Abstract
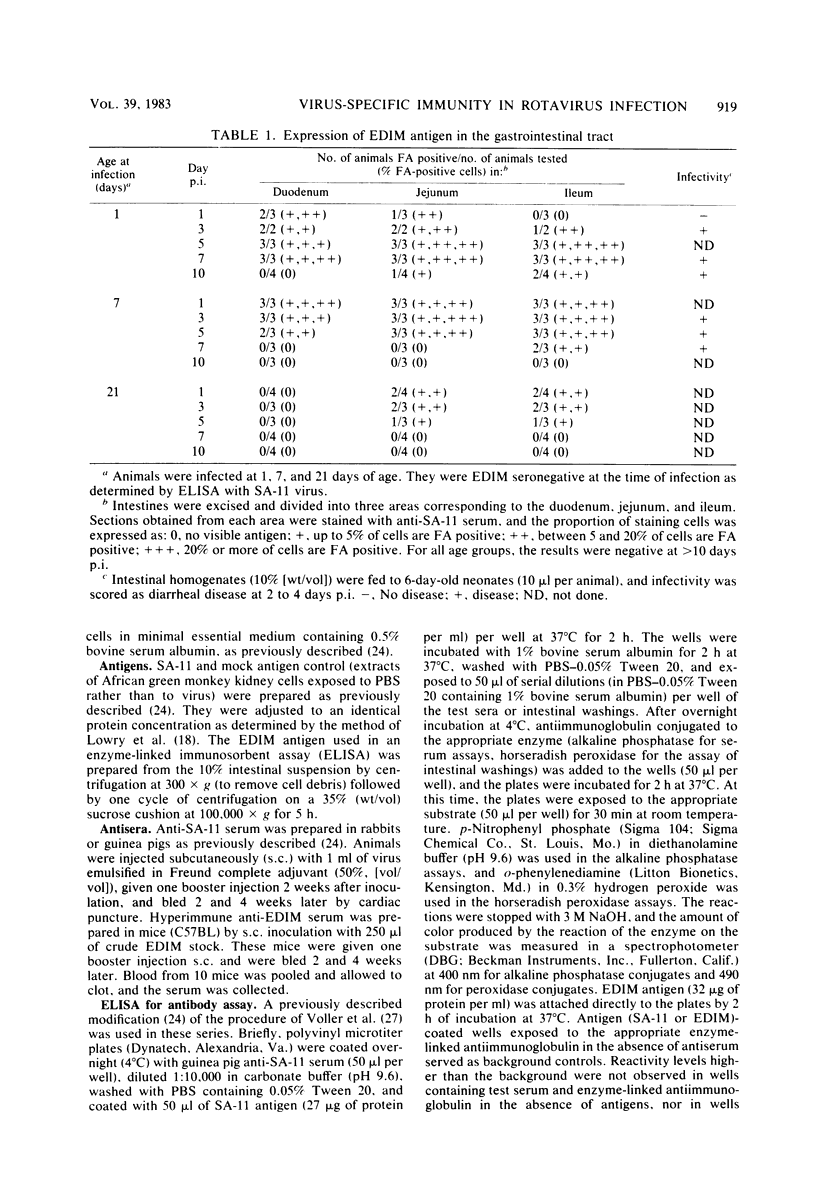
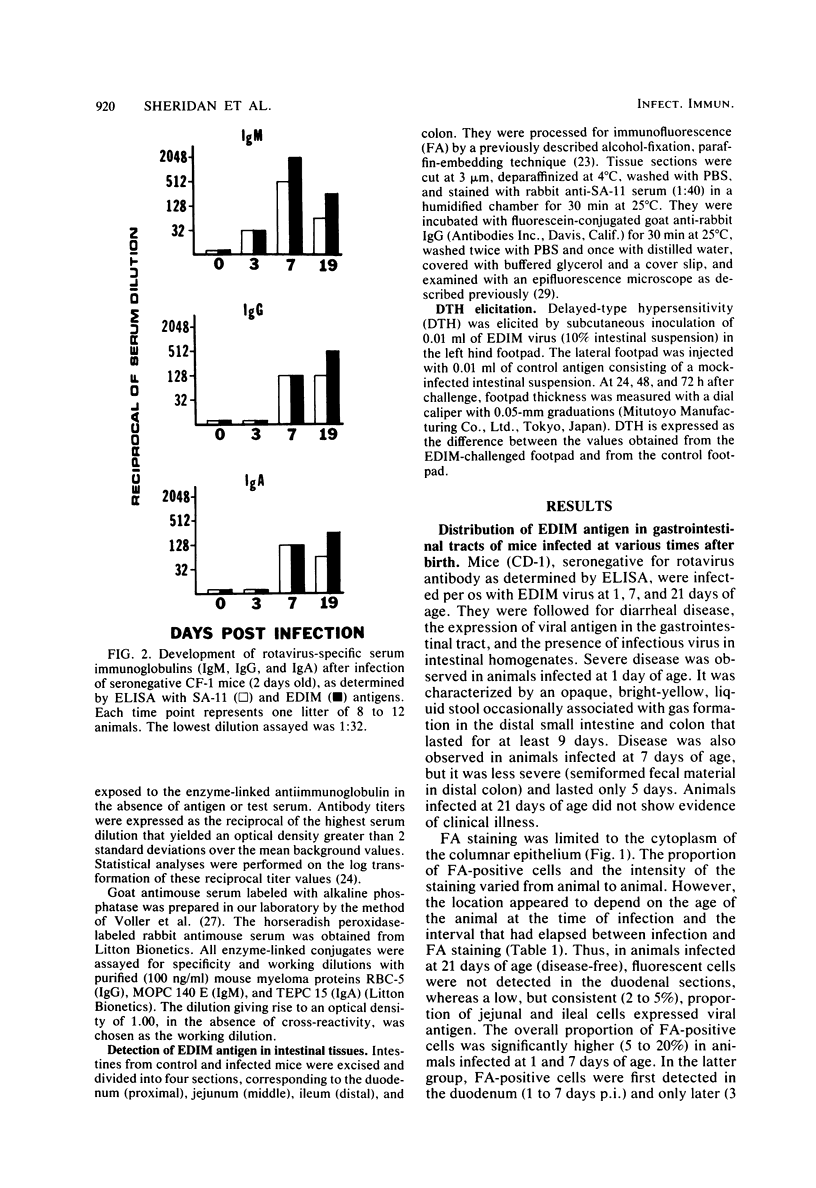
