Abstract
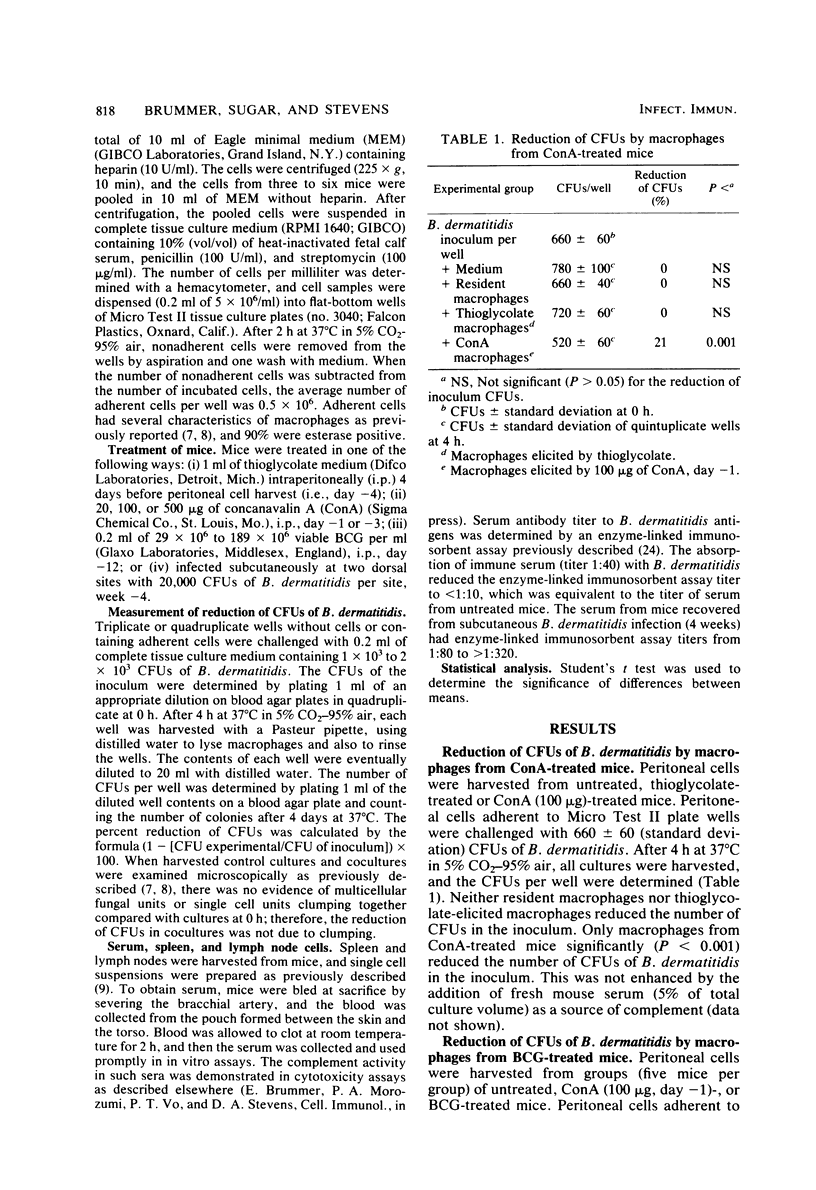
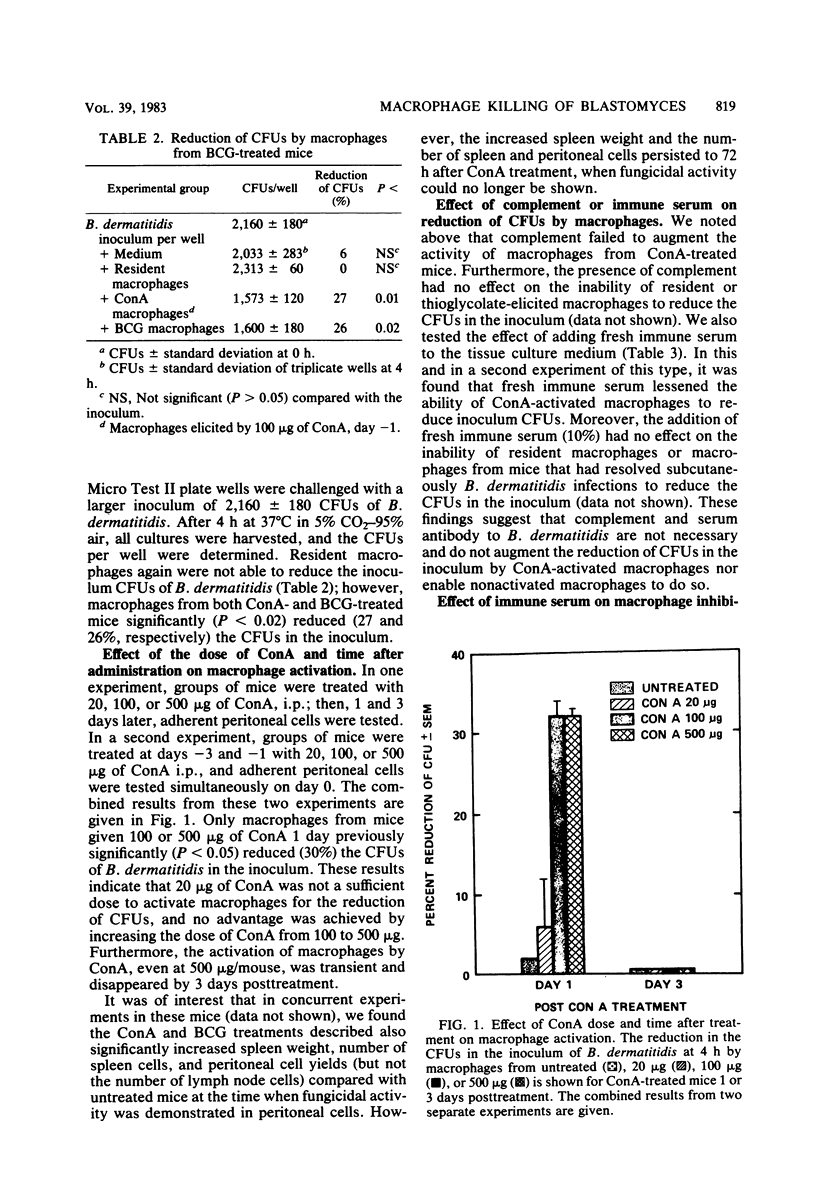
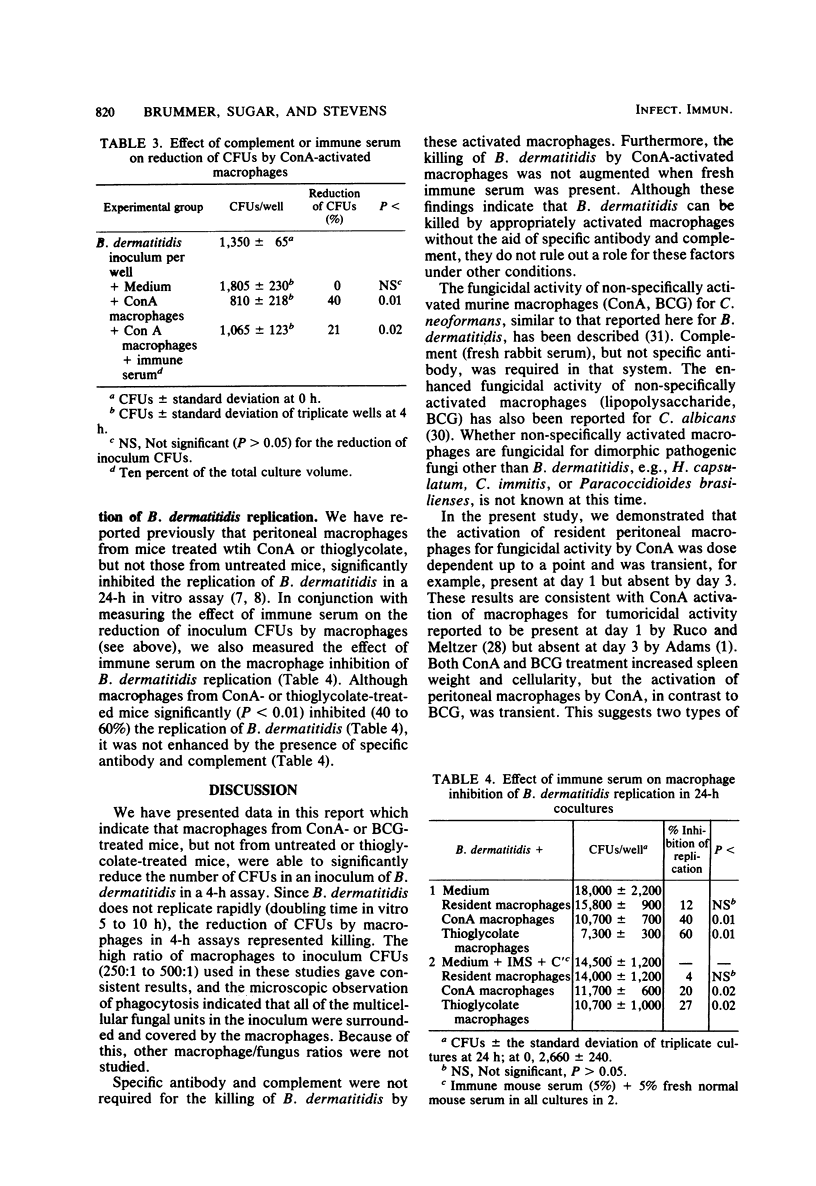
With a new short-term assay, where the reduction of CFUs in the inoculum could be measured, we investigated the killing of the dimorphic fungal pathogen Blastomyces dermatitidis in its yeast phase by murine peritoneal macrophages. Peritoneal macrophages from concanavalin A- or Mycobacterium bovis BCG-treated mice, but not resident or thioglycolate-elicited macrophages, significantly reduced the CFUs of B. dermatitidis in the inoculum. The activation of peritoneal macrophages for fungicidal activity by concanavalin A treatment was shown to be dose dependent and transient, i.e., absent after 72 h. These results indicate that it is possible for murine peritoneal macrophages to kill B. dermatitidis in vitro. The addition of specific antibody or complement or both did not enhance the killing of B. dermatitidis by these nonspecifically activated macrophages.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. Effector mechanisms of cytolytically activated macrophages. I. Secretion of neutral proteases and effect of protease inhibitors. J Immunol. 1980 Jan;124(1):286–292. [PubMed] [Google Scholar]
- Alexander J., Smith C. C. Growth of Mycobacterium lepraemurium in nonstimulated and stimulated mouse peritoneal-derived and bone marrrow-derived macrophages in vitro. Infect Immun. 1978 Dec;22(3):631–636. doi: 10.1128/iai.22.3.631-636.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell P. F., Krikszens A. Influence of anti-slime glycolipoprotein serum on the interaction between Pseudomonas aeruginosa and macrophages. Infect Immun. 1980 Mar;27(3):777–783. doi: 10.1128/iai.27.3.777-783.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Benjamini E., Pappagianis D. Role of lymphocytes in macrophage-induced killing of Coccidioides immitis in vitro. Infect Immun. 1981 Nov;34(2):347–353. doi: 10.1128/iai.34.2.347-353.