Abstract
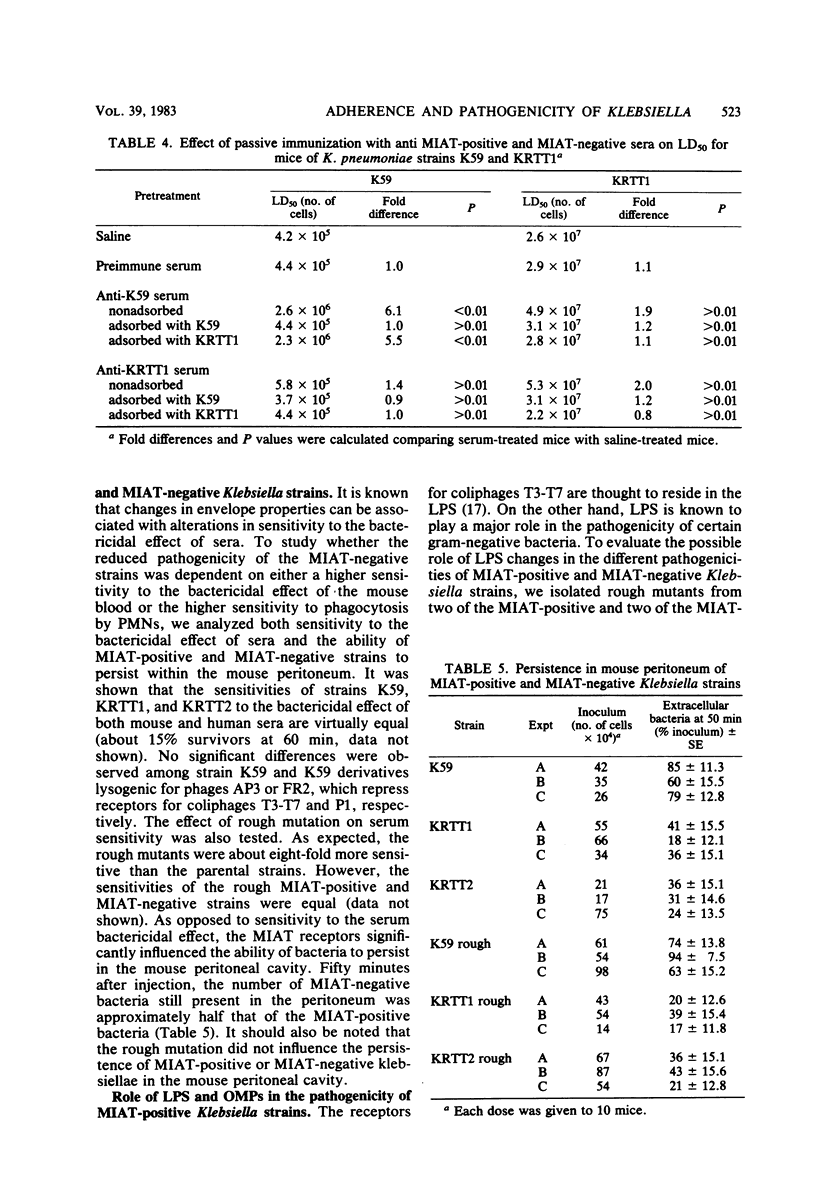
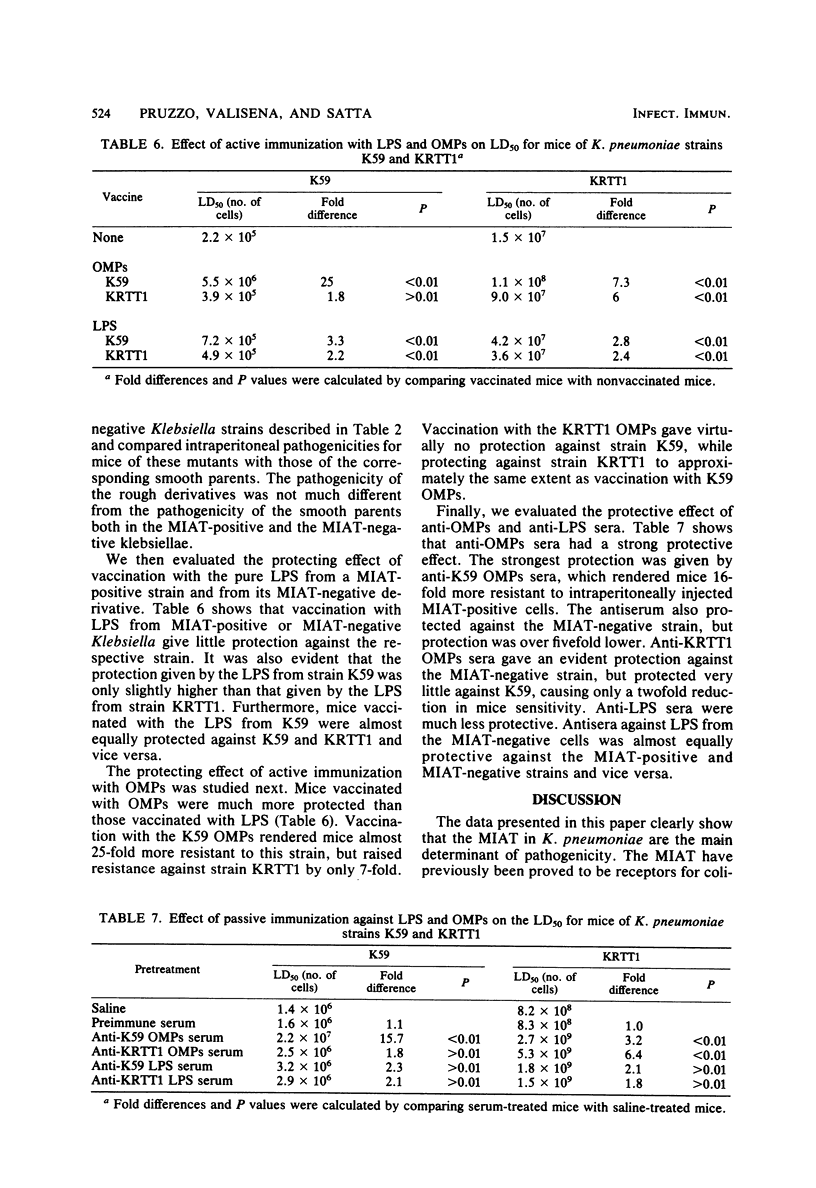
We have shown previously that Klebsiella pneumoniae receptors for coliphages T3 and T7 also mediate mannose-inhibitable adherence to human epithelial cells and protect bacteria from phagocytosis and intracellular killing by human polymorphonuclear cells. In this paper we analyze the possible role of such mannose-inhibitable adhesins and T3-T7 receptors (MIAT) in K. pneumoniae intraperitoneal pathogenicity for mice. We showed that intraperitoneal pathogenicity for mice of four different Klebsiella strains (one laboratory and three wild-type) that carry the MIAT was approximately 60-fold higher than that of four derivative strains that lost such receptors by spontaneous mutation. The MIAT could be repressed by Klebsiella phage AP3 lysogenic conversion. Two laboratory and two wild-type strains converted by phage AP3 were also approximately 60-fold less pathogenic for mice than parental strains and showed a pathogenicity level equal to that of the MIAT-negative mutants. Studies of protection in mice with anti-whole cell antisera showed that passive immunization against MIAT-positive cells was more protective than immunization against MIAT-negative cells. Studies of protection in mice by both active and passive immunization with lipopolysaccharide and purified outer membrane proteins have shown that the proteins are the most protective outer membrane components. Since it has been shown previously that the Klebsiella receptors for T3-T7 have a proteic component and that an outer membrane protein is missing in the strains resistant to T3-T7 (C. Pruzzo et al., in R. C. Berkely (ed.), Microbial Adhesion to Surfaces, 1980); the latter finding further supports the role of MIAT in the pathogenicity of Klebsiella for mice.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson M., Medalia O., Schori L., Mirelman D., Sharon N., Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J Infect Dis. 1979 Mar;139(3):329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- Bachhuber M., Brill W. J., Howe M. M. Use of bacteriophage Mu to isolate deletions in the his-nif region of Klebsiella pneumoniae. J Bacteriol. 1976 Dec;128(3):749–753. doi: 10.1128/jb.128.3.749-753.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit Z., Ofek I., Goldman R., Mirelman D., Sharon N. Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun. 1977 Sep 9;78(1):455–460. doi: 10.1016/0006-291x(77)91276-1. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Blumenstock E., Jann K. Adhesion of piliated Escherichia coli strains to phagocytes: differences between bacteria with mannose-sensitive pili and those with mannose-resistant pili. Infect Immun. 1982 Jan;35(1):264–269. doi: 10.1128/iai.35.1.264-269.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth J. A., Hendley J. O., Mandell G. L. Attachment and ingestion of gonococci human neutrophils. Infect Immun. 1975 Mar;11(3):512–516. doi: 10.1128/iai.11.3.512-516.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader R. C., Davis C. P. Effect of piliation on Klebsiella pneumoniae infection in rat bladders. Infect Immun. 1980 Nov;30(2):554–561. doi: 10.1128/iai.30.2.554-561.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Dean E. A., Morgan R. L., Moon H. W. Immunization of suckling pigs against enterotoxigenic Escherichia coli-induced diarrheal disease by vaccinating dams with purified K99 or 987P pili: antibody production in response to vaccination. Infect Immun. 1980 Aug;29(2):824–826. doi: 10.1128/iai.29.2.824-826.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxen H., Valtonen M., Mäkelä P. H. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun. 1979 Sep;25(3):857–862. doi: 10.1128/iai.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A. Bacteriophage receptors. Annu Rev Microbiol. 1973;27:205–241. doi: 10.1146/annurev.mi.27.100173.001225. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Holme T. Evaluation of some extraction methods for the preparation of bacterial lipopolysaccharides for structural analysis. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(5):751–759. doi: 10.1111/j.1699-0463.1972.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Mangan D. F., Snyder I. S. Mannose-sensitive interaction of Escherichia coli with human peripheral leukocytes in vitro. Infect Immun. 1979 Nov;26(2):520–527. doi: 10.1128/iai.26.2.520-527.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medearis D. N., Jr, Camitta B. M., Heath E. C. Cell wall composition and virulence in Escherichia coli. J Exp Med. 1968 Sep 1;128(3):399–414. doi: 10.1084/jem.128.