Abstract
One important aspect of climate change is the increase in average temperature, which will not only have direct physiological effects on all species but also indirectly modifies abundances, interaction strengths, food-web topologies, community stability and functioning. In this theme issue, we highlight a novel pathway through which warming indirectly affects ecological communities: by changing their size structure (i.e. the body-size distributions). Warming can shift these distributions towards dominance of small- over large-bodied species. The conceptual, theoretical and empirical research described in this issue, in sum, suggests that effects of temperature may be dominated by changes in size structure, with relatively weak direct effects. For example, temperature effects via size structure have implications for top-down and bottom-up control in ecosystems and may ultimately yield novel communities. Moreover, scaling up effects of temperature and body size from physiology to the levels of populations, communities and ecosystems may provide a crucially important mechanistic approach for forecasting future consequences of global warming.
Keywords: food webs, metabolic theory, allometric scaling, global change, ecological networks
1. Introduction
Climate change may become one of the major drivers affecting the diversity, composition, structure and functioning of ecological communities over the next several decades. Specific changes will include shifts in the means and variability of ecologically important factors, including temperature, precipitation, irradiance and wind. While all of these aspects of climate change are likely to have profound effects on natural communities, with potential feedbacks from communities to climate, the focus of this theme issue is on warming. Global surface temperature increased by 0.74°C on average over the last century (1906–2005) with greater warming on land than on oceans [1]. Future warming is likely to be between 1.1°C and 6.4°C by the end of the twenty-first century, depending on the projection scenario used [1]. Predicted increases in global mean temperature conceal, of course, considerable regional and local variation. For example, land surfaces, mountain ranges and arctic regions are experiencing stronger increases in temperature than other areas [1]. A pressing issue is predicting the effect of increased temperature for ecosystems across the globe.
Among the various pathways by which temperature affects ecological communities, the increase in rate of biochemical reactions caused by warming is particularly well known. Increases in temperature directly affect all individuals in a community, particularly ectotherms (figure 1a), by accelerating the speed of all biochemical reactions that compose their cellular metabolism [2]. This heated metabolism has knock-on effects on the physiological rates, including growth, reproduction, respiration and mortality [2–5].
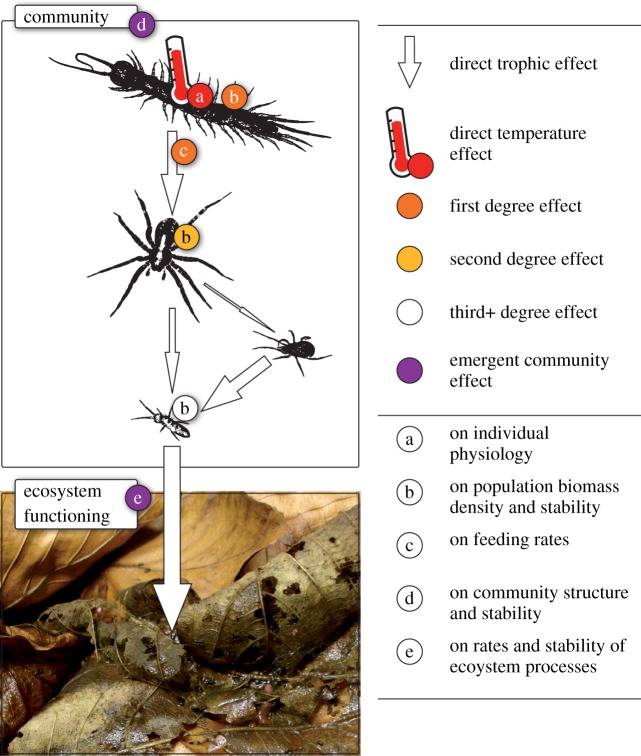
Figure 1.
Direct temperature effects on individual physiology (red, a) affect population biomass density and stability (orange, b) and feeding interactions (orange, c), cascading through the food web on trophically adjacent (yellow, b) and remote populations (white, b) with knock-on consequences for community structure and stability (purple, d) and ecosystem functioning (purple, e).
Climate change will affect patterns and processes of species and food webs in a variety of ways besides metabolic rates, such as modification of dispersal rates, spatial decoupling of interactions and shifted phenology resulting in a rearrangement of species interactions [6]. This theme issue, however, focuses primarily on metabolic consequences of warming as one of the most fundamental and universal impacts at the level of individual organisms, and at higher levels of organization, such as populations, communities and ecosystems. Additionally, metabolic process rates are strongly constrained by individual body masses [3,5], which indicate an important interaction between body masses and environmental temperature that is rooted in cellular metabolism. Ultimately, the metabolic and physiological effects of climate change on higher levels of ecological organization may provide a mechanistic and predictive understanding how increasing temperature may modify natural ecological communities. This theme issue integrates novel approaches for addressing the consequences of climate change in complex, size-structured ecosystems. This includes the development of novel concepts [7–10], model analyses [11–13] and empirical studies in marine [14,15], freshwater [16,17] and terrestrial ecosystems [18–22]. In the following, we will introduce the framework of this theme issue by describing the complexity and the size structure of natural communities and how they might interact with climate change in determining top-down or bottom-up control, community stability and spatial processes.
2. Food webs and indirect warming effects
Trophic consumer–resource interactions provide the backbone of ecological and evolutionary dynamics, as all species must acquire resources to survive and reproduce. These fundamentally important energetic interactions among species compose complex food webs, in which all species of a community are directly (by a consumer–resource interaction; figure 1: arrows) or indirectly (over multiple consumer–resource interactions) linked to each other. In these complex networks, direct temperature effects on the physiology of a species (figure 1: a) will modify the growth and mortality conditions of all other species of the community resulting in different biomass and abundance densities (figure 1: b). Hence, direct temperature effects at the physiology level (figure 1: red a) can cause changes at the population level of the same species (figure 1: orange b), species that are directly linked (figure 1: yellow b) and also species that are only indirectly linked (figure 1: white b). Henceforth, we will refer to all effects that are not mediated by direct modifications of a species' physiology as indirect warming effects.
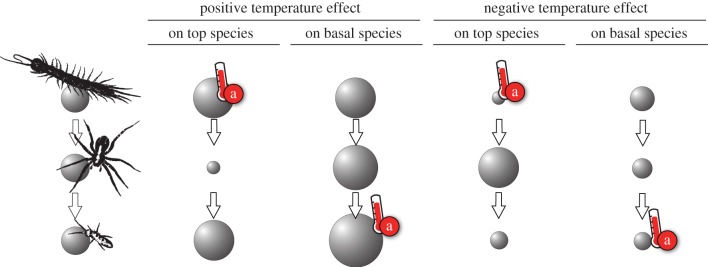
Understanding the potential for cascading effects to spread through a food web requires knowledge on how pairs of species are trophically connected. In food webs, there are often relatively short paths between pairs of species: on average, any pair of species is separated by only two links (i.e. there is one intermediary species) [23,24]. Despite a general decay in interaction strength with the number of links involved [25], these short paths imply that climatic effects on a species will lead to indirect effects on almost all other species within the food web. Interestingly, these cascading effects of external stressors can be much stronger than the immediate effects of stressors on particular species [26]. This can cause apparently idiosyncratic effects, because the consequences of positive or negative direct warming effects have different indirect implications depending on (i) which species is directly affected (e.g. top or basal species) and (ii) whether the cascade is bottom-up or top-down (see figure 2 for an illustration). For instance, a negative response of the basal species in the food chain of figure 2 may reflect a direct negative warming effect on the basal or the top species, and a positive response of the top species can be associated with a direct positive warming effect on the top or the basal species (figure 2). Predicting the likelihood and strength of these ‘thermal cascades’ will depend on our understanding of warming effects on network structure [27] and species interaction strengths [28,29].