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Holmberg C. A. In vitro response of alveolar macrophages to infection with Coccidioides immitis. Infect Immun. 1980 May;28(2):594–600. doi: 10.1128/iai.28.2.594-600.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E., Morozumi P. A., Philpott D. E., Stevens D. A. Virulence of fungi: correlation of virulence of Blastomyces dermatitidis in vivo with escape from macrophage inhibition of replication in vitro. Infect Immun. 1981 May;32(2):864–871. doi: 10.1128/iai.32.2.864-871.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E., Morozumi P. A., Stevens D. A. Macrophages and fungi: in vitro effects of method of macrophage induction, activation by different stimuli, and soluble factors on Blastomyces. J Reticuloendothel Soc. 1980 Dec;28(6):507–518. [PubMed] [Google Scholar]
- Brummer E., Vris T. W., Lawrence H. S. A microculture system for the measurement of antigen-induced murine lymphocyte proliferation: advantages of 5% horse serum and 5 X 10(-5) M mercaptoethanol. J Immunol Methods. 1977;17(3-4):319–327. doi: 10.1016/0022-1759(77)90114-4. [DOI] [PubMed] [Google Scholar]
- Cummings N. P., Pabst M. J., Johnston R. B., Jr Activation of macrophages for enhanced release of superoxide anion and greater killing of Candida albicans by injection of muramyl dipeptide. J Exp Med. 1980 Dec 1;152(6):1659–1669. doi: 10.1084/jem.152.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect Immun. 1973 Feb;7(2):231–236. doi: 10.1128/iai.7.2.231-236.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filice G. A., Beaman B. L., Remington J. S. Effects of activated macrophages on Nacardia asteroides. Infect Immun. 1980 Feb;27(2):643–649. doi: 10.1128/iai.27.2.643-649.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser-Smith E. B., Waters R. V., Matthews T. R. Correlation between in vivo anti-Pseudomonas and anti-Candida activities and clearance of carbon by the reticuloendothelial system for various muramyl dipeptide analogs, using normal and immunosuppressed mice. Infect Immun. 