barriers and induces pluripotency in minimal conditions. Curr Biol. 2011;21:65–71. doi: 10.1016/j.cub.2010.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira CF, Fisher AG. 2009. Heterokaryon-based reprogramming for pluripotency. Curr Protoc Stem Cell Biol, Chapter 4:Unit 4B.1.
- 22.Yi F, Pereira L, Merrill BJ. Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem Cells. 2008;26:1951–1960. doi: 10.1634/stemcells.2008-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodda DJ, et al. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280:24731–24737. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- 24.van den Berg DL, et al. Estrogen-related receptor beta interacts with Oct4 to positively regulate Nanog gene expression. Mol Cell Biol. 2008;28:5986–5995. doi: 10.1128/MCB.00301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Fang F, Liou YC, Ng HH. Zfp143 regulates Nanog through modulation of Oct4 binding. Stem Cells. 2008;26:2759–2767. doi: 10.1634/stemcells.2008-0398. [DOI] [PubMed] [Google Scholar]
- 26.Niwa H, Ogawa K, Shimosato D, Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature. 2009;460:118–122. doi: 10.1038/nature08113. [DOI] [PubMed] [Google Scholar]
- 27.Chia NY, et al. A genome-wide RNAi screen reveals determinants of human embryonic stem cell identity. Nature. 2010;468:316–320. doi: 10.1038/nature09531. [DOI] [PubMed] [Google Scholar]
- 28.Ding L, et al. A genome-scale RNAi screen for Oct4 modulators defines a role of the Paf1 complex for embryonic stem cell identity. Cell Stem Cell. 2009;4:403–415. doi: 10.1016/j.stem.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Hu G, et al. A genome-wide RNAi screen identifies a new transcriptional module required for self-renewal. Genes Dev. 2009;23:837–848. doi: 10.1101/gad.1769609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huangfu D, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dovey OM, Foster CT, Cowley SM. Histone deacetylase 1 (HDAC1), but not HDAC2, controls embryonic stem cell differentiation. Proc Natl Acad Sci USA. 2010;107:8242–8247. doi: 10.1073/pnas.1000478107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu G, Wade P. 2012. NuRD and pluripotency: A complex balancing act. Cell Stem Cell 10:497–503.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.