Figure 2.
Thermal cascades in a tri-trophic food chain: temperature may affect populations positively or negatively. These direct effects (red, a) trigger thermal cascades on populations on lower or higher trophic levels. Sizes of the nodes represent changes in population biomass densities relative to original food chain (left panel).
In addition to physiological accelerations, warming can have effects on the interaction strengths between species (figure 1: c) driven by digestion, movement, behaviour and encounter rates [30,31]. While biochemical accelerations of digestion rates increase the maximum feeding rates of consumers and decrease the time they require for handling resources, behavioural responses to warming drive the rates of movement, encounter, attack and also interference competition among consumer individuals [28,29,32,33]. Two contributions to this theme issue analyse consumer–resource interaction strengths, one focusing on a marine benthic community [15] and the other on a global database of nonlinear functional responses across different ecosystem types [10]. Interestingly, both studies support prior findings [28,29,32] that increases in feeding rates with temperature are generally weaker than those of respiration, but also that many species show considerable variation in their mass-feeding rate scalings with temperature [10,15]. This indicates that warming should cause net energy losses, which might explain decreases in population densities with warming [34]. Moreover, nonlinear deviations from simple exponential Arrhenius relationships indicate that warming effects go beyond simple accelerations of physiological processes [10,33]. In particular, behavioural responses cause nonlinear deviations that need to be taken into account for understanding how warming effects on interaction strength (i) differ across species [15] and (ii) interact with constraints of body masses [10].
Overall, predicting the consequences of warming requires knowledge of the topology of the interaction network as well as interaction strengths [25,35–37], which are both closely linked to the body masses of the species and the community size structure [38–46]. Subsequently, we will first describe the concept of size-structured communities and then illustrate its interaction with warming effects.
3. Size structure of natural communities
Food webs can contain hundreds of species and thousands of interactions. However, they exhibit a surprisingly consistent topological architecture across different ecosystem types [47–51] and evolutionary time [52]. This conserved architecture is mediated by fundamental constraints on who can consume whom, leading to a pattern of strongly hierarchically ordered sets of species that feed on nearly contiguous ranges of mostly lower ranked species [53,54]. While this ordering is likely influenced by various factors, one of the strongest drivers may be organismal size determining, for example, that many species feed on resources smaller than themselves (figure 3), and feed on all taxa between a specific minimum and maximum size [41,46,55–57]. Some types of consumers break these rules; for example, it is obvious that parasitoids and parasites feed on organisms larger than themselves [39,58–60]. In general, however, predator–prey communities exhibit a consistent size structure (see figure 3 for an illustration) characterized by (i) increases in body masses with trophic levels (i.e. predators become larger along food chains), (ii) decreases in predator–prey size ratios with trophic levels (i.e. predators and their prey become more similarly sized along food chains), while (iii) generality (i.e. the number of links to prey) and vulnerability (i.e. the number of links to predators) increase and decrease, respectively, with body mass [39,42,43,45,61]. In consequence, large and rare species usually occupy high trophic levels, consume many prey species and have few predators, whereas small and abundant species are found at low trophic levels with few links to prey but many links to predators (figure 3). Hence, many characteristics of how species are embedded in complex food webs are determined by their sizes [45,46,57,62], which stimulated the development of allometric network models [41].
Figure 3.
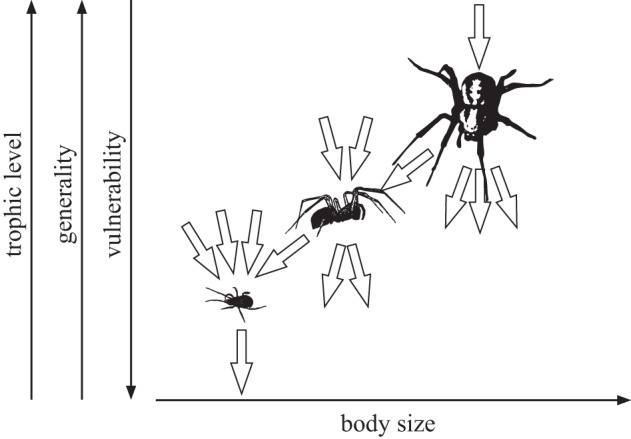
Body size determines the trophic position and network environment of a species: with increasing body size the trophic level and the number of prey species (generality) increases and the number of predators (vulnerability) decreases.
These allometric studies include taxonomically defined species that are characterized by their population-averaged body masses thus ignoring variance in body masses within populations. During their ontogenetic development, however, individuals often increase in size by several orders of magnitude, which can lead to drastic changes in diet during an individual's life. This has led to a ‘size spectrum’ approach to food web ecology [63–65]. In this approach, individuals differ only in body size (taxonomic differences are ignored), individuals grow at a rate determined by how much they eat, and what they eat is determined by their size. Studies bridging the gap between purely size-based and purely taxonomic descriptions of ecosystems are beginning to emerge and show that, for example, predicting the strengths of trophic interactions [66] or the robustness of food webs [67] benefits from analysis of both taxonomy and size.
The systematic size structure of natural communities is crucially important for maintaining network persistence (i.e. decreasing the likelihood that dynamics lead to extinctions) [61,68–70], preventing competitive exclusion processes among basal species such as plants [69], and buffering against unstable enrichment effects [71] and secondary extinctions waves [72]. However, the question of how warming interacts with size structure has been relatively unexplored. The contributions to this theme issue bridge this gap. As many qualitative aspects of size structure (figure 3) equally characterize marine, freshwater and terrestrial ecosystems [42,43], this may allow generalizing the findings across these diverse community types. In the following, we introduce the contributions to this theme issue on effects of warming in size-structured communities in sections on top-down or bottom-up control, changes in community stability and interactions with spatial patterns and processes.
