Abstract
Adaptive accounts of modern low human fertility argue that small family size maximizes the inheritance of socioeconomic resources across generations and may consequently increase long-term fitness. This study explores the long-term impacts of fertility and socioeconomic position (SEP) on multiple dimensions of descendant success in a unique Swedish cohort of 14 000 individuals born during 1915–1929. We show that low fertility and high SEP predict increased descendant socioeconomic success across four generations. Furthermore, these effects are multiplicative, with the greatest benefits of low fertility observed when SEP is high. Low fertility and high SEP do not, however, predict increased descendant reproductive success. Our results are therefore consistent with the idea that modern fertility limitation represents a strategic response to the local costs of rearing socioeconomically competitive offspring, but contradict adaptive models suggesting that it maximizes long-term fitness. This indicates a conflict in modern societies between behaviours promoting socioeconomic versus biological success. This study also makes a methodological contribution, demonstrating that the number of offspring strongly predicts long-term fitness and thereby validating use of fertility data to estimate current selective pressures in modern populations. Finally, our findings highlight that differences in fertility and SEP can have important long-term effects on the persistence of social inequalities across generations.
Keywords: demographic transition, multigenerational, fertility, socioeconomic position, reproductive success, quality–quantity trade-off
1. Introduction
Evolutionary anthropologists argue that the physiological, cognitive and cultural mechanisms regulating human reproduction have evolved by natural selection to channel accumulated resources into the maximization of inclusive fitness (i.e. production of genetic descendants) [1,2]. Supporting this proposition, male socioeconomic success has been reported to be positively associated with reproductive success across a range of ‘traditional’ pre-industrial societies [3]. There is also some evidence that fertility patterns in traditional societies approximate local optima for maximizing fitness in the presence of resource allocation trade-offs between offspring quantity and quality [4–6, but see 7]; that is, trade-offs between the number of descendants and their ability to reproduce in turn. By contrast, in ‘modern’ post-industrial societies that have undergone demographic transition (i.e. the sequential decline in mortality and fertility observed with population-level socioeconomic development [8]), the lowest recorded fertility rates in human history now coincide with unprecedented material prosperity. This immediately seems at odds with adaptive models, because such prosperity ought to enable individuals to rear more children should they desire to do so [9]. Furthermore, in modern societies, the anticipated within-population positive associations between socioeconomic and reproductive success have been attenuated or even reversed [3,10–12]. Why this shift occurs is poorly understood by both evolutionary and non-evolutionary social scientists [11,13], but a persistent idea is that modernization favours reduced fertility by increasing the costs of rearing socioeconomically competitive offspring [1,14–16]. Consistent with this view, many studies indicate that low fertility substantially advances offspring education and wealth in modern societies [17–19]. There is also evidence that such benefits emerge with or are magnified by socioeconomic development [2,20], although direct tests of this hypothesis are rare [14].
Rising quantity–quality trade-offs with regard to the socioeconomic success of offspring have been incorporated into alternative evolutionary models of the demographic transition. Firstly, Kaplan has suggested that evolved psychological mechanisms may be maladaptive in the face of such novel costs of reproduction, favouring low fertility even if these do not enhance offspring quality in more direct ways (i.e. survival, mating or fertility) [1,21]. This model argues that humans have undergone selection for psychological mechanisms that lead them to strive for the culturally recognized goals of wealth and status, and to balance fertility against these goals, because until recently such advantages closely predicted offspring survival and reproduction. Modernization, however, combines (i) increased scope for socioeconomic competition between individuals, owing to engagement with modern labour-market economies with (ii) novel conditions where offspring survival is virtually guaranteed and where few individuals have insufficient resources to reproduce. This ‘maladaptive’ hypothesis is supported by studies showing that low fertility in modern populations advances offspring educational attainment and/or wealth, but does not increase offspring survival or fertility [22–25]. In contrast, other researchers have argued that immediate deficits in reproductive success may eventually yield adaptive increases in long-term fitness provided strong socioeconomic advantage is transmitted across generations [26]. This second, ‘adaptive’ hypothesis is supported by a number of formal theoretical models [26–29], but a dearth of high-quality multigenerational data means that empirical tests are lacking. This study provides a powerful test of these competing hypotheses by considering associations between reproductive and socioeconomic success in a modern society, over both the short- and long-term. To do this, we use data from the Uppsala Multigenerational Birth Cohort study (UBCoS), a unique Swedish dataset that tracks 14 000 individuals born in the early 1900s and all their descendants to the present day.
Understanding relationships between fertility, socioeconomic advantage and long-term descendant socioeconomic and reproductive success is also of wider importance for the biological and social sciences. Firstly, multigenerational analyses can provide crucial validation for research into long-term patterns of natural selection. In recent years, there has been much interest in using fertility data to estimate the direction and strength of natural selection currently acting in modern human populations [12,30–32]. Among the most consistent findings is that, using lifetime number of offspring as a measure of reproductive success, both sexes are under selection for earlier age at first birth in both traditional and modern populations [31]. One study with unusually rich physiological data also reported selection in modern US women for shorter height, lower total cholesterol and lower systolic blood pressure, leading the authors to conclude that ‘natural selection is acting slowly and gradually on traits of medical importance and on life history traits’ [30, p. 1790]. Many of these studies, however, assume that lifetime number of offspring is an effective proxy for long-term genetic fitness, an assumption that would be invalidated if high fertility compromised descendant reproductive success. Our data enable us to examine this assumption explicitly by quantifying relationships between short- and long-term fitness. Secondly, multigenerational data are required to assess the long-term implications of modern inequalities in fertility and socioeconomic position (SEP). It is already well known that high parental fertility carries important costs for offspring health, education and socioeconomic success in modern societies [19]. Conversely, even within well-functioning welfare states, high parental SEP is a strong predictor of positive outcomes across a multiple domains of child and adult well-being [33]. Recent studies have also suggested that these effects interact, with the benefits of fertility limitation being particularly large in high SEP families [2]. Very little, however, is known about how fertility or SEP affect quantity and quality of grandchildren and later descendants. There is also very little research examining how far any long-term effects of fertility may be mediated by differences in the socioeconomic success of intervening generations, or vice versa.
