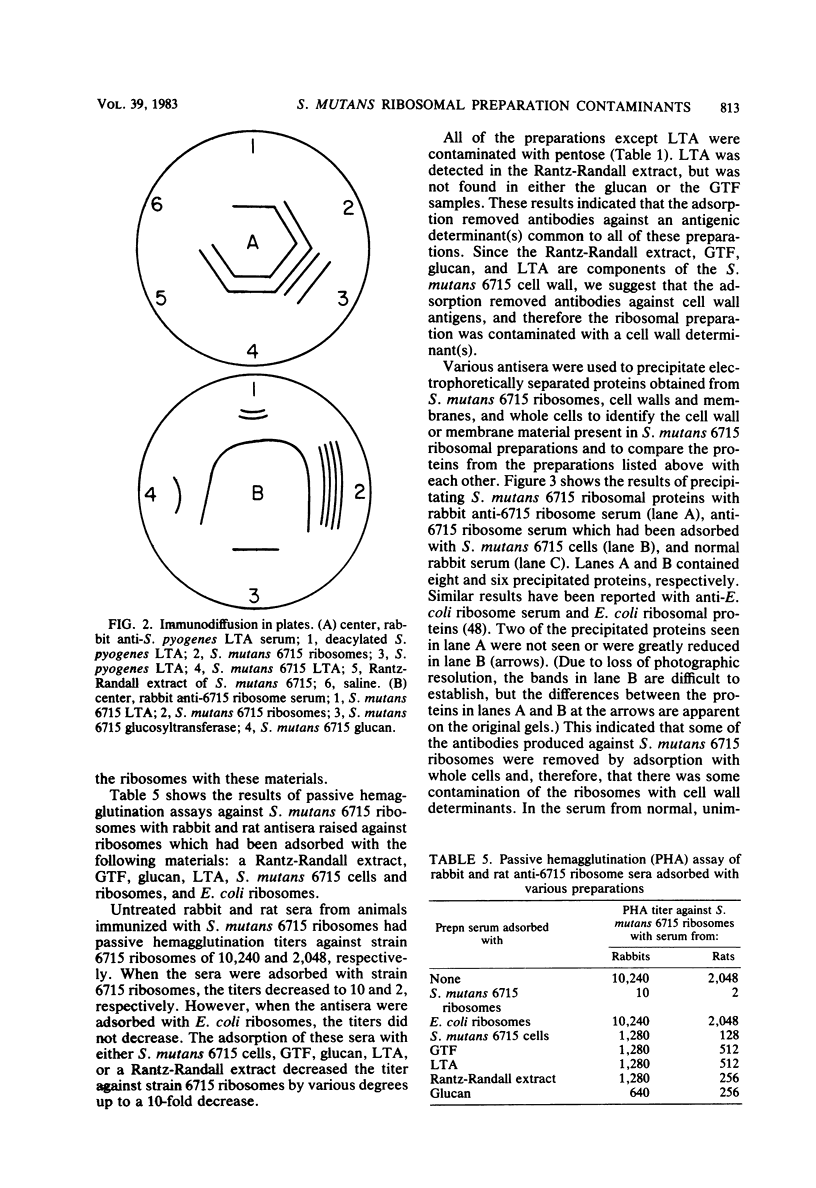
Abstract
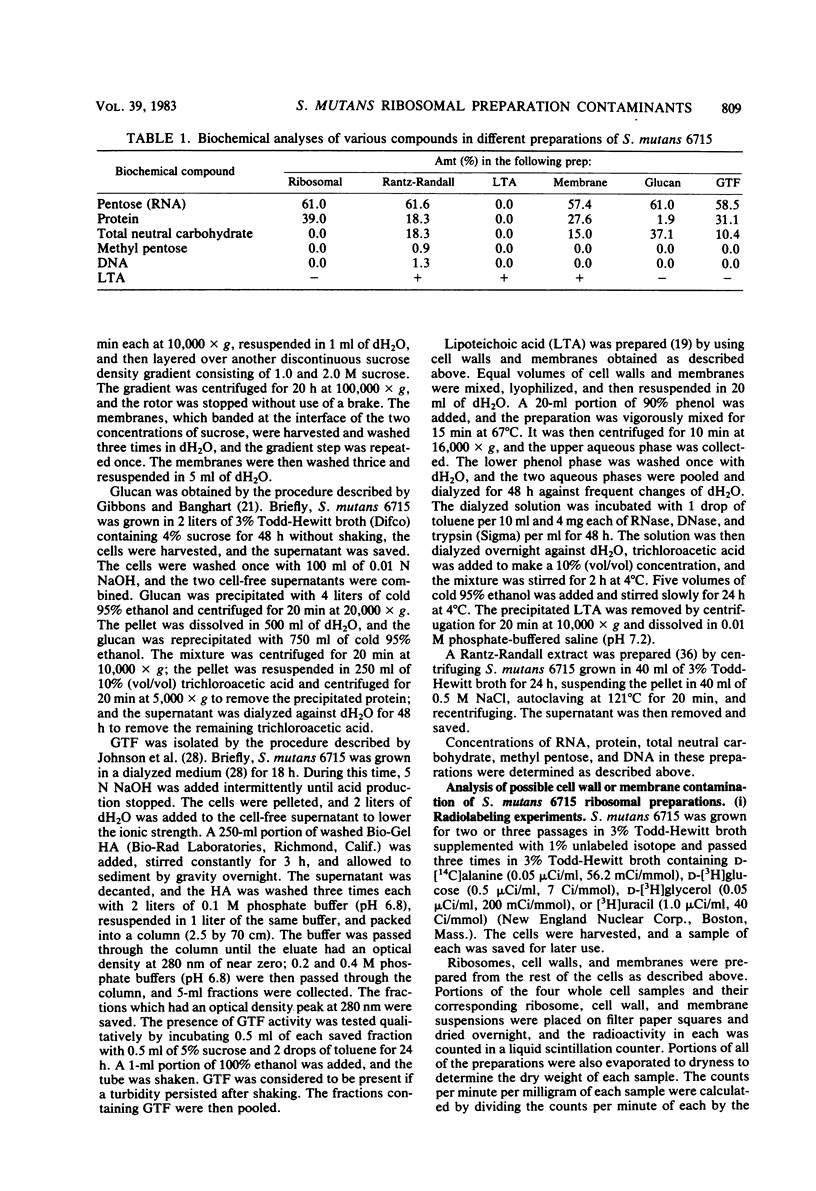
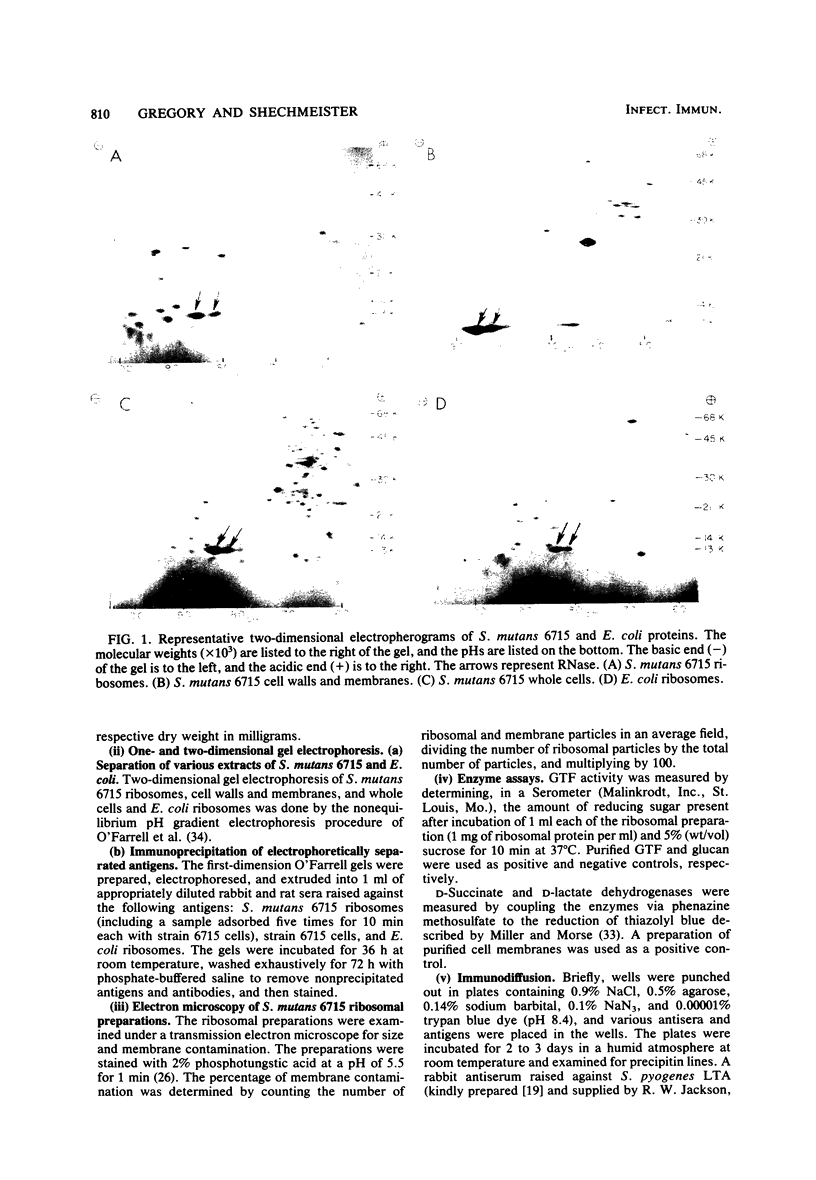
A ribosomal preparation from a cariogenic strain of Streptococcus mutans was examined for cell wall and membrane contamination. A biochemical characterization established that the preparation contained 61.0% RNA and 39.0% protein. Carbohydrate was not detected by phenol-sulfuric acid or methyl pentose assays. Glucosyltransferase and D-succinate dehydrogenase, which are cell wall- and membrane-associated enzymes, respectively, were not found. However, D-lactate dehydrogenase, another membrane-associated enzyme, was present in the preparation. A comparison of two-dimensional gel electropherograms of a mixture of cell walls and membranes and the S. mutans ribosomal preparation revealed contamination of the latter sample with at least six cell wall- or membrane-associated proteins. Adsorption of a rabbit antiserum raised against the ribosomal preparation with whole S. mutans cells abrogated antibodies directed against at least two proteins from the ribosomal preparation. Immunodiffusion plates showed reactivity of this antiserum against preparations of purified lipoteichoic acid from Streptococcus pyogenes and S. mutans. Adsorption of rat and rabbit antisera against the ribosomal preparation with the cell wall-derived materials glucosyltransferase, lipoteichoic acid, glucan, and a Rantz-Randall extract reduced the concentration of antibodies against the ribosomes by as much as 10-fold. These data indicated that the preparation was contaminated with at least six cell wall proteins, one cell membrane-associated enzyme, and lipoteichoic acid.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerman C. R., Eisenstein T. K. Correlation of the duration and magnitude of protection against Salmonella infection afforded by various vaccines with antibody titers. Infect Immun. 1980 Feb;27(2):435–443. doi: 10.1128/iai.27.2.435-443.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au C. C., Eisenstein T. K. Nature of the cross-protective antigen in subcellular vaccines of Streptococcus pneumoniae. Infect Immun. 