1982 Jan;35(1):105–110. doi: 10.1128/iai.35.1.105-110.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry L. O., Remington J. S. Resistance against Cryptococcus conferred by intracellular bacteria and protozoa. J Infect Dis. 1971 Jan;123(1):22–31. doi: 10.1093/infdis/123.1.22. [DOI] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD D. H. INTRACELLULAR GROWTH OF HISTOPLASMA CAPSULATUM. J Bacteriol. 1965 Feb;89:518–523. doi: 10.1128/jb.89.2.518-523.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. P., Schmid E. S., Carrington C. C., Stevens D. A. Mouse model of pulmonary blastomycosis: utility, simplicity, and quantitative parameters. Am Rev Respir Dis. 1978 Apr;117(4):695–703. doi: 10.1164/arrd.1978.117.4.695. [DOI] [PubMed] [Google Scholar]
- Howard D. H., Otto V. Experiments on lymphocyte-mediated cellular immunity in murine histoplasmosis. Infect Immun. 1977 Apr;16(1):226–231. doi: 10.1128/iai.16.1.226-231.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. Defect in intracellular killing of Staphylococcus aureus within alveolar macrophages in Sendai virus-infected murine lungs. J Clin Invest. 1976 Jun;57(6):1533–1539. doi: 10.1172/JCI108423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Goldstein E., Lewis J. P., Lippert W., Warshauer D. Murine pulmonary alveolar macrophages: rates of bacterial ingestion, inactivation, and destruction. J Infect Dis. 1976 Mar;133(3):310–320. doi: 10.1093/infdis/133.3.310. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Ferrari L. G., Patterson-Delafield J., Sorrell T. Fungicidal activity of rabbit alveolar and peritoneal macrophages against Candida albicans. Infect Immun. 1980 Jun;28(3):1001–1008. doi: 10.1128/iai.28.3.1001-1008.