(a). Research aims and hypotheses
Our primary aim is to examine how and why parental fertility and SEP affect short- and long-term descendant socioeconomic and reproductive success. Specifically, we test the following hypotheses: (i) that high parental SEP and low parental fertility increase descendant socioeconomic success across generations; (ii) that high parental SEP and low parental fertility increase descendant reproductive success across generations; (iii) that high parental SEP and low parental fertility interact such that the benefits of fertility limitation are greatest in high SEP families; and (iv) that following hypotheses i–ii, long-term reproductive success is maximized at an intermediate fertility level. We also address the methodological aim of examining the validity of lifetime number of offspring as a measure of long-term reproductive success in modern low-fertility societies, and compare its performance with the alternative measures of number of grandchildren or great-grandchildren.
In testing these hypotheses using data from across four generations, we extend previous research in post-demographic transition populations that has focused solely upon effects in the offspring generation [22–24] (but see [32] for an examination of longer-term outcomes in a pre-demographic transition population). The UBCoS dataset also provides a number of additional advantages. First, it is a large, population-based cohort that is known to be representative of the Swedish population at large in terms of infant mortality and fertility [25]. By contrast, previous studies of modern populations have used smaller and potentially biased samples, such as opportunistic sampling of men (only) at service stations [23] or drawing study populations from US military personnel and German physicians [24]. Secondly, UBCoS suffers from remarkably little loss to follow-up, allowing us to trace 96 per cent of all cohort members (and all their registered descendants) up to 2009. Finally, high-quality data are available on an unusually wide range of measures for each individual, enabling us to examine effects upon descendant survival, marriage, reproduction, school achievement, educational continuation and family income.
2. Methods
(a). Sample selection and early-life characteristics
Our sample comprises all live births at the Uppsala University Hospital between 1915 and 1929. This hospital delivered an estimated 75 per cent of births in Uppsala city and 50 per cent of births in surrounding rural parishes. From a total of 14 192 births, 13 811 (98%) were successfully traced through parish archives until death, emigration or until being assigned a unique personal number in 1947. Of these, we excluded 139 cohort members (henceforth ‘G1s’ from ‘generation one’) who emigrated permanently before reaching 60 years in age, leaving a study population of 13 672 (7178 male) G1s. This birth cohort is representative of Sweden nationally in terms of infant mortality and fertility [25], albeit with a somewhat higher proportion of infants from urban areas (46% versus 31% nationally [34]).
Archived obstetric records provided data on G1 birthweight, gestational age and twin/triplet status. These records also provided information on cohort members' parents (‘G0s’), including mother's age, marital status and household head SEP (henceforth ‘parental SEP’: further details in electronic supplementary material, S1). Finally, we assigned parental fertility as being equal to the total number of children (including deceased children) belonging to the parents' household in the 1930 census. Where this information was not available (47% of sample), we instead used the mother's maximum-recorded parity in obstetric records (r = 0.89 for correlation with the census data: details in electronic supplementary material, S1).
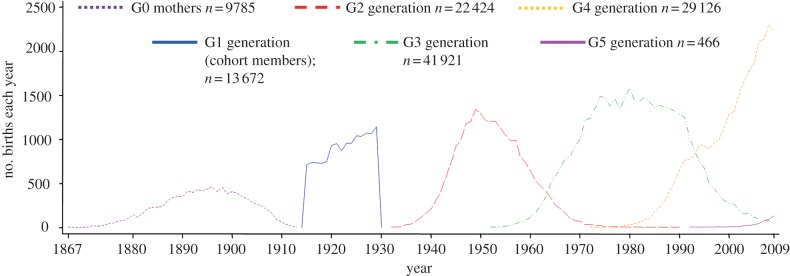
We supplemented this data on parents (G0s) and offspring (G1s) by linking G1s to all biological descendants born up to 31 December 2009, using the Swedish Multigenerational Register (estimated completeness 97.7% for paternity, 99.6% for maternity: see [25]). As judged by the distribution of birth years, the grandchild (‘G2s’) and great-grandchild (‘G3s’) generations were essentially complete by 2009; the great-great-grandchild generation (‘G4s’) was in a relatively early phase; and the great-great-great-grandchild generation (‘G5s’) had just begun (figure 1).
Figure 1.
Distribution of year of birth for UBCoS cohort members, parents and biological descendants. Data presented on G0 mothers only, because we did not have data on the age of G0 fathers.
(b). Descendant socioeconomic and reproductive success
We operationalized descendant ‘quality’ in terms of both socioeconomic success (which is expected to have indirectly increased descendants' ability to reproduce in our evolutionary past) and also in terms of more direct measures of reproductive success. For all G1s, G2s and G3s we used Swedish Register data to assign three indicators of socioeconomic success: (i) school marks: standardized average marks across all compulsory subjects in elementary school (collected age 10 in G1s, age 16 in descendants); (ii) entering university: ever entering university or equivalent, if aged 21 or over; and (iii) family income: disposable family income, standardized each calendar year by age and sex and then averaged across all available calendar years in which the descendant was aged 21–65. We also used three more direct measures of reproductive success: (iv) survival to age 16; (v) mating success: marriage before age 40, if survived to age 16; and (vi) fertility: number of offspring up to 2009. Further details of calculation are in electronic supplementary material, S1, including details of instances where some measures were not available for all generations. From these individual-level outcomes, we then generated averages across all available descendants in each generation (e.g. mean G1 school marks, proportion of G1s surviving to age 16).
(c). Estimated fitness of G1s
The increasing overlap between generations (figure 1) motivated us to create a measure of long-term fitness that combined descendants across generations. For each descendant, we calculated their reproductive value as their expected total number of additional future offspring given their sex, age and parity in 2009. We used a lifetable approach that assumed the continuation of 2009 mortality and fertility rates in the total Swedish population (electronic supplementary material, S1). We then multiplied this expected number of future offspring by the descendant's coefficient of relatedness to the G1 cohort member, i.e. 0.5 for G2s, 0.25 for G3s, 0.125 for G4s and 0.0625 for G5s. By summing this product across all descendants, we obtained the estimated direct fitness of each G1. This can be interpreted as the expected number of future times in which each living descendant would pass on the G1's genes to the next generation.