4. Warming and top-down control
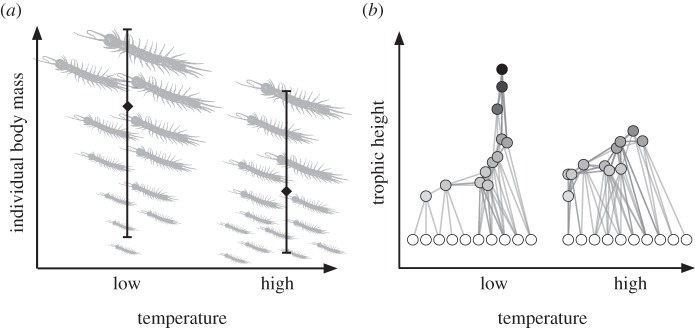
Warming often induces decreases in individual body masses within and across populations [73,74] suggesting that warmed communities may be composed of more abundant, smaller species (figure 4a). While these smaller species are expected to exhibit more rapid population oscillations and lower community stability [75], they will also have different diets, which will likely modify network structure (figure 4b). For instance, by using a temperature-based extension of the allometric diet-breadth model, Petchey et al. [27,41] suggest that future networks may be characterized by smaller species with less links, more intra-guild predation and lower trophic levels (figure 4b). These network modifications may have severe implications, as top-down control should be weakened by intraguild predation, which may negatively impact ecosystem functioning [76]. Locally or temporally, however, warming can also have positive effects on population-averaged body sizes, for example, by favouring larger individuals in particular seasons [17]. The interaction of warming with the size structure of the community thus needs to be specified for the geographical location and season. One contribution to this theme issue presents a replicated drought experiment to simulate climate change effects on stream food webs [16]. Drought simplified the food webs by reducing species richness and the number of trophic links. Interestingly, two classes of species were particularly prone to extinction: (i) the largest species, and (ii) those that were rare for their size (i.e. rare after accounting for effects of their body size, [16]).
Figure 4.
Warming modifies the community size structure. (a) Warming decreases individual and population-averaged body masses. (b) This decreases the trophic positions of the populations within the food web thus leading to food webs with lower average trophic levels and more omnivory.
Warming may also alter predator–prey size ratios, but as a result of differential range shifts of predators and prey [21]. Prey of vertebrate species migrating in altitude are larger than prey species already inhabiting this area, whereas predators that expand their ranges in altitude are not different in size than the natives [21]. This leads to decreases in predator–prey body-size ratios, which have similar implications for the community size structure as the loss of large species, and this is ultimately likely to affect community dynamics [61,68,75].
The loss of large-bodied populations in food webs—as may result from warming [73,74]—may cause secondary-extinction avalanches [72,77,78]. Climate change may thus induce trophic cascades where the loss of species (in particular large ones) has alternating positive and negative effects on the trophic levels below (figure 2). These trophic cascades generally structure biomass distributions across populations in different ecosystem types [79]. Warming generally increases the individual consumption rates [10,15], which may explain the intensified trophic cascade in the planktonic food web of a pond mesocosm experiment [17]. However, the lack of a warming effect on the benthic trophic cascade in the same experiment illustrates that species interactions are often driven by more complex constraints than simple metabolic changes.
One issue specific to terrestrial ecosystems is that effects of warming on size structure may differ between the below- and above-ground compartments. While small species profit from warming in the above-ground part, increasing temperatures may benefit larger species in the below-ground realm [8]. This is exemplified in this theme issue for the boreal–temperate ecotone of the Great Lakes Region, USA, [8], where moose replacement by small deer is associated with changes in below-ground communities from small mesofaunal detritivores to dominance by larger earthworms. Interestingly, these changes in the size structure may impose top-down control on plant communities and thus indirectly determine the community structure [8].
In addition, warming may induce more subtle indirect effects that do not involve species' extinctions. For instance, warming causes shifts in species' phenologies, which can lead to trophic cascades if consumer and resource phenologies become temporally desynchronized [80]. This concept is supported by analyses of a 10-year-dataset of a host–parasitoid network with aphids as the basal species [18]. Distinct seasonal patterns in parasitoid activity suggest how climate change may alter feeding behaviour thus modifying the indirect interaction network. The analyses show how climate change may shift behavioural patterns with a strong feedback on biological control [18].
Another aspect of indirect effects is warming-induced shifts towards lower individual body sizes in top-predator populations. These shifts in population size structure change consumption strengths [10] and thus induce cascading effects on lower trophic levels without extinctions of top predators in a marine experiment [14]. In that experiment, an allometrically induced cascade was transmitted over four trophic levels and ultimately caused an increase in algal biomass. Interestingly, this suggests that warming may have profound consequences for ecosystem functioning prior to extinctions [14]. In the same vein, an aquatic mesocosm experiment demonstrated that long-term changes in the size structure had knock-on effects on community metabolism and biogeochemical fluxes that were stronger than the direct consequences of warming [9].
Thus, in the short-term, warming will induce physiological and behavioural responses that manifest as altered consumption patterns [10]. The resulting shifts in the size structure of the community towards populations of smaller individuals can induce trophic cascades that modify biomass distribution across size classes and trophic levels [14]. These modifications can lead to the loss of existing interactions and the emergence of novel ones, leading to different food-web structures [7]. Hence, understanding the interplay of direct warming effects with indirect effects of modified community size structures may provide a generalized understanding of climate change impacts on natural communities. Overall, these studies suggest that warming of ecosystems should lead to accelerated feeding rates and consequently stronger top-down control over short time-scales, whereas food-web re-organization may ultimately lead to weaker top-down control of smaller consumers with more intra-guild predation (figure 4).
5. Warming and bottom-up control
Increases in atmospheric carbon dioxide will modify the growth conditions for autotrophic plant species. Plant species generally respond to increased carbon supply by a higher production rate and higher carbon contents in their tissue, which modifies their stoichiometry (e.g. higher C/N and C/P ratios) [81]. Among many other major impacts of climate change, both higher production rates and lower stoichiometric quality of the basal plant species may impose bottom-up control on natural communities.
Changes in basal resource quality can greatly modify the feeding and growth conditions for herbivores [82] and detritivores [19]. Understanding bottom-up consequences of climate change thus requires bridging the gap between metabolic theory, which quantifies effects of environmental temperature on physiological processes, and stoichiometric theory, which conceptualizes constraints of elemental concentrations on growth. Moreover, the reaction of terrestrial decomposers to variation in resource stoichiometry depends on metabolic constraints [19]. While small-bodied decomposers and cool temperatures lead to avoidance of poor resources, increases in decomposer body mass or temperature cause higher metabolic rates, which triggers compensatory feeding on poorer resources. Strikingly, this implies that warming may lead to stronger decomposition of the poorest resources, which may accelerate the release of carbon from terrestrial pools [19].
Increased productivity at the base of food webs can increase the energy supply for higher trophic-level consumers, thus supporting more diverse communities, but it can also destabilize dynamics [71]. In consequence, low- and high-productivity communities may gain and lose, respectively, species by these enrichment effects. Crucially, these enrichment effects on complex communities can vary dramatically with the size structure of the community [71]. One contribution to this theme issue uses food-chain models to study the interactive effects of nutrient enrichment and warming in communities with and without size structure [11]. Warming induces inverse enrichment effects in size-structured communities leading to lower biomass densities and dampened oscillations. Surprisingly, classic paradox of enrichment effects can thus be diminished by warming, which is consistent with recent experimental data [83]. However, the stabilizing effects of warming are much more pronounced in size-structured (consumers larger than their resources) compared with unstructured communities (consumer and resources similarly sized) [11].