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. L., Isaacson R. E., Moon H. W., Brinton C. C., To C. C. Immunization of suckling pigs against enterotoxigenic Escherichia coli-induced diarrheal disease by vaccinating dams with purified 987 or K99 pili: protection correlates with pilus homology of vaccine and challenge. Infect Immun. 1978 Dec;22(3):771–777. doi: 10.1128/iai.22.3.771-777.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh N., Furukawa H., Mizushima S. Role of lipopolysaccharide and outer membrane protein of Escherichia coli K-12 in the receptor activity for bacteriophage T4. J Bacteriol. 1978 Nov;136(2):693–699. doi: 10.1128/jb.136.2.693-699.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Bacterial adherence. Adv Intern Med. 1980;25:503–532. [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Pruzzo C., Debbia E. A., Satta G. Identification of the major adherence ligand of Klebsiella pneumoniae in the receptor for coliphage T7 and alteration of Klebsiella adherence properties by lysogenic conversion. Infect Immun. 1980 Nov;30(2):562–571. doi: 10.1128/iai.30.2.562-571.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzzo C., Debbia E., Satta G. Mannose-inhibitable adhesins and T3-T7 receptors of Klebsiella pneumoniae inhibit phagocytosis and intracellular killing by human polymorphonuclear leukocytes. Infect Immun. 1982 Jun;36(3):949–957. doi: 10.1128/iai.36.3.949-957.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Fulbright R. S., Eads M. E., Sawyer W. D. Ethylenediaminetetraacetic acid-sensitive antiphagocytic activity of Neisseria gonorrhoeae. Infect Immun. 1977 Mar;15(3):817–827. doi: 10.1128/iai.15.3.817-827.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter J. M., Jones G. W. Protection against enteric disease caused by Escherichia coli--a model for vaccination with a virulence determinant? Nature. 1973 Apr 20;242(5399):531–532. doi: 10.1038/242531a0. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Fontana R., Canepari P., Botta G. Peptidoglycan synthesis in cocci and rods of a pH-dependent, morphologically conditional mutant of Klebsiella pneumoniae. J Bacteriol. 1979 Feb;137(2):727–734. doi: 10.1128/jb.137.2.727-734.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Fontana R. Characterization of a conditional mutant with altered envelope showing pH-dependent morphology and temperature-dependent division. J Gen Microbiol. 1974 Jan;80(1):51–63. doi: 10.1099/00221287-80-1-51. [DOI] [PubMed] [Google Scholar]