(d). Statistical analysis
We used multivariable regression to investigate the effect of G0 parental fertility and SEP upon the (average) socioeconomic and reproductive success of their G1, G2 and G3 descendants. To facilitate comparisons of effect sizes across generations and across outcomes, we standardized all outcomes for each generation and used these in linear regression analysis. The only exception was for our three binary outcomes (G1 survival, entering university and marriage), for which we used logistic regression and converted the log-odds to effect sizes [35]. We adjusted all analyses for G1 birthweight, gestational age, twin/triplet status, mother's age, mother's marital status and birth year (all correlation coefficients between early-life characteristics were less than 0.5: see electronic supplementary material, S2). We calculated CIs using robust standard errors clustered by G0 mother. We combined males and females, unless there was evidence of a sex interaction (p < 0.05), but present sex-stratified analyses in electronic supplementary material, S3. We also tested for interactions between G0 parental fertility and SEP. All tests for interaction are reported in electronic supplementary material, S3, and all significant interactions are reported in the text and/or figures.
To explore mediation across generations, we fitted linear structural equation models for the G2 and G3 outcomes, using the same independent variables and including as mediators (i) intervening SEP (e.g. G1's adult education) and (ii) intervening fertility (e.g. G1's number of offspring). We fitted these models with a robust maximum-likelihood estimator using Gaussian integration with 24 quadrature points (see electronic supplementary material, S3).
We addressed our aim of validating total number of children as a predictor of long-term fitness in two ways. Firstly, we calculated the Pearson's correlation between G1 number of offspring (G2 generation) and total estimated fitness. We compared this with the correlations observed for G1 number of grandchildren (G3s) and great-grandchildren (G4s). Secondly, we used multivariable linear regression analyses to compare the early-life predictors of these four measures of G1 reproductive success, standardizing outcomes to facilitate comparisons. Our purpose in running these analyses was the methodological aim of establishing whether these four alternative measures generated similar findings. See Goodman & Koupil [25] for a detailed consideration of which early-life characteristics predict reproductive success, how this differs by sex and which mortality/mating/fertility pathways mediate associations.
All analyses handled missing data on early-life characteristics (0–3.5% missing data) under an assumption of missing at random. We used maximum-likelihood estimation in MPlus and used multiple imputation by chained equations in Stata (five imputations).
3. Results
Among the G0s, average fertility was 3.2 offspring. Their children, the G1 cohort members, had a mean of 1.7 offspring (2.3 for those with at least one offspring), and these grandchildren (G2s) had in turn a mean of 1.8 offspring (2.3 if at least one offspring). As of 2009, these great-grandchildren (G3s) had a mean of 0.7 offspring (1.9 if at least one offspring).
(a). Fertility, socioeconomic position and descendant socioeconomic success
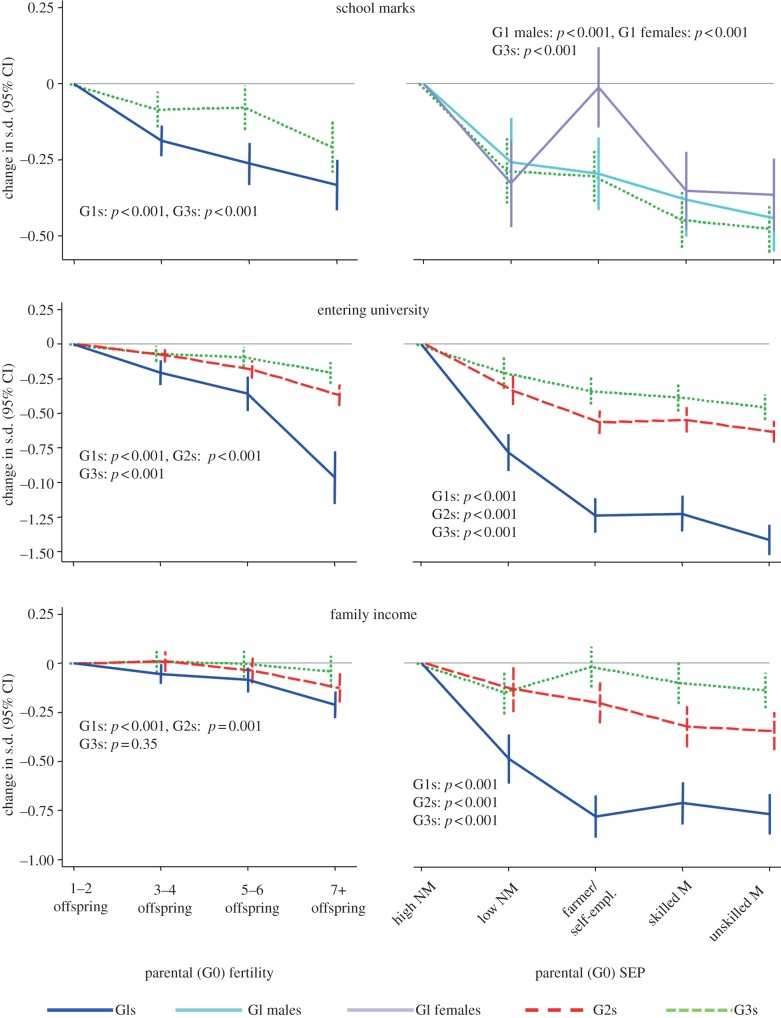
Figure 2 presents the adjusted effects of parental fertility and SEP upon our measures of descendant socioeconomic success. The underlying correlation coefficients are presented in electronic supplementary material, S2, while electronic supplementary material, S3 tabulates the data and also presents R2 values, raw means and percentages, unadjusted analyses and sex-stratified analyses. Among both male and female G1 cohort members, both lower parental fertility and higher parental SEP were independently associated with substantially higher school marks, educational level and family income. The effects of parental SEP were particularly large. For example, high/mediate non-manual SEP versus unskilled manual SEP was associated with an adjusted effect size of 0.41 standard deviations for school marks; 1.41 for entrance to university; and 0.77 for family income (figure 2). The corresponding effect sizes for parental fertility of 7 or more offspring versus 1–2 offspring were 0.33, 0.96 and 0.21 (figure 2, left-hand). There was also evidence that, as predicted, the benefits of low parental fertility were particularly large for G1s born into families of high SEP (p≤0.03 for interaction for all three measures of G1 socioeconomic success; see electronic supplementary material, S3 and see also figure 3 for a graph of the interaction with respect to the educational level). Moreover, most of these effects persisted to the G2s and G3s, including interactions with respect to educational level. There was strong evidence that all the multigenerational effects presented in figure 2 were substantially mediated by intervening SEP, with the magnitude of this indirect path always being at least half that of the total effect (see electronic supplementary material, S3). By contrast, there was always little or no evidence of an indirect path via intervening fertility.