1981 Jan;31(1):160–168. doi: 10.1128/iai.31.1.160-168.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. E., Hill W. E., Larson C. L. Delayed hypersensitivity reactions provoked by ribosomes from acid-fast bacilli. I. Ribosomal isolation, characterization, delayed hypersensitivity, and specificity. Infect Immun. 1972 Sep;6(3):258–265. doi: 10.1128/iai.6.3.258-265.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen W. H., Cohen B., Cole M. F., Colman G. Immunization against dental caries. Br Dent J. 1975 Jul 15;139(2):45–58. [PubMed] [Google Scholar]
- Cooper H. R., Chorpenning F. W., Rosen S. Preparation and chemical composition of the cell walls of Streptococcus mutans. Infect Immun. 1975 Apr;11(4):823–828. doi: 10.1128/iai.11.4.823-828.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Wannemuehler M. J., Miller R. D., Fedyk M. F. Role of outer envelope contamination in protection elicited by ribosomal preparations against Neisseria gonorrhoeae infection. Infect Immun. 1981 Apr;32(1):173–179. doi: 10.1128/iai.32.1.173-179.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- Dussourd d'Hinterland L. Ribosomal vaccines: introduction to the study of ribosomal vaccines. Arzneimittelforschung. 1980;30(1A):122–125. [PubMed] [Google Scholar]
- Eisenstein T. K. Evidence for O antigens as the antigenic determinants in "ribosomal" vaccines prepared from Salmonella. Infect Immun. 1975 Aug;12(2):364–377. doi: 10.1128/iai.12.2.364-377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feit C., Tewari R. P. Immunogenicity of Ribosomal Preparations from Yeast Cells of Histoplasma capsulatum. Infect Immun. 1974 Nov;10(5):1091–1097. doi: 10.1128/iai.10.5.1091-1097.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedel B. A., Jackson R. W. Immunogenicity of a purified and carrier-complexed streptococcal lipoteichoic acid. Infect Immun. 1976 Jun;13(6):1585–1590. doi: 10.1128/iai.13.6.1585-1590.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Some Immunochemical Properties of Dextransucrase and Invertase from Streptococcus mutans. Infect Immun. 1974 Nov;10(5):985–990. doi: 10.1128/iai.10.5.985-990.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. B. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol. 1967 Jan;12(1):11–23. doi: 10.1016/0003-9969(67)90137-9. [DOI] [PubMed] [Google Scholar]