These theoretical model analyses are consistent with the results of a freshwater experiment in which warming reduced enrichment effects on benthic and pelagic autotrophs while strengthening the top-down effects of fish species [17]. In a field manipulation of the temperature and nitrogen load experienced by a host–parasitoid food web, however, increasing temperature or nitrogen yielded higher host densities and consequently more generalized feeding by parasitoids. Interestingly, increasing temperature and nitrogen simultaneously did not yield an additive response in host density or parasitoid generality, suggesting that they do not interact linearly [20]. Differences in whether warming counteracts enrichment [17] or if both effects are non-additive [20] may be explained by the presence of a strongly size-structured predator–prey community in the aquatic experiment of the former and the lack thereof in the host–parasitoid community of the latter study as suggested by model analyses [11]. Interestingly, the size structure of host–parasitoid communities may be strengthened by warming [18,20], which may modify interactions with enrichment. In conclusion, understanding how warming modifies bottom-up control by basal resource quality (i.e. stoichiometry) or quantity (i.e. productivity) depends strongly on the size structure of the community.
6. Warming and ecosystem stability
Although the consistent size structure of communities has been studied over several decades [38,39,56,84,85], its importance for their dynamic stability has been unravelled only more recently [61,64,68,69,75,86]. Decreasing metabolic rates with increasing body masses and trophic levels yield patterns of weaker interactions at the top [86]. These systematic patterns in interaction strengths across trophic levels places communities in a domain of relatively high stability that corresponds to natural body-mass patterns [61]. Accounting for effects of warming on metabolic rates yields similar stability domains for simulated food chains across a temperature axis [11]. This study demonstrated that warming can dampen population oscillations by reducing predator–prey interaction strengths. Surprisingly, this finding contrasts with prior model analyses demonstrating that warming should destabilize population dynamics [87,88]. In contrast to those prior studies, Binzer et al. [11] use empirical relationships between the populations' biological rates and warming [10]. However, decreases in interaction strengths with warming, which stabilize population dynamics, may ultimately cause predator starvation [28]. The stability implications of warming may thus represent a double-edged sword [11]. Similarly, synthetic models of size-spectrum communities and ocean-biogeochemical processes can be used to predict the consequences of warming for community-level patterns such as primary and fish production in marine ecosystems [13]. Interestingly, fish production depends more on primary production than on direct effects of temperature, thus stressing the importance of thermal bottom-up cascades (figure 2) in marine systems. Predicted declines of 30–60% in fish production will impose severe threats on the security of the future food supply [13]. These studies suggest that warming may have complex effects on community-level patterns (figure 1d) that feed back to determine population persistence, with high-trophic-level species being most threatened.
7. Warming and spatial processes
While top-down and bottom-up control, as well as food-web stability, structure local communities, ecosystems are also affected by spatial processes. In particular, habitat fragmentation is one of the major global-change drivers of ecosystem changes, and dispersal of individuals between local communities may have severe implications for their structure and functioning. While fragmentation effects on communities have been intensively explored [89–91], interactions between warming and fragmentation have received less attention. Generally, warming should increase movement rates of ectotherm organisms [4], which should lead to higher dispersal rates between local habitats. However, warming may also increase the hostility of the landscape matrix surrounding the local habitats thus yielding higher extinction rates during dispersal. In an analysis of a spatially and dynamically explicit metacommunity model, community persistence was highest at intermediate levels of dispersal [12]. Persistence decreased towards low dispersal rates, because local bottom-up extinction cascades were not balanced by re-invasions. However, high dispersal rates also decreased persistence due to high mortality experienced by the dispersing individuals [12]. Perdomo et al. [22] complement this theoretical approach by addressing interactions between habitat fragmentation and warming in a laboratory experiment with moss communities. They applied a temperature shock event and studied the recolonization of the micro-arthropod communities on satellite islands from a source mainland at different temperatures. The study highlights the value of dispersal for the recovery of habitat patches affected by catastrophic climatic events, and shows that community assembly can depend on the degree of habitat isolation and on temperature. Interestingly, communities impacted by these stressors showed size-structures unlike those seen in field communities: A few large, prey species became highly dominant in abundance [22]. This illustrates that warming may strongly interact with spatial processes such as dispersal in structuring ecological communities.
8. Conclusions
The contributions to this theme issue demonstrate that warming will have strong implications for ecological communities. Warming directly accelerates individual metabolic rates [2–5,15,92], which causes increased feeding rates [10,15]. Over the short term, this can lead to increased top-down control and trophic cascades. However, increases in feeding rates with warming are generally weaker than those of metabolism [15,28,29], which should lead to reduced consumer densities and long-term reductions in top-down control and trophic cascades. Interestingly, experimental results are mixed, with increases and decreases of trophic cascade strength in the planktonic and benthic food web, respectively [17]. Moreover, shifts in the body-mass distributions of populations can be triggered by warming or drought of freshwater ecosystems [17,73,93]. These modifications in the community size structure can relax top-down control causing modifications in primary production [14], secondary production [13], community metabolism and biogeochemical fluxes exceeding the direct consequences of warming [9].
In addition, warming also interacts with the community size structure in determining bottom-up control. While warming ameliorates nutrient enrichment effects in size-structured communities, this effect disappears in communities with less variance in body size such as host–parasitoid systems [11,17,20]. Moreover, compensatory feeding on stoichiometric poor resources may be increased by decomposer body mass as well as warming leading to accelerated release of CO2 from soil ecosystems, particularly in size-structured communities [19].
The results presented in this theme issue suggest that warming modifies the size structure of communities with effects on top-down and bottom-up control that exceed the direct temperature effects. These indirect warming effects can lead to severely changed community compositions [7,8,22], food-web topologies [16,21], predator–prey size ratios [21], network dynamics [11] and drastically altered ecosystem functioning [9,13]. Integration of temperature effects with modifications of the community size structure can thus yield a more mechanistic and predictive understanding how global warming may restructure ecosystems. The interplay between conceptual, theoretical and empirical approaches addressing the consequences of warming in this theme issue may serve as a role model for future interdisciplinary projects tackling other implications of climate change.
Acknowledgments
This research was supported by a Research Network Programme of the European Science Foundation on body size and ecosystem dynamics (SIZEMIC) and the workshop on ‘Changing climate, physiological adaptation, ecosystem resilience and body-size constraints’ that was partially financed by the German Research Foundation (JA 1726/3-1) as well as the Cluster of Excellence CliSAP (EXC177), University of Hamburg, funded through the DFG. U.B. is funded by the German Research Foundation (BR 2315/13). J.A.D. received support from NSF (DBI-0850373).
References
- 1.IPCC 2007. Climate change 2007: Synthesis report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Core Writing Team, R. K. Pachauri & A. Reisinger). Geneva, Switzerland: IPCC.