Figure 2.
Effect of parental (G0) fertility and SEP upon descendant socioeconomic success. CI, confidence interval; s.d., standard deviations; SEP, socioeconomic position; high NM, high/mediate non-manual; low NM, low non-manual; farmer/self-empl., farmer or self-employed; skilled M, skilled manual; unskilled M, unskilled manual. p-values are from regression models adjusting for G1 early-life characteristics, and are for heterogeneity for SEP and for linear trend for parental fertility. See electronic supplementary material, S3 for numbers of individuals, for raw data and unadjusted analyses.
Figure 3.
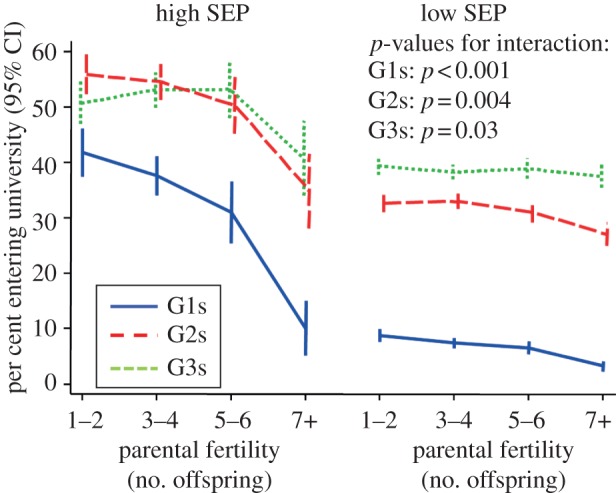
Interactions between parental (G0) fertility and SEP in predicting descendant entrance to university. CI, confidence interval; SEP, socioeconomic position. ‘high SEP’ defined as high, mediate and low non-manual SEP; ‘low SEP’ defined as skilled manual, unskilled manual, farmer or self-employed SEP. p-values for interactions are from regression models adjusting for G1 early-life characteristics; full results of all tests for interactions in electronic supplementary material, S3.
These results therefore supported hypotheses 1 and 3: lower parental fertility and higher parental SEP increased offspring educational and socioeconomic quality, particularly when low fertility and high SEP coincided; and these advantages were in turn transmitted to subsequent generations. Nevertheless, the size of these effects generally attenuated across generations as did the proportion of variation explained. For example, R2 values indicated that an additional 11.2 per cent of variation in G1 education was explained by parental SEP and 2.0 per cent by parental fertility, as compared with 3.2 per cent and 1.1 per cent for G2 educational level, and 1.6 per cent and 0.3 per cent for G3 educational level (see electronic supplementary material, S3).
(b). Fertility, socioeconomic position and descendant reproductive success
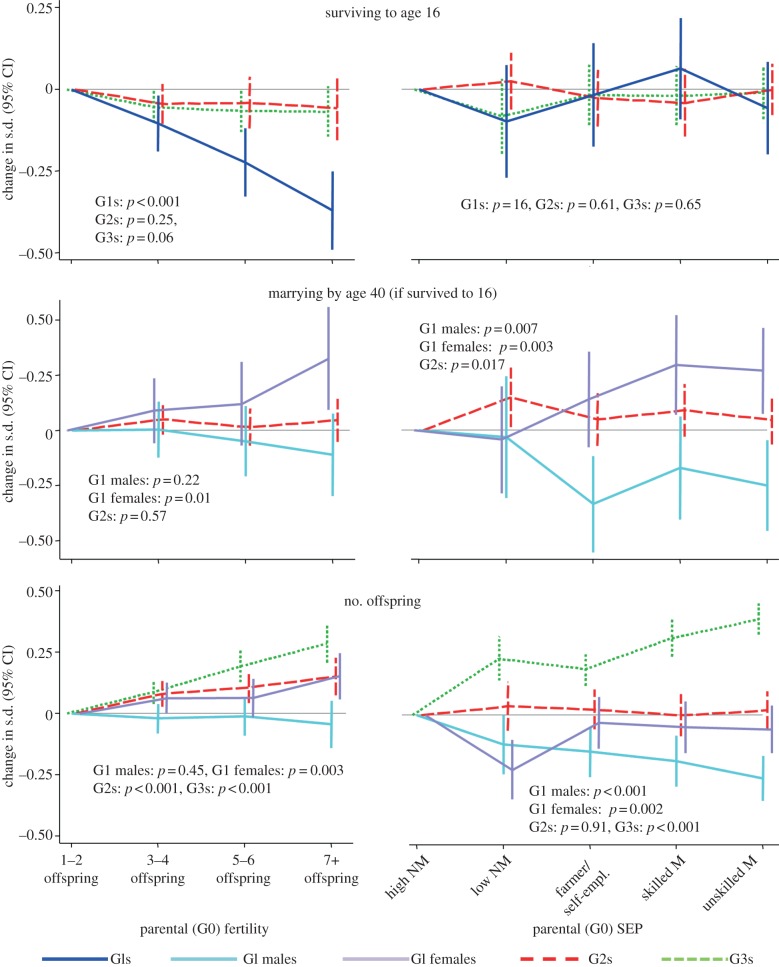
Figure 4 demonstrates that, contrary to our second hypothesis, low parental fertility and high parental SEP either did not affect reproductive success beyond the G1 generation (survival to age 16, marriage by age 40, G2 fertility) or if anything showed a negative effect in subsequent generations (G3 fertility). For example, higher parental SEP predicted more offspring among G1s, particularly among G1 males (a sex interaction driven by high rates of childlessness among low SEP men: see electronic supplementary material, S3) and also particularly among G1s from smaller families (p < 0.001 for interaction: see electronic supplementary material, S3). Higher parental SEP had no effect upon total number of offspring among G2s, however, and predicted fewer offspring among G3s.