- Satta G., Pruzzo C., Debbia E., Calegari L. Lysogenic conversion in Klebsiella pneumoniae: system which requires active immunity regulation for expression of the conversion phenomenon. J Virol. 1978 Dec;28(3):786–794. doi: 10.1128/jvi.28.3.786-794.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Pruzzo C., Debbia E., Fontana R. Close association between shape alteration and loss of immunity to superinfection in a wild-type Klebsiella pneumoniae stable lysogen which can be both immune and nonimmune to superinfection. J Virol. 1978 Dec;28(3):772–785. doi: 10.1128/jvi.28.3.772-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Cohen L. S. Antipili antibody affords protection against experimental ascending pyelonephritis. J Clin Invest. 1979 Jul;64(1):333–336. doi: 10.1172/JCI109458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Ofek I. Influence of pili on the virulence of Proteus mirabilis in experimental hematogenous pyelonephritis. J Infect Dis. 1978 Nov;138(5):664–667. doi: 10.1093/infdis/138.5.664. [DOI] [PubMed] [Google Scholar]
- Svanborg-Edén C., Jodal U. Attachment of Escherichia coli to urinary sediment epithelial cells from urinary tract infection-prone and healthy children. Infect Immun. 1979 Dec;26(3):837–840. doi: 10.1128/iai.26.3.837-840.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Young D., King G. Studies on Gonococcus infection. X. Pili and leukocyte association factor as mediators of interactions between gonococci and eukaryotic cells in vitro. Infect Immun. 1975 Jun;11(6):1352–1361. doi: 10.1128/iai.11.6.1352-1361.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen V. V. Mouse virulence of Salmonella strains: the effect of different smooth-type O side-chains. J Gen Microbiol. 1970 Dec;64(3):255–268. doi: 10.1099/00221287-64-3-255. [DOI] [PubMed] [Google Scholar]
- Väisänen V., Elo J., Tallgren L. G., Siitonen A., Mäkelä P. H., Svanborg-Edén C., Källenius G., Svenson S. B., Hultberg H., Korhonen T. Mannose-resistant haemagglutination and P antigen recognition are characteristic of Escherichia coli causing primary pyelonephritis. Lancet. 1981 Dec 19;2(8260-61):1366–1369. doi: 10.1016/s0140-6736(81)92796-3. [DOI] [PubMed] [Google Scholar]
- Wilson J. H., Luftig R. B., Wood W. B. Interaction of bacteriophage T4 tail fiber components with a lipopolysaccharide fraction from Escherichia coli. J Mol Biol. 1970 Jul 28;51(2):423–434. doi: 10.1016/0022-2836(70)90152-x. [DOI] [PubMed] [Google Scholar]