- 2.Gillooly J. F., Brown J. H., West G. B., Savage V. M., Charnov E. L. 2001. Effects of size and temperature on metabolic rate. Science 293, 2248–2251 10.1126/science.1061967 (doi:10.1126/science.1061967) [DOI] [PubMed] [Google Scholar]
- 3.Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 10.1890/03-9000 (doi:10.1890/03-9000) [DOI] [Google Scholar]
- 4.Peters R. H. 1983. The ecological implications of body size. New York, NY: Cambridge University Press [Google Scholar]
- 5.Ehnes R. B., Rall B. C., Brose U. 2011. Phylogenetic grouping, curvature and metabolic scaling in terrestrial invertebrates. Ecol. Lett. 14, 993–1000 10.1111/j.1461-0248.2011.01660.x (doi:10.1111/j.1461-0248.2011.01660.x) [DOI] [PubMed] [Google Scholar]
- 6.Montoya J., Raffaelli D. 2010. Climate change, biotic interactions and ecosystem services. Phil. Trans. R. Soc. B 365, 2013–2018 10.1098/rstb.2010.0114 (doi:10.1098/rstb.2010.0114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lurgi M., López B. C., Montoya J. M. 2012. Novel communities from climate change. Phil. Trans. R. Soc. B 367, 2913–2922 10.1098/rstb.2012.0238 (doi:10.1098/rstb.2012.0238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frelich L. E., Peterson R. O., Dovčiak M., Reich P. B., Vucetich J. A., Eisenhauer N. 2012. Trophic cascades, invasive species and body-size hierarchies interactively modulate climate change responses of ecotonal temperate–boreal forest. Phil. Trans. R. Soc. B 367, 2955–2961 10.1098/rstb.2012.0235 (doi:10.1098/rstb.2012.0235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yvon-Durocher G., Allen A. P. 2012. Linking community size structure and ecosystem functioning using metabolic theory. Phil. Trans. R. Soc. B 367, 2998–3007 10.1098/rstb.2012.0246 (doi:10.1098/rstb.2012.0246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rall B. C., Brose U., Hartvig M., Kalinkat G., Schwarzmüller F., Vucic-Pestic O., Petchey O. L. 2012. Universal temperature and body-mass scaling of feeding rates. Phil. Trans. R. Soc. B 367, 2923–2934 10.1098/rstb.2012.0242 (doi:10.1098/rstb.2012.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binzer A., Guill C., Brose U., Rall B. C. 2012. The dynamics of food chains under climate change and nutrient enrichment. Phil. Trans. R. Soc. B 367, 2935–2944 10.1098/rstb.2012.0230 (doi:10.1098/rstb.2012.0230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eklöf A., Kaneryd L., Münger P. 2012. Climate change in metacommunities: dispersal gives double-sided effects on persistence. Phil. Trans. R. Soc. B 367, 2945–2954 10.1098/rstb.2012.0234 (doi:10.1098/rstb.2012.0234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanchard J. L., Jennings S., Holmes R., Harle J., Merino G., Allen J. I., Holt J., Dulvy N. K., Barange M. 2012. Potential consequences of climate change for primary production and fish production in large marine ecosystems. Phil. Trans. R. Soc. B 367, 2979–2989 10.1098/rstb.2012.0231 (doi:10.1098/rstb.2012.0231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jochum M., Schneider F. D., Crowe T. P., Brose U., O'Gorman E. J. 2012. Climate-induced changes in bottom-up and top-down processes independently alter a marine ecosystem. Phil. Trans. R. Soc. B 367, 2962–2970 10.1098/rstb.2012.0237 (doi:10.1098/rstb.2012.0237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Twomey M., Brodte E., Jacob U., Brose U., Crowe T. P., Emmerson M. C. 2012. Idiosyncratic species effects confound size-based predictions of responses to climate change. Phil. Trans. R. Soc. B 367, 2971–2978 10.1098/rstb.2012.0244 (doi:10.1098/rstb.2012.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodward G., Brown L. E., Edwards F. K., Hudson L. N., Milner A. M., Reuman D. C., Ledger M. E. 2012. Climate change impacts in multispecies systems: drought alters food web size structure in a field experiment. Phil. Trans. R. Soc. B 367, 2990–2997 10.1098/rstb.2012.0245 (doi:10.1098/rstb.2012.0245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shurin J. B., Clasen J. L., Greig H. S., Kratina P., Thompson P. L. 2012. Warming shifts top-down and bottom-up control of pond food web structure and function. Phil. Trans. R. Soc. B 367, 3008–3017 10.1098/rstb.2012.0243 (doi:10.1098/rstb.2012.0243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henri D. C., Seager D., Weller T., van Veen F. J. F. 2012. Potential for climate effects on the size-structure of host–parasitoid indirect interaction networks. Phil. Trans. R. Soc. B 367, 3018–3024 10.1098/rstb.2012.0236 (doi:10.1098/rstb.2012.0236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ott D., Rall B. C., Brose U. 2012. Climate change effects on macrofaunal litter decomposition: the interplay of temperature, body masses and stoichiometry. Phil. Trans. R. Soc. B 367, 3025–3032 10.1098/rstb.2012.0240 (doi:10.1098/rstb.2012.0240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Sassi C., Staniczenko P. P. A., Tylianakis J. M. 2012. Warming and nitrogen affect size structuring and density dependence in a host–parasitoid food web. Phil. Trans. R. Soc. B 367, 3033–3041 10.1098/rstb.2012.0233 (doi:10.1098/rstb.2012.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lurgi M., López B. C., Montoya J. M. 2012. Climate change impacts on body size and food web structure on mountain ecosystems. Phil. Trans. R. Soc. B 367, 3050–3057 10.1098/rstb.2012.0239 (doi:10.1098/rstb.2012.0239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perdomo G., Sunnucks P., Thompson R. M. 2012. The role of temperature and dispersal in moss-microarthropod community assembly after a catastrophic event. Phil. Trans. R. Soc. B 367, 3042–3049 10.1098/rstb.2012.0241 (doi:10.1098/rstb.2012.0241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montoya J. M., Sole R. V. 2002. Small world patterns in food webs. J. Theor. Biol. 214, 405–412 10.1006/jtbi.2001.2460 (doi:10.1006/jtbi.2001.2460) [DOI] [PubMed] [Google Scholar]