Figure 4.
Effect of parental (G0) fertility and SEP upon descendant reproductive success. For abbreviations see figure 2.
These intergenerational effects on number of offspring persisted after excluding childless individuals, and were once again mediated to a substantial degree by intervening SEP and hardly at all by intervening fertility (see electronic supplementary material, S3). Further exploratory analyses indicated that the crucial, socioeconomically patterned factor was the longer generation time in the descendants of G0 parents of high SEP. Age at first childbearing was 27.2 versus 25.8 years for G1s descended from G0s of high versus low SEP; 27.6 versus 26.0 years for G2s; and 27.4 versus 26.8 years for G3s (all p < 0.001). The result was that by 2009 the G3 descendants of high SEP lineages were on average younger and had also started child-bearing later. In path analyses, there was little or no evidence that any direct association remained between G0 SEP and G3 fertility once G1, G2 and G3 age at first childbearing were included as mediators (see electronic supplementary material, S3).
(c). Optimal fertility levels for maximizing long-term reproductive success
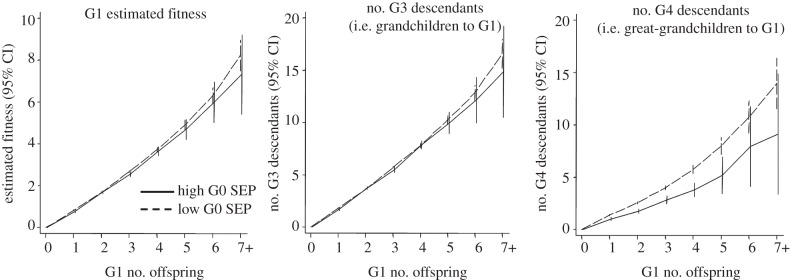
The shorter generation time of low SEP lineages meant that, at any given number of children, G1s from high SEP lineages had fewer G4 descendants (an incomplete generation) despite having a comparable number of G3 descendants (a complete generation). Figure 5 illustrates this, and also demonstrates that in neither SEP group did intermediate G1 fertility maximize number of G3 descendants, number of G4 descendants or the G1s total ‘estimated fitness’. Instead, all associations were essentially linear across the full range of G1 fertility, with no suggestion of an inverted U-shaped relationship or even of any flattening of the line at the high end. This therefore provided evidence against our fourth hypothesis that intermediate fertility would maximize long-term reproductive success. It also indicated that the optimum number of offspring was far above the observed population mean (1.7 offspring for all G1s, 2.3 for those with at least one child).
Figure 5.
Association between G1 fertility and subsequent reproductive success, stratified by parental (G0) SEP.
(d). Measuring long-term fitness
Finally, we turned to our methodological aim of examining the validity of total number of offspring as a measure of long-term fitness in modern societies. As illustrated in figure 5, these variables were strongly correlated among G1 cohort members, with a Pearson's correlation coefficient (r) of 0.84 and an R2 value of 0.71 (i.e. 71% of the variance explained). Number of grandchildren showed an even stronger correlation with total estimated fitness (r = 0.97, R2 = 0.94), but number of great-grandchildren showed a notably weaker correlation (r = 0.73, R2 = 0.53: results similar when stratified by sex, SEP or parental fertility, see electronic supplementary material, S3). This weaker correlation results from the fact that the G4 generation is incomplete, and its size is therefore subject to additional ‘noise’ introduced by differences in the birth year of the G1s and in the age at which the G1s and their descendants reproduced.
The effects of this additional noise were also apparent when we examined which G1 early-life characteristics independently predicted our four alternative measures of G1 fitness (table 1; sex-stratified results in electronic supplementary material, S3; see also [25]). Once again, number of children performed well relative to our measure of total estimated fitness, generally showing similar effect sizes and significance levels. The only exception was an underestimation of the advantage of high parental fertility, an advantage that was comparatively smaller in the first generation because it was partly offset by higher mortality (figure 4; see also [25]). Number of grandchildren performed even better, showing identical substantive findings and near-identical effect sizes. By contrast, number of great-grandchildren showed several substantial differences, including much stronger advantages associated with female sex and higher parental fertility; a novel positive association with unmarried mother status; and a reversal of the direction of the effect of SEP. These discrepancies were again driven by differences in generation length, which on average was shorter for female G1s (mean age first childbearing 24.6 years versus 27.4 in G1 males), for G1s from larger families (25.6 years in families of size 5 or more versus 26.2 years in families of size 1–4), for G1s with unmarried mothers (25.3 years versus 26.2 years in ever-married mothers) and for G1s, G2 and G3s alike from low SEP lineages (see above).
Table 1.
Early-life predictors of four alternative measures of reproductive success among G1 cohort members, born 1915–1929 (n = 13 672). For abbreviations see figure 2. Regression coefficients from linear regression, adjusting for all variables in column plus year of birth. Variables in bold are p ≤ 0.05; see electronic supplementary file S3 for confidence intervals.
