Abstract
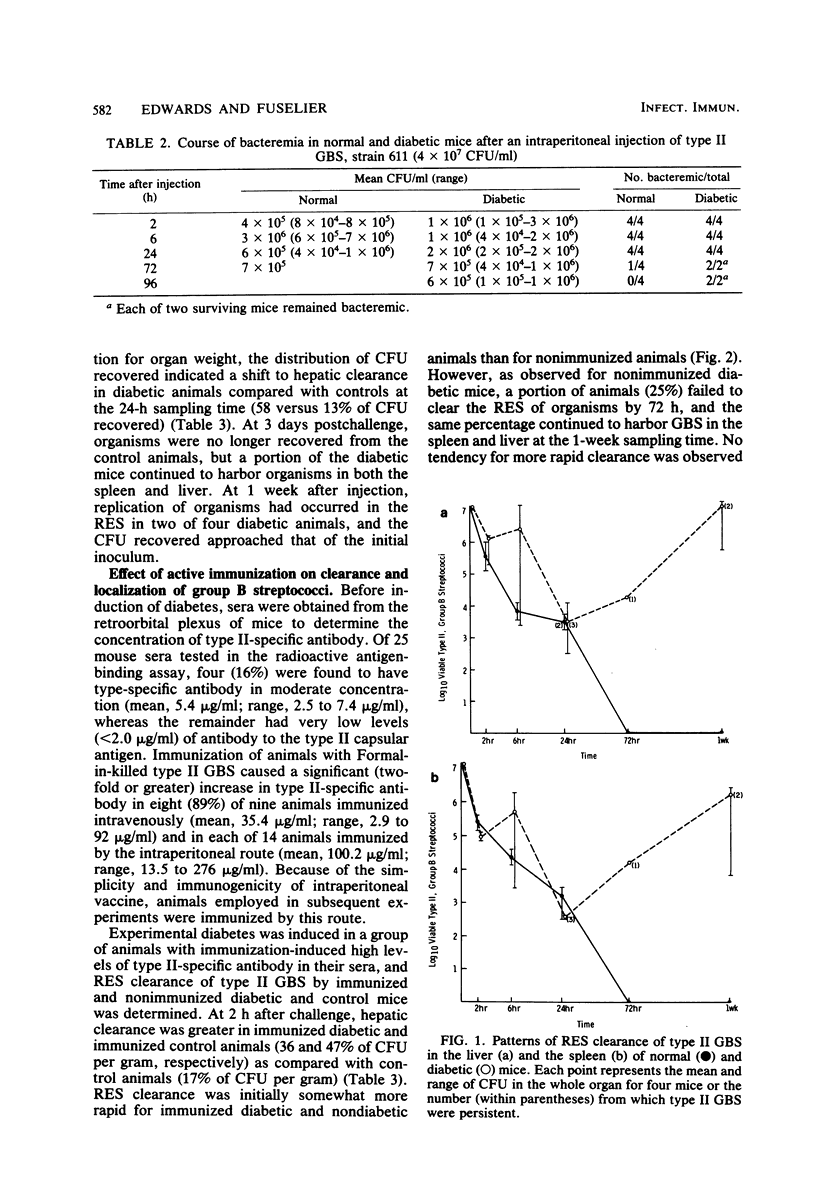
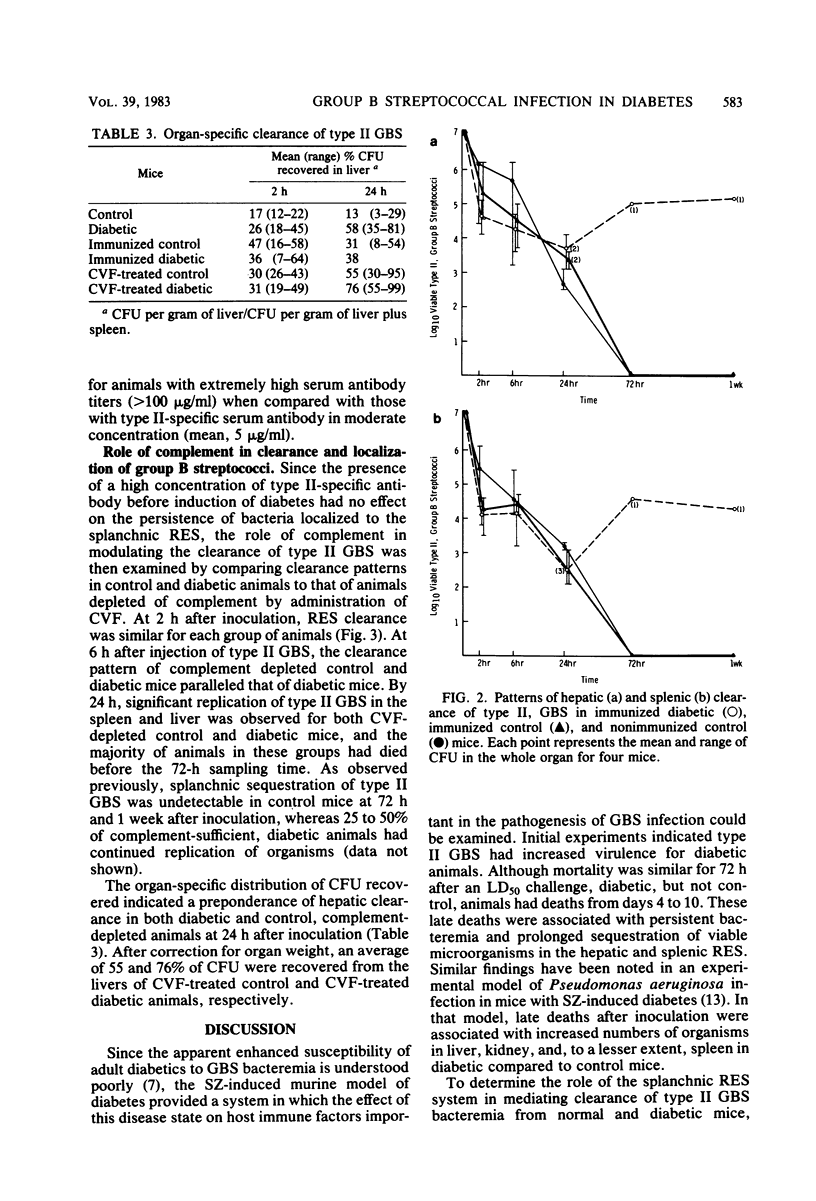
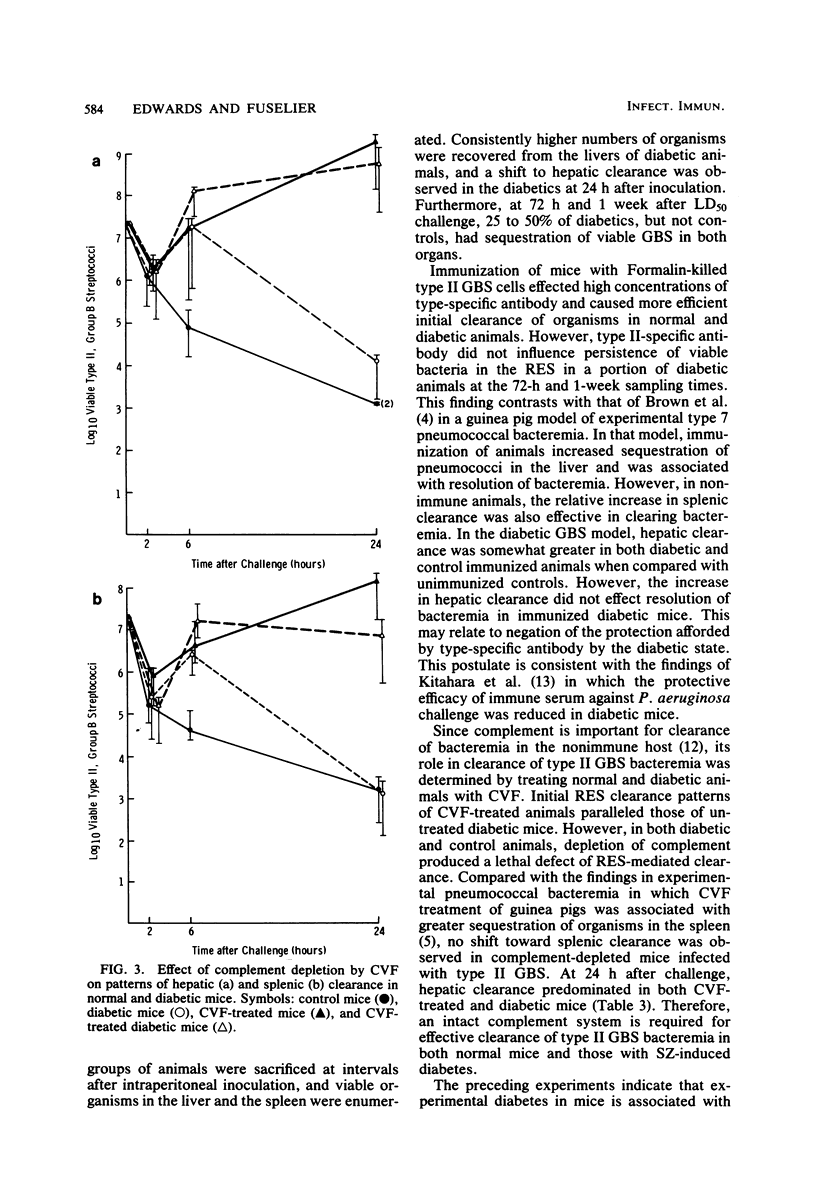
Since diabetes mellitus predisposes adults to group B streptococcal (GBS) bacteremia, a murine model of streptozotocin-induced diabetes and type II GBS bacteremia was developed to assess certain immune factors which might influence susceptibility to infection. In diabetic mice, the 50% lethal dose for two strains of type II GBS was significantly lower (greater than 1 log10 decrease in CFU per milliliter) than in control animals. This enhanced virulence of GBS for diabetic animals was associated with prolonged bacteremia, persistent sequestration of organisms in the splanchnic reticuloendothelial system, and a shift from splenic to hepatic clearance. Although immunization of control and diabetic animals resulted in high concentrations of type-specific serum antibody, it had no effect on late reticuloendothelial system sequestration in diabetics. In contrast, depletion of complement by treatment of mice with cobra venom factor blocked reticuloendothelial system clearance and resulted in fatal infection in both diabetic and control mice. These results indicate that neither type-specific antibody nor an intact complement system is adequate for effective clearance of type II GBS bacteremia in mice with experimentally induced diabetes. This clearance deficit could be the result of a defect in hepatocyte membrane receptors necessary for removal of this encapsulated microorganism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal M. K. Streptozotocin: mechanisms of action: proceedings of a workshop held on 21 June 1980, Washington, DC. FEBS Lett. 1980 Oct 20;120(1):1–3. doi: 10.1016/0014-5793(80)81031-3. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Chow A. W., Anthony B. F., Guze L. B. Serious infections in adults due to group B streptococci. Clinical and serotypic characterization. Am J Med. 1976 Oct;61(4):498–503. doi: 10.1016/0002-9343(76)90329-6. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Hosea S. W., Frank M. M. Reticuloendothelial clearance of radiolabelled pneumococci in experimental bacteremia: correlation of changes in clearance rates, sequestration patterns, and opsonization requirements at different phases of the bacterial growth cycle. J Reticuloendothel Soc. 1981 Jul;30(1):23–31. [PubMed] [Google Scholar]
- Brown E. J., Hosea S. W., Frank M. M. The role of complement in the localization of pneumococci in the splanchnic reticuloendothelial system during experimental bacteremia. J Immunol. 1981 Jun;126(6):2230–2235. [PubMed] [Google Scholar]
- Drachman R. H., Root R. K., Wood W. B., Jr Studies on the effect of experimental nonketotic diabetes mellitus on antibacterial defense. I. Demonstration of a defect in phagocytosis. J Exp Med. 1966 Aug 1;124(2):227–240. doi: 10.1084/jem.124.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand G., Dumont J. P., Appel M., Durand D., Davy J., Féger J., Agneray J. Effect of streptozotocin diabetes on sialic acid content and glycoprotein binding of isolated hepatocytes. Horm Metab Res. 1980;12(6):247–251. doi: 10.1055/s-2007-996259. [DOI] [PubMed] [Google Scholar]
- Dworzack D. L., Hodges G. R., Barnes W. G., Rosett W. Group B streptococcal infections in adult males. Am J Med Sci. 1979 Jan-Feb;277(1):67–73. doi: 10.1097/00000441-197901000-00008. [DOI] [PubMed] [Google Scholar]
- EICKHOFF T. C., KLEIN J. O., DALY A. K., INGALL D., FINLAND M. NEONATAL SEPSIS AND OTHER INFECTIONS DUE TO GROUP B BETA-HEMOLYTIC STREPTOCOCCI. N Engl J Med. 1964 Dec 10;271:1221–1228. doi: 10.1056/NEJM196412102712401. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Current concepts in immunology: the alternative pathway of complement--a system for host resistance to microbial infection. N Engl J Med. 1980 Jul 31;303(5):259–263. doi: 10.1056/NEJM198007313030505. [DOI] [PubMed] [Google Scholar]
- Kitahara Y., Ishibashi T., Harada Y., Takamoto M., Tanaka K. Reduced resistance to Pseudomonas septicaemia in diabetic mice. Clin Exp Immunol. 1981 Mar;43(3):590–598. [PMC free article] [PubMed] [Google Scholar]
- Lancefield R. C., McCarty M., Everly W. N. Multiple mouse-protective antibodies directed against group B streptococci. Special reference to antibodies effective against protein antigens. J Exp Med. 1975 Jul 1;142(1):165–179. doi: 10.1084/jem.142.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner P. I., Gopalakrishna K. V., Wolinsky E., McHenry M. C., Tan J. S., Rosenthal M. Group B streptococcus (S. agalactiae) bacteremia in adults: analysis of 32 cases and review of the literature. Medicine (Baltimore) 1977 Nov;56(6):457–473. doi: 10.1097/00005792-197711000-00001. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Wilkinson H. W. Analysis of group B streptococcal types associated with disease in human infants and adults. J Clin Microbiol. 1978 Feb;7(2):176–179. doi: 10.1128/jcm.7.2.176-179.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