- Green B. A., Johnson W. Immunogenicity of ribosomes from enzymatically lysed Streptococcus pyogenes. Infect Immun. 1980 Feb;27(2):424–430. doi: 10.1128/iai.27.2.424-430.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory R. L., Shechmeister I. L. Humoral and cell-mediated responses to a ribosomal preparation from Streptococcus mutans. Infect Immun. 1982 Dec;38(3):1094–1101. doi: 10.1128/iai.38.3.1094-1101.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoops P., Prather N. E., Berry J., Ravel J. M. Evidence for an extrinsic immunogen in effective ribosomal vaccines from Salmonella typhimurium. Infect Immun. 1976 Apr;13(4):1184–1192. doi: 10.1128/iai.13.4.1184-1192.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M., Machardy S. M., Sheppard A. J., Woods N. C. Evidence for an immunological relationship between Streptococcus mutans and human cardiac tissue. Infect Immun. 1980 Feb;27(2):576–588. doi: 10.1128/iai.27.2.576-588.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R., Gregory B., Naylor J., Actor P. Isolation of protective somatic antigen from Vibrio cholerae (Ogawa) ribosomal preparations. Infect Immun. 1972 Aug;6(2):156–161. doi: 10.1128/iai.6.2.156-161.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. C., Bozzola J. J., Shechmeister I. L., Shklair I. L. Biochemical study of the relationship of extracellular glucan to adherence and cariogenicity in Streptococcus mutans and an extracellular polysaccharide mutant. J Bacteriol. 1977 Jan;129(1):351–357. doi: 10.1128/jb.129.1.351-357.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. Ribosomal vaccines. II. Specificity of the immune response to ribosomal ribonucleic acid and protein isolated from Salmonella typhimurium. Infect Immun. 1973 Sep;8(3):395–400. doi: 10.1128/iai.8.3.395-400.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn M., Tewari R. P., Solotorovsky M. Immunoprotective activity of ribosomes from Haemophilus influenzae. Infect Immun. 