| per cent/mean (s.d.) among G1s | regression coefficients from linear regression, standardizing all outcomes |
||||
|---|---|---|---|---|---|
| total estimated fitness | no. G2 children | no. G3 grandchildren | no. G3 great-grandchildren | ||
| R2 = 0.017 | R2 = 0.026 | R2 = 0.017 | R2 = 0.049 | ||
| female sex | 47% | 0.08 | 0.09 | 0.09 | 0.22 |
| birthweight, change per kg | 3.4 (0.6) | 0.13 | 0.16 | 0.14 | 0.08 |
| preterm birth | 9% | −0.07 | −0.14 | −0.07 | −0.05 |
| twin/triplet status | 3% | 0.01 | −0.01 | 0.01 | 0.02 |
| mother's age, change per decade | 28.4 (6.5) | −0.09 | −0.08 | −0.09 | −0.10 |
| unmarried mother | 20% | 0.00 | 0.00 | 0.00 | 0.06 |
| family size 1–2 | 28% | 0 | 0 | 0 | 0 |
| family size 3–4 | 39% | 0.05 | 0.02 | 0.05 | 0.08 |
| family size 5–6 | 19% | 0.07 | 0.02 | 0.06 | 0.14 |
| family size 7+ | 14% | 0.12 | 0.05 | 0.11 | 0.24 |
| high NM parent SEP | 9% | 0 | 0 | 0 | 0 |
| low NM parent SEP | 7% | −0.12 | −0.16 | −0.12 | 0.08 |
| farmer/self-empl. | 19% | −0.05 | −0.09 | −0.06 | 0.12 |
| skilled M parent SEP | 15% | −0.09 | −0.12 | −0.10 | 0.17 |
| unskilled M parent SEP | 50% | −0.10 | −0.16 | −0.12 | 0.20 |
Thus, number of children and, even better, number of grandchildren were strongly correlated with long-term estimated fitness and yielded similar substantive findings with respect to the early-life predictors of long-term fitness. Moreover, these two completed generations performed better than a more recent but incomplete generation (great-grandchildren), which showed a weaker correlation and which generated biased effect sizes for several characteristics. These findings suggest that, at least with respect to modern societies, researchers seeking to estimate long-term fitness should use the youngest completed generation available. Furthermore, if total number of offspring (‘lifetime fertility’) is the youngest generation available, then this seems generally to provide an adequate proxy for longer-term fitness.
4. Discussion
Our study contributes substantially to current understanding of the adaptive status of key phenotypic traits and the long-term dynamics of natural selection in modern human populations. Using high-quality multigenerational data from Sweden (1915–2009), we estimate for the first time the effects of fertility and SEP on multiple dimensions of descendant success and across four generations. Across two generations (i.e. from parents to offspring), we replicate previous findings in demonstrating that low family SEP predicts lower fertility, an effect that (in line with previous research [12,36]) is particularly strong in males and is largely driven by higher rates of childlessness. Beyond two generations, however, we find no evidence for a predicted life-history trade-off between the quantity and quality of descendants: relatively high fertility did not compromise the survival, mating success or reproductive success of grandchildren or great-grandchildren. This adds important support to previous studies reporting similar results in other post-demographic transition European and American populations, but with shorter follow-up (offspring generation only) and using smaller and less representative samples [23,24]. Taken together, these findings suggest that fertility limitation in modern populations is unlikely to increase direct fitness even in the long term. This contradicts adaptive accounts of fertility limitation that have previously been supported through a series of theoretical models [26–29]. Our study therefore adds to the evidence that a satisfying evolutionary account of the demographic transition may require perspectives that explicitly model pathways to maladaptive decision-making, including adaptive lags in the face of environmental mismatch [11].
We do, however, find strong support for the prediction that fertility limitation in modern societies enhances descendant socioeconomic success. Thus, our results indicate that reproductive behaviours that promote biological success (i.e. long-term genetic fitness) are in conflict with those that promote descendant socioeconomic success in modern populations. Specifically, we find that both low parental fertility and high parental SEP independently predict higher school marks, educational level and income, and this is generally true in male and female descendants alike (see [37] for a discussion of the one minor exception, concerning school marks in the children of farmers). Moreover, these associations persist up to at least the great-grandchild generation, reflecting the advantage of starting one's own offspring on a favourable socioeconomic trajectory.
We also demonstrate for the first time a multigenerational interaction between SEP and fertility, such that the socioeconomic benefits of low fertility were especially large in groups that already had high SEP. This finding adds to a number of recent studies indicating that demographic modernization is associated with increased socioeconomic pay-offs to fertility limitation for the wealthiest families (reviewed in [2]). These differential consequences of low and high fertility across socioeconomic groups may stem from several related mechanisms. Kaplan suggests that direct wealth transfers and investments in skill-acquisition in modern economies dramatically increase a descendant's ability to generate new wealth and further invest in their own status, leading to magnified returns to strategies of low fertility and high parental investment [1]. Simultaneously, socioeconomic advantage may reduce the negative impact of extrinsic risks (e.g. environmental shocks) and so increase the relative importance of parental investment. Finally, Downey distinguishes between ‘base’ and ‘surplus’ forms of specifically educational investment [7]. In modern societies, he argues, base investments in schooling are covered by the welfare state and so available to low SEP families irrespective of family size [17]. High SEP families have, however, potentially also got access to expensive surplus investments (e.g. extra tuition or private schooling), which are consequently more subject to resource dilution effects as family size increases. Whichever mechanisms apply, our findings support both evolutionary [1,2,21] and non-evolutionary [14,16] accounts of the demographic transition that view modern fertility limitation as motivated by the socioeconomic advantages it bestows on offspring.
We also make a broader methodological contribution to the study of natural selection in human populations. Our findings provide the best empirical evidence to date that the total number of offspring is a valid proxy for long-term fitness in modern low-fertility societies, with the two measures being highly correlated and generally yielding similar substantive findings regarding the correlates of reproductive success. Most studies in this field have data on only one or two generations [31]. For studies with longer-term follow-up, we exemplify how generations increasingly overlap with time and show one method for combining information across all generations in a single measure of estimated fitness (the sum of each descendant's reproductive value multiplied their coefficient of relatedness to the index cohort member). If data are lacking for this single summary measure, we find that the size of completed generations provides a better proxy for long-term fitness than the size of a more recent but incomplete generation. This is because the latter is also affected by factors that predicted generation time, highlighting our somewhat counterintuitive finding that, although earlier child-bearing has consistently been found to increase reproductive success at the individual level [31], earlier average child-bearing does not necessarily increase reproductive success at the lineage level. Getting a ‘head start’ by earlier childbearing is only expected to confer a selective advantage if (i) earlier child-bearing also predicts a greater total number of offspring and/or (ii) if average fertility levels are above the replacement rate of 2.1 (i.e. if the population is expanding). By contrast if both early- and late-child-bearing lineages have similar total fertility (as was the case in our study for high SEP and low SEP lineages), and if the population as a whole has below-replacement fertility (which currently applies to more than half the world's population [8]), then shorter generation time may simply speed up a lineage's trajectory towards extinction. Yet despite not affecting reproductive success, earlier average childbearing may still increase the speed of selective responses in low versus high SEP lineages, and may therefore still be relevant for understanding the dynamics of current evolution in modern populations.