- 24.Williams R. J., Martinez N. D., Berlow E. L., Dunne J. A., Barabási A.-L. 2002. Two degrees of separation in complex food webs. Proc. Natl Acad. Sci. USA 99, 12 913–12 916 10.1073/pnas.192448799 (doi:10.1073/pnas.192448799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berlow E. L., Dunne J. A., Martinez N. D., Stark P. B., Williams R. J., Brose U. 2009. Simple prediction of interaction strengths in complex food webs. Proc. Natl Acad. Sci. USA 106, 187–191 10.1073/pnas.0806823106 (doi:10.1073/pnas.0806823106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ives A. R., Cardinale B. J. 2004. Food-web interactions govern the resistance of communities after non-random extinctions. Nature 429, 174–177 10.1038/nature02515 (doi:10.1038/nature02515) [DOI] [PubMed] [Google Scholar]
- 27.Petchey O., Brose U., Rall B. 2010. Predicting the effects of temperature on food web connectance. Phil. Trans. R. Soc. B 365, 2081–2091 10.1098/rstb.2010.0011 (doi:10.1098/rstb.2010.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rall B., Vucic-Pestic O., Ehnes R., Emmerson M., Brose U. 2010. Temperature, predator–prey interaction strength and population stability. Glob. Change Biol. 16, 2145–2157 10.1111/j.1365-2486.2009.02124.x (doi:10.1111/j.1365-2486.2009.02124.x) [DOI] [Google Scholar]
- 29.Vucic-Pestic O., Ehnes R. B., Rall B. C., Brose U. 2011. Warming up the system: higher predator feeding rates but lower energetic efficiencies. Glob. Change Biol. 17, 1301–1310 10.1111/j.1365-2486.2010.02329.x (doi:10.1111/j.1365-2486.2010.02329.x) [DOI] [Google Scholar]
- 30.Jeschke J. M., Kopp M., Tollrian R. 2002. Predator functional responses: Discriminating between handling and digesting prey. Ecol. Monogr. 72, 95–112 10.1890/0012-9615(2002)072[0095:PFRDBH]2.0.CO;2 (doi:10.1890/0012-9615(2002)072[0095:PFRDBH]2.0.CO;2) [DOI] [Google Scholar]
- 31.Gergs A., Ratte H. 2009. Predicting functional response and size selectivity of juvenile Notonecta maculata foraging on Daphnia magna. Ecol. Model. 220, 3331–3341 10.1016/j.ecolmodel.2009.08.012 (doi:10.1016/j.ecolmodel.2009.08.012) [DOI] [Google Scholar]
- 32.Lang B., Rall B. C., Brose U., Lang B., Rall B. C., Brose U. 2012. Warming effects on consumption and intraspecific interference competition depend on predator metabolism, warming effects on consumption and intraspecific interference competition depend on predator metabolism. J. Anim. Ecol. 81, 516–523 10.1111/j.1365-2656.2011.01931.x (doi:10.1111/j.1365-2656.2011.01931.x) [DOI] [PubMed] [Google Scholar]
- 33.Englund G., Öhlund G., Hein C. L., Diehl S. 2011. Temperature dependence of the functional response. Ecol. Lett. 14, 914–921 10.1111/j.1461-0248.2011.01661.x (doi:10.1111/j.1461-0248.2011.01661.x) [DOI] [PubMed] [Google Scholar]
- 34.Meehan T. D. 2006. Energy use and animal abundance in litter and soil communities. Ecology 87, 1650–1658 10.1890/0012-9658(2006)87[1650:EUAAAI]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[1650:EUAAAI]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 35.Yodzis P. 1988. The indeterminacy of ecological interactions as perceived through perturbation experiments. Ecology 69, 508–515 10.2307/1940449 (doi:10.2307/1940449) [DOI] [Google Scholar]
- 36.Novak M., Wootton J. T., Doak D. F., Emmerson M., Estes J. A., Tinker M. T. 2011. Predicting community responses to perturbations in the face of imperfect knowledge and network complexity. Ecology 92, 836–846 10.1890/10-1354.1 (doi:10.1890/10-1354.1) [DOI] [PubMed] [Google Scholar]
- 37.Brose U., Berlow E. L., Martinez N. D. 2005. Scaling up keystone effects from simple to complex ecological networks. Ecol. Lett. 8, 1317–1325 10.1111/j.1461-0248.2005.00838.x (doi:10.1111/j.1461-0248.2005.00838.x) [DOI] [Google Scholar]
- 38.Cohen J. E., Pimm S. L., Yodzis P., Saldana J. 1993. Body sizes of animal predators and animal prey in food webs. J. Anim. Ecol. 62, 67–78 10.2307/5483 (doi:10.2307/5483) [DOI] [Google Scholar]
- 39.Brose U., et al. 2006. Consumer–resource body-size relationships in natural food webs. Ecology 87, 2411–2417 10.1890/0012-9658(2006)87[2411:CBRINF]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[2411:CBRINF]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 40.Brose U., Ehnes R. B., Rall B. C., Vucic-Pestic O., Berlow E. L., Scheu S. 2008. Foraging theory predicts predator–prey energy fluxes. J. Anim. Ecol. 77, 1072–1078 10.1111/j.1365-2656.2008.01408.x (doi:10.1111/j.1365-2656.2008.01408.x) [DOI] [PubMed] [Google Scholar]
- 41.Petchey O. L., Beckerman A. P., Riede J. O., Warren P. H. 2008. Size, foraging, and food web structure. Proc. Natl Acad. Sci. USA 105, 4191–4196 10.1073/pnas.0710672105 (doi:10.1073/pnas.0710672105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riede J. O., Brose U., Ebenman B., Jacob U., Thompson R., Townsend C. R., Jonsson T. 2011. Stepping in Elton's footprints: a general scaling model for body masses and trophic levels across ecosystems. Ecol. Lett. 14, 169–178 10.1111/j.1461-0248.2010.01568.x (doi:10.1111/j.1461-0248.2010.01568.x) [DOI] [PubMed] [Google Scholar]
- 43.Digel C., Riede J. O., Brose U. 2011. Body sizes, cumulative and allometric degree distributions across natural food webs. Oikos 120, 503–509 10.1111/j.1600-0706.2010.18862.x (doi:10.1111/j.1600-0706.2010.18862.x) [DOI] [Google Scholar]
- 44.Aljetlawi A. A., Sparrevik E., Leonardsson K. 2004. Prey–predator size-dependent functional response: derivation and rescaling to the real world. J. Anim. Ecol. 73, 239–252 10.1111/j.0021-8790.2004.00800.x (doi:10.1111/j.0021-8790.2004.00800.x) [DOI] [Google Scholar]