1977 Feb;15(2):453–460. doi: 10.1128/iai.15.2.453-460.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McGhee J. R., Michalek S. M. Immunobiology of dental caries: microbial aspects and local immunity. Annu Rev Microbiol. 1981;35:595–638. doi: 10.1146/annurev.mi.35.100181.003115. [DOI] [PubMed] [Google Scholar]
- Miller R. D., Morse S. A. Binding of progesterone to Neisseria gonorrhoeae and other gram-negative bacteria. Infect Immun. 1977 Apr;16(1):115–123. doi: 10.1128/iai.16.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- RANTZ L. A., RANDALL E. Use of autoclaved extracts of hemolytic streptococci for serological grouping. Stanford Med Bull. 1955 May;13(2):290–291. [PubMed] [Google Scholar]
- Riottot M. M., Fournier J. M. Immunoprotective activity of capsular polysaccharide in Klebsiella pneumoniae ribosomal preparations does not involve ribonucleic acid. Infect Immun. 1981 Oct;34(1):126–130. doi: 10.1128/iai.34.1.126-130.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riottot M. M., Fournier J. M., Jouin H. Direct evidence for the involvement of capsular polysaccharide in the immunoprotective activity of Klebsiella pneumoniae ribosomal preparations. Infect Immun. 1981 Jan;31(1):71–77. doi: 10.1128/iai.31.1.71-77.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riottot M., Fournier J. M., Pillot J. Capsular serotypic specificity of the protection conferred on mice by Klebsiella pneumoniae ribosomal preparations. Infect Immun. 1979 May;24(2):476–482. doi: 10.1128/iai.24.2.476-482.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalla W. O., Johnson W. Immunogenicity of ribosomal vaccines isolated from group A, type 14 Streptococcus pyogenes. Infect Immun. 1975 Jun;11(6):1195–1202. doi: 10.1128/iai.11.6.1195-1202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A., Ebersole J. L. Local and systemic antibody response to oral administration of glucosyltransferase antigen complex. Infect Immun. 1980 May;28(2):441–450. doi: 10.1128/iai.28.2.441-450.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Nisengard R. J., Bergey E. J. Binding of streptococcal antigens to muscle tissue in vitro. Infect Immun. 