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meléndez M., González M. C., Reid M., Fuentes C., Castillo D. Immunity to antigenically related salmonellae: effects of humoral factors on the bactericidal activity of normal and immune peritoneal exudate cells. Infect Immun. 1978 Dec;22(3):640–643. doi: 10.1128/iai.22.3.640-643.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T. G., Friedman L. In vitro phagocytosis and intracellular fate of variously encapsulated strains of Cryptococcus neoformans. Infect Immun. 1972 Apr;5(4):491–498. doi: 10.1128/iai.5.4.491-498.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozumi P. A., Halpern J. W., Stevens D. A. Susceptibility differences of inbred strains of mice to blastomycosis. Infect Immun. 1981 Apr;32(1):160–168. doi: 10.1128/iai.32.1.160-168.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa R. T., Sekiguchi R., Yokota T. Stimulation by conditioned medium of L-929 fibroblasts, E. coli lipopolysaccharide, and muramyl dipeptide of candidacidal activity of mouse macrophages. Cell Immunol. 1980 Jul 15;53(1):116–124. doi: 10.1016/0008-8749(80)90431-1. [DOI] [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Defective tumoricidal capacity of macrophages from C3H/HeJ mice. J Immunol. 1978 Jan;120(1):329–334. [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Macrophage activation for tumor cytotoxicity: induction of tumoricidal macrophages by supernatants of PPD-stimulated Bacillus Calmette-Guérin-immune spleen cell cultures. J Immunol. 1977 Sep;119(3):889–896. [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD W. B., Jr Phagocytosis, with particular reference to encapsulated bacteria. Bacteriol Rev. 1960 Mar;24(1):41–49. doi: 10.1128/br.24.1.41-49.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnack E., Bisno A. L., Beachey E. H. Hyaluronate capsule prevents attachment of group A streptococci to mouse peritoneal macrophages. Infect Immun. 1981 Mar;31(3):985–991. doi: 10.1128/iai.31.3.985-991.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