- 45.Williams R. J., Anandanadesan A., Purves D. 2010. The probabilistic niche model reveals the niche structure and role of body size in a complex food web. PLoS ONE 5, e12092. 10.1371/journal.pone.0012092 (doi:10.1371/journal.pone.0012092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zook A. E., Eklof A., Jacob U., Allesina S. 2011. Food webs: Ordering species according to body size yields high degree of intervality. J. Theor. Biol. 271, 106–113 10.1016/j.jtbi.2010.11.045 (doi:10.1016/j.jtbi.2010.11.045) [DOI] [PubMed] [Google Scholar]
- 47.Dunne J. A., Williams R. J., Martinez N. D. 2002. Food-web structure and network theory: The role of connectance and size. Proc. Natl Acad. Sci. USA 99, 12 917–12 922 10.1073/pnas.192407699 (doi:10.1073/pnas.192407699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunne J. A., Williams R. J., Martinez N. D. 2004. Network structure and robustness of marine food webs. Mar. Ecol. Prog. Ser. 273, 291–302 10.3354/meps273291 (doi:10.3354/meps273291) [DOI] [Google Scholar]
- 49.Riede J. O., Rall B. C., Banasek-Richter C., Navarrete S., Wieters E. A., Emmerson M.C., Jacob U., Brose U. 2010. Scaling of food-web properties with diversity and complexity across ecosystems. Adv. Ecol. Res. 42, 139–169 10.1016/B978-0-12-381363-3.00003-4 (doi:10.1016/B978-0-12-381363-3.00003-4) [DOI] [Google Scholar]
- 50.Montoya J. M., Pimm S. L., Sole R. V. 2006. Ecological networks and their fragility. Nature 442, 259–264 10.1038/nature04927 (doi:10.1038/nature04927) [DOI] [PubMed] [Google Scholar]
- 51.Ings T. C., et al. 2009. Ecological networks: beyond food webs. J. Anim. Ecol. 78, 253–269 10.1111/j.1365-2656.2008.01460.x (doi:10.1111/j.1365-2656.2008.01460.x) [DOI] [PubMed] [Google Scholar]
- 52.Dunne J. A., Williams R. J., Martinez N. D., Wood R. A., Erwin D. H. 2008. Compilation and network analyses of Cambrian food webs. PLoS Biol. 6, 693–708 10.1371/journal.pbio.0060102 (doi:10.1371/journal.pbio.0060102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams R. J., Martinez N. D. 2000. Simple rules yield complex food webs. Nature 404, 180–183 10.1038/35004572 (doi:10.1038/35004572) [DOI] [PubMed] [Google Scholar]
- 54.Stouffer D. B., Camacho J., Guimera R., Ng C. A., Amaral L. A. N. 2005. Quantitative patterns in the structure of model and empirical food webs. Ecology 86, 1301–1311 10.1890/04-0957 (doi:10.1890/04-0957) [DOI] [Google Scholar]
- 55.Loeuille N., Loreau M. 2005. Evolutionary emergence of size-structured food webs. Proc. Natl Acad. Sci. USA 102, 5761–5766 10.1073/pnas.0408424102 (doi:10.1073/pnas.0408424102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brose U. 2010. Body-mass constraints on foraging behaviour determine population and food-web dynamics. Funct. Ecol. 24, 28–34 10.1111/j.1365-2435.2009.01618.x (doi:10.1111/j.1365-2435.2009.01618.x) [DOI] [Google Scholar]
- 57.Stouffer D., Rezende E., Amaral L. 2011. The role of body mass in diet contiguity and food-web structure. J. Anim. Ecol. 80, 632–639 10.1111/j.1365-2656.2011.01812.x (doi:10.1111/j.1365-2656.2011.01812.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen J. E., Jonsson T., Muller C. B., Godfray H. C. J., Savage V. M. 2005. Body sizes of hosts and parasitoids in individual feeding relationships. Proc. Natl Acad. Sci. USA 102, 684–689 10.1073/pnas.0408780102 (doi:10.1073/pnas.0408780102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lafferty K. D., Dobson A. P., Kuris A. M. 2006. Parasites dominate food web links. Proc. Natl Acad. Sci. USA 103, 11 211–11 216 10.1073/pnas.0604755103 (doi:10.1073/pnas.0604755103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beckerman A. P., Petchey O. L. 2009. Infectious food webs. J. Anim. Ecol. 78, 493–496 10.1111/j.1365-2656.2009.01538.x (doi:10.1111/j.1365-2656.2009.01538.x) [DOI] [PubMed] [Google Scholar]
- 61.Otto S. B., Rall B. C., Brose U. 2007. Allometric degree distributions facilitate food-web stability. Nature 450, 1226–1229 10.1038/nature06359 (doi:10.1038/nature06359) [DOI] [PubMed] [Google Scholar]
- 62.Thierry A., Petchey O. L., Beckerman A. P., Warren P. H., Williams R. J. 2011. The consequences of size dependent foraging for food web topology. Oikos 120, 493–502 10.1111/j.1600-0706.2010.18861.x (doi:10.1111/j.1600-0706.2010.18861.x) [DOI] [Google Scholar]
- 63.Law R., Plank M. J., James A., Blanchard J. L. 2009. Size-spectra dynamics from stochastic predation and growth of individuals. Ecology 90, 802–811 10.1890/07-1900.1 (doi:10.1890/07-1900.1) [DOI] [PubMed] [Google Scholar]
- 64.Blanchard J. L., Law R., Castle M. D., Jennings S. 2010. Coupled energy pathways and the resilience of size-structured food webs. Theor. Ecol. 4, 289–300 10.1007/s12080-010-0078-9 (doi:10.1007/s12080-010-0078-9) [DOI] [Google Scholar]
- 65.Hartvig M., Andersen K. H., Beyer J. E. 2011. Food web framework for size-structured populations. J. Theor. Biol. 272, 113–122 10.1016/j.jtbi.2010.12.006 (doi:10.1016/j.jtbi.2010.12.006) [DOI] [PubMed] [Google Scholar]
- 66.Rall B., Kalinkat G., Ott D., Vucic-Pestic O., Brose U. 2011. Taxonomic versus allometric constraints on non-linear interaction strengths. Oikos 120, 483–492 10.1111/j.1600-0706.2010.18860.x (doi:10.1111/j.1600-0706.2010.18860.x) [DOI] [Google Scholar]
- 67.Rudolf V. H. W., Lafferty K. D. 2011. Stage structure alters how complexity affects stability of ecological networks. Ecol. Lett. 14, 75–79 10.1111/j.1461-0248.2010.01558.x (doi:10.1111/j.1461-0248.2010.01558.x) [DOI] [PubMed] [Google Scholar]
- 68.Heckmann L., Drossel B., Brose U., Guill C. 2012. Interactive effects of body-size structure and adaptive foraging on food-web stability. Ecol. Lett. 15, 243–250 10.1111/j.1461-0248.2011.01733.x (doi:10.1111/j.1461-0248.2011.01733.x) [DOI] [PubMed] [Google Scholar]
- 69.Brose U. 2008. Complex food webs prevent competitive exclusion among producer species. Proc. R. Soc. B 275, 2507–2514 10.1098/rspb.2008.0718 (doi:10.1098/rspb.2008.0718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Emmerson M. C., Raffaelli D. 2004. Predator–prey body size, interaction strength and the stability of a real food web. J. Anim. Ecol. 73, 399–409 10.1111/j.0021-8790.2004.00818.x (doi:10.1111/j.0021-8790.2004.00818.x) [DOI] [Google Scholar]