Our analyses highlight the value of using multigenerational datasets to examine evolutionary perspectives on human health and behaviour [31,32]. They do, however, leave a number of questions unaddressed, which warrant future investigation. Firstly, we do not have access to information on the genetic basis of the traits we examine. These data are necessary to evaluate fully whether the fitness consequences of fertility and SEP are leading to new trajectories of genetic change over time [30]. Secondly, our focus here has been upon the long-term fitness implications of wealth and fertility for one particular generation (the G0s). Future analyses are required to model in more detail the underlying processes of social (im)mobility across generations, to explore how these interact with reproductive decisions such as age at first childbearing, and to examine how these effects may be changing over time. Similarly, because our hypotheses concern average effects of parental fertility and SEP upon descendant quality, we have not examined in detail potential differences in the dynamics of quality transmission across different types of descendants (e.g. males versus females [22]). Finally, although broadly representative of early twentieth-century Sweden, our birth cohort captures only one ‘index’ generation from a particular part of one Western country. Replication of these findings using multigenerational data in other settings is therefore required to draw firm conclusions about the generalizability of our results. Yet insofar as our findings across two generations are generally consistent with previous research in other high-income countries, there is some reason to believe that our novel multigenerational findings may also apply in other, similar populations. Indeed, one might expect the socioeconomic effects we observe to be stronger in settings that do not enjoy Sweden's unusually strong welfare state and unusually low levels of income inequality [33]. Thus, from a broader social policy perspective, our findings highlight the continued need for policies that equalize opportunities across children in modern societies [33], including with respect to characteristics such as family size which typically receive far less attention than socioeconomic differences.
Acknowledgements
UBCoS was approved by the Regional Ethics committee in Stockholm (dnr 03-117, dnr 04-944T and dnr 2009/1115-32) and is funded by the Swedish Research Council (grant no. 2006-7498) and Swedish Council for Working Life and Social Research (grant no. 2007-1010). The latter organization also funds I.K., while the Leverhulme Trust funds D.W.L. Contact Ilona Koupil (ilona.koupil@chess.su.se) for details of how to access UBCoS data. Many thanks to A. Makolli, B. Modin, I. Ljungkvist, L. Svensson L. Holmberg R. Mohsen and R. Österman for contributions to data collection and management.
References
- 1.Kaplan H. S. 1996. A theory of fertility and parental investment in traditional and modern human societies. Yearb. Phys. Anthropol. 39, 91–135 (doi:10.1002/(SICI)1096-8644(1996)23+<91::AID-AJPA4>3.0.CO;2-C) [DOI] [Google Scholar]
- 2.Lawson D. W., Mace R. 2011. Parental investment and the optimization of human family size. Phil. Trans. R. Soc. B 366, 333–343 10.1098/rstb.2010.0297 (doi:10.1098/rstb.2010.0297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hopcroft R. L. 2006. Sex status and reproductive success in the contemporary United States. Evol. Hum. Behav. 27, 104–120 10.1016/j.evolhumbehav.2005.07.004 (doi:10.1016/j.evolhumbehav.2005.07.004) [DOI] [Google Scholar]
- 4.Strassmann B. I., Gillespie B. 2002. Life-history theory, fertility and reproductive success in humans. Proc. R. Soc. Lond. B 269, 553–562 10.1098/rspb.2001.1912 (doi:10.1098/rspb.2001.1912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borgerhoff Mulder M. 2000. Optimizing offspring: the quantity–quality tradeoff in agropastoral Kipsigis. Evol. Hum. Behav. 21, 391–410 10.1016/S1090-5138(00)00054-4 (doi:10.1016/S1090-5138(00)00054-4) [DOI] [PubMed] [Google Scholar]
- 6.Gillespie D. O. S., Russell A. F., Lummaa V. 2008. When fecundity does not equal fitness: evidence of an offspring quantity versus quality trade-off in pre-industrial humans. Proc. R. Soc. B 275, 713–722 10.1098/rspb.2007.1000 (doi:10.1098/rspb.2007.1000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawson D. W., Alvergne A., Gibson M. A. In press. The life history trade-off between fertility and child survival. Proc. R. Soc. B (doi:10.1098/rspb.2012.1635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee R. 2003. The demographic transition: three centuries of fundamental change. J. Econ. Perspect. 17, 167–190 10.1257/089533003772034943 (doi:10.1257/089533003772034943) [DOI] [Google Scholar]
- 9.Vining D. R. 1986. Social versus reproductive success: the central theoretical problem of human sociobiology. Behav. Brain Sci. 9, 167–216 10.1017/S0140525X00021968 (doi:10.1017/S0140525X00021968) [DOI] [Google Scholar]
- 10.Perusse D. 1993. Cultural and reproductive success in industrial-societies: testing the relationship at the proximate and ultimate levels. Behav. Brain Sci. 16, 267–283 10.1017/S0140525X00029939 (doi:10.1017/S0140525X00029939) [DOI] [Google Scholar]
- 11.Borgerhoff Mulder M. 1998. The demographic transition: are we any closer to an evolutionary explanation? Trends Ecol. Evol. 13, 266–270 10.1016/S0169-5347(98)01357-3 (doi:10.1016/S0169-5347(98)01357-3) [DOI] [PubMed] [Google Scholar]
- 12.Nettle D., Pollet T. V. 2008. Natural selection on male wealth in humans. Am. Nat. 172, 658–666 10.1086/591690 (doi:10.1086/591690) [DOI] [PubMed] [Google Scholar]
- 13.Caldwell J. C., Caldwell B. K., Caldwell P., McDonald P. F., Schindlmayr T. 2006. Demographic transition theory. Dordrecht, The Netherlands: Springer [Google Scholar]
- 14.Van Bavel J., Moreels S., Van de Putte B., Matthijs K. 2011. Family size and intergenerational social mobility during the fertility transition: evidence of resource dilution from the city of Antwerp in nineteenth century Belgium. Demogr. Res. 24, 313–343 10.4054/DemRes.2011.24.14 (doi:10.4054/DemRes.2011.24.14) [DOI] [Google Scholar]
- 15.Robinson W. C. 1997. The economic theory of fertility over three decades. Popul. Stud. 51, 63–74 10.1080/0032472031000149736 (doi:10.1080/0032472031000149736) [DOI] [PubMed] [Google Scholar]
- 16.Becker G. S. 1991. A treatise on the family, 2nd edn Cambridge, MA: Harvard University Press [Google Scholar]
- 17.Downey D. B. 2001. Number of siblings and intellectual development. The resource dilution explanation. Am. Psychol. 56, 497–504 10.1037/0003-066X.56.6-7.497 (doi:10.1037/0003-066X.56.6-7.497) [DOI] [PubMed] [Google Scholar]
- 18.Steelman L., Powell B., Werum R., Carter S. 2002. Reconsidering the effects of sibling configuration: recent advances and challenges. Annu. Rev. Sociol. 28, 243–269 10.1146/annurev.soc.28.111301.093304 (doi:10.1146/annurev.soc.28.111301.093304) [DOI] [Google Scholar]
- 19.Lawson D. W., Mace R. 2010. Optimizing modern family size. Hum. Nat. 21, 39–61 10.1007/s12110-010-9080-6 (doi:10.1007/s12110-010-9080-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maralani V. 2008. The changing relationship between family size and educational attainment over the course of socioeconomic development: evidence from Indonesia. Demography 45, 693–717 10.1353/dem.0.0013 (doi:10.1353/dem.0.0013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan H., Lancaster J. B., Tucker W. T., Anderson K. G. 2002. Evolutionary approach to below replacement fertility. Am. J. Hum. Biol. 14, 233–256 10.1002/ajhb.10041 (doi:10.1002/ajhb.10041) [DOI] [PubMed] [Google Scholar]
- 22.Kaptijn R., Thomese F., van Tilburg T. G., Liefbroer A. C., Dorly J. H. D. 2010. Low fertility in contemporary humans and the mate value of their children: sex-specific effects on social status indicators. Evol. Hum. Behav. 31, 59–68 10.1016/j.evolhumbehav.2009.07.007 (doi:10.1016/j.evolhumbehav.2009.07.007) [DOI] [Google Scholar]
- 23.Kaplan H. S., Lancaster J. B., Bock J. A., Johnson S. E. 1995. Fertility and fitness among Albuquerque men: a competitive labour market theory. In Human reproductive decisions (ed. Dunbar R. I. M.), pp. 96–136 London, UK: St Martin's Press [Google Scholar]
- 24.Mueller U. 2001. Is there a stabilizing selection around average fertility in modern human populations? Popul. Dev. Rev. 27, 469–498 10.1111/j.1728-4457.2001.00469.x (doi:10.1111/j.1728-4457.2001.00469.x) [DOI] [Google Scholar]
- 25.Goodman A., Koupil I. 2009. Social and biological determinants of reproductive success in Swedish males and females born 1915–1929. Evol. Hum. Behav. 30, 329–341 10.1016/j.evolhumbehav.2009.03.007 (doi:10.1016/j.evolhumbehav.2009.03.007) [DOI] [Google Scholar]
- 26.McNamara J. M., Houston A. I. 2006. State and value: a perspective from behavioural ecology. In Social information transmission and human biology (eds Wells J. C. K., Strickland S. S., Laland K. N.), pp. 59–88 London, UK: Taylor and Francis [Google Scholar]
- 27.Hill S. E., Reeve H. K. 2005. Low fertility in humans as the evolutionary outcome of snowballing resource games. Behav. Ecol. 16, 398–402 10.1093/beheco/ari001 (doi:10.1093/beheco/ari001) [DOI] [Google Scholar]
- 28.Boone J. L., Kessler K. L. 1999. More status or more children? Social status, fertility reduction, and long-term fitness. Evol. Hum. Behav. 20, 257–277 10.1016/S1090-5138(99)00011-2 (doi:10.1016/S1090-5138(99)00011-2) [DOI] [Google Scholar]
- 29.Mace R. 1998. The coevolution of human fertility and wealth inheritance strategies. Phil. Trans. R. Soc. Lond. B 353, 389–397 10.1098/rstb.1998.0217 (doi:10.1098/rstb.1998.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byars S. G., Ewbank D., Govindaraju D. R., Stearns S. C. 2010. Natural selection in a contemporary human population. Proc. Natl Acad. Sci. USA 107, 1787–1792 10.1073/pnas.0906199106 (doi:10.1073/pnas.0906199106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stearns S. C., Byars S. G., Govindaraju D. R., Ewbank D. 2010. Measuring selection in contemporary human populations. Nat. Rev. Genet. 11, 611–622 10.1038/nrn2908 (doi:10.1038/nrn2908) [DOI] [PubMed] [Google Scholar]
- 32.Courtiol A., Pettay J. E., Jokela M., Rotkirch A., Lummaa V. 2012. Natural and sexual selection in a monogamous historical human population. Proc. Natl Acad. Sci. USA 109, 8044–8049 10.1073/pnas.1118174109 (doi:10.1073/pnas.1118174109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO 2008. Closing the gap in a generation: health equity through action on the social determinants of health. Geneva, Switzerland: World Health Organization [Google Scholar]
- 34.Mosher W. D. 1980. Demographic responses and demographic transitions: a case study of Sweden. Demography 17, 395–412 10.2307/2061153 (doi:10.2307/2061153) [DOI] [PubMed] [Google Scholar]
- 35.Chinn S. 2000. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat. Med. 19, 3127–3131 (doi:10.1002/1097-0258(20001130)19:22<3127::AID-SIM784>3.0.CO;2-M) [DOI] [PubMed] [Google Scholar]
- 36.Fieder M., Huber S. 2007. The effects of sex and childlessness on the association between status and reproductive output in modern society. Evol. Hum. Behav. 28, 392–398 10.1016/j.evolhumbehav.2007.05.004 (doi:10.1016/j.evolhumbehav.2007.05.004) [DOI] [Google Scholar]
- 37.Goodman A., Gisselmann M. D., Koupil I. 2010. Birth outcomes and early-life social characteristics predict unequal educational outcomes: consistency across Swedish cohorts born 1915–1929 and 1973–1980. Longitudinal Life Course Studies 1, 315–316 [Google Scholar]