1980 Feb;27(2):604–613. doi: 10.1128/iai.27.2.604-613.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Nisengard R. J., Pelonero L., Drinnan A. J. Immunological cross-reactivity of oral non-streptococcal bacteria with mammalian tissue. Infect Immun. 1979 May;24(2):532–538. doi: 10.1128/iai.24.2.532-538.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen C. L., Johnson W. Humoral immunity to Streptococcus pneumoniae induced by a pneumococcal ribosomal protein fraction. Infect Immun. 1976 Aug;14(2):345–354. doi: 10.1128/iai.14.2.345-354.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman M. A., Smith D. J. Effects of local immunization with glucosyltransferase fractions from Streptococcus mutans on dental caries in rats and hamsters. J Immunol. 1977 Feb;118(2):710–720. [PubMed] [Google Scholar]
- Thomas D. W., Weiss E. Response of mice to injection of ribosomal fraction from group B Neisseria meningitidis. Infect Immun. 1972 Sep;6(3):355–363. doi: 10.1128/iai.6.3.355-363.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H. C., Snyder I. S. Protection against pneumococcal infection by a ribosomal preparation. Infect Immun. 1971 Jan;3(1):16–23. doi: 10.1128/iai.3.1.16-23.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Rijn I., Bleiweis A. S., Zabriskie J. B. Antigens in Streptococcus mutans cross reactive with human heart muscle. J Dent Res. 1976 Apr;55(Spec No):C59–C64. doi: 10.1177/002203457605500326011. [DOI] [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J. Isolation and partial characterization of an immunogenic moiety obtained from Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):140–148. doi: 10.1128/jb.100.1.140-148.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. IMMUNOGENIC ACTIVITY OF A RIBOSOMAL FRACTION OBTAINED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1965 May;89:1291–1298. doi: 10.1128/jb.89.5.1291-1298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. The effect of metabolic inhibitors and hydroxylamine on the immune response in mice to mycobacterial ribonucleic acid vaccines. J Immunol. 1974 Jan;112(1):271–284. [PubMed] [Google Scholar]