- 71.Rall B. C., Guill C., Brose U. 2008. Food-web connectance and predator interference dampen the paradox of enrichment. Oikos 117, 202–213 10.1111/j.2007.0030-1299.15491.x (doi:10.1111/j.2007.0030-1299.15491.x) [DOI] [Google Scholar]
- 72.Riede J. O., Binzer A., Brose U., de Castro F., Curtsdotter A., Rall B. C., Eklöf A. 2011. Size-based food web characteristics govern the response to species extinctions. Basic Appl. Ecol. 12, 581–589 10.1016/j.baae.2011.09.006 (doi:10.1016/j.baae.2011.09.006) [DOI] [Google Scholar]
- 73.Daufresne M., Lengfellner K., Sommer U. 2009. Global warming benefits the small in aquatic ecosystems. Proc. Natl Acad. Sci. USA 106, 12 788–12 793 10.1073/pnas.0902080106 (doi:10.1073/pnas.0902080106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yvon-Durocher G., Montoya J. M., Trimmer M., Woodward G. 2011. Warming alters the size spectrum and shifts the distribution of biomass in freshwater ecosystems. Glob. Change Biol. 17, 1681–1694 10.1111/j.1365-2486.2010.02321.x (doi:10.1111/j.1365-2486.2010.02321.x) [DOI] [Google Scholar]
- 75.Brose U., Williams R. J., Martinez N. D. 2006. Allometric scaling enhances stability in complex food webs. Ecol. Lett. 9, 1228–1236 10.1111/j.1461-0248.2006.00978.x (doi:10.1111/j.1461-0248.2006.00978.x) [DOI] [PubMed] [Google Scholar]
- 76.Schneider F. D., Scheu S., Brose U. 2012. Body mass constraints on feeding rates determine the consequences of predator loss. Ecol. Lett. 15, 436–443 10.1111/j.1461-0248.2012.01750.x (doi:10.1111/j.1461-0248.2012.01750.x) [DOI] [PubMed] [Google Scholar]
- 77.Binzer A., Brose U., Curtsdotter A., Eklöf A., Rall B. C., Riede J. O., de Castro F. 2011. The susceptibility of species to extinctions in model communities. Basic Appl. Ecol. 12, 590–599 10.1016/j.baae.2011.09.002 (doi:10.1016/j.baae.2011.09.002) [DOI] [Google Scholar]
- 78.Curtsdotter A., Binzer A., Brose U., de Castro F., Ebenman B., Eklöf A., Riede J. O., Thierry A., Rall B. C. 2011. Robustness to secondary extinctions: comparing trait-based sequential deletions in static and dynamic food webs. Basic Appl. Ecol. 12, 571–580 10.1016/j.baae.2011.09.008 (doi:10.1016/j.baae.2011.09.008) [DOI] [Google Scholar]
- 79.Borer E. T., Seabloom E. W., Shurin J. B., Anderson K. E., Blanchette C. A., Broitman B., Cooper S. D., Halpern B. S. 2005. What determines the strength of a trophic cascade? Ecology 86, 528–537 10.1890/03-0816 (doi:10.1890/03-0816) [DOI] [Google Scholar]
- 80.Otto S. B., Berlow E. L., Rank N. E., Smiley J., Brose U. 2008. Predator diversity and identity drive interaction strength and trophic cascades in a food web. Ecology 89, 134–144 10.1890/07-0066.1 (doi:10.1890/07-0066.1) [DOI] [PubMed] [Google Scholar]
- 81.Sterner R. W., Elser J. J. 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton, NJ: Princeton University Press [Google Scholar]
- 82.Hillebrand H., et al. 2009. Herbivore metabolism and stoichiometry each constrain herbivory at different organizational scales across ecosystems. Ecol. Lett. 12, 516–527 10.1111/j.1461-0248.2009.01304.x (doi:10.1111/j.1461-0248.2009.01304.x) [DOI] [PubMed] [Google Scholar]
- 83.Kratina P., Greig H., Thompson P. L., Carvalho-Pereira T. S. A., Shurin J. B. 2012. Warming modifies trophic cascades and eutrophication in experimental freshwater communities. Ecology 93, 1421–1430 10.1890/11-1595.1 (doi:10.1890/11-1595.1) [DOI] [PubMed] [Google Scholar]
- 84.Elton C. 1927. Animal ecology. London, UK: Sidgewick & Jackson [Google Scholar]
- 85.Woodward G., Ebenman B., Ernmerson M., Montoya J. M., Olesen J. M., Valido A., Warren P. H. 2005. Body size in ecological networks. Trends Ecol. Evol. 20, 402–409 10.1016/j.tree.2005.04.005 (doi:10.1016/j.tree.2005.04.005) [DOI] [PubMed] [Google Scholar]
- 86.Kartascheff B., Heckmann L., Drossel B., Guill C. 2009. Why allometric scaling enhances stability in food web models? Theor. Ecol. 3, 195–208 10.1007/s12080-009-0063-3 (doi:10.1007/s12080-009-0063-3) [DOI] [Google Scholar]
- 87.Vasseur D. A., McCann K. S. 2005. A mechanistic approach for modeling temperature-dependent consumer–resource dynamics. Am. Nat. 166, 184–198 10.1086/431285 (doi:10.1086/431285) [DOI] [PubMed] [Google Scholar]
- 88.Emmerson M., Bezemer M., Hunter M. D., Jones T. H. 2005. Global change alters the stability of food webs. Glob. Change Biol. 11, 490–501 10.1111/j.1365-2486.2005.00919.x (doi:10.1111/j.1365-2486.2005.00919.x) [DOI] [Google Scholar]
- 89.Gonzalez A., Lawton J. H., Gilbert F. S., Blackburn T. M., Evans-Freke I. 1998. Metapopulation dynamics, abundance, and distribution in a microecosystem. Science 281, 2045–2047 10.1126/science.281.5385.2045 (doi:10.1126/science.281.5385.2045) [DOI] [PubMed] [Google Scholar]
- 90.Gonzalez A., Chaneton E. J. 2002. Heterotroph species extinction, abundance and biomass dynamics in an experimentally fragmented microecosystem. J. Anim. Ecol. 71, 594–602 10.1046/j.1365-2656.2002.00625.x (doi:10.1046/j.1365-2656.2002.00625.x) [DOI] [Google Scholar]
- 91.Staddon P., Lindo Z., Crittenden P. D., Gilbert F., Gonzalez A. 2010. Connectivity, non-random extinction and ecosystem function in experimental metacommunities. Ecol. Lett. 13, 543–552 10.1111/j.1461-0248.2010.01450.x (doi:10.1111/j.1461-0248.2010.01450.x) [DOI] [PubMed] [Google Scholar]
- 92.Kolokotrones T., Savage V., Deeds E. J., Fontana W. 2010. Curvature in metabolic scaling. Nature 464, 753–756 10.1038/nature08920 (doi:10.1038/nature08920) [DOI] [PubMed] [Google Scholar]
- 93.Dossena M., Yvon-Durocher G., Grey J., Montoya J. M., Perkins D. M., Trimmer M., Woodward G. 2012. Warming alters community size structure and ecosystem functioning. Proc. R. Soc. B 279, 3011–3019 10.1098/rspb.2012.0394 (doi:10.1098/rspb.2012.0394) [DOI] [PMC free article] [PubMed] [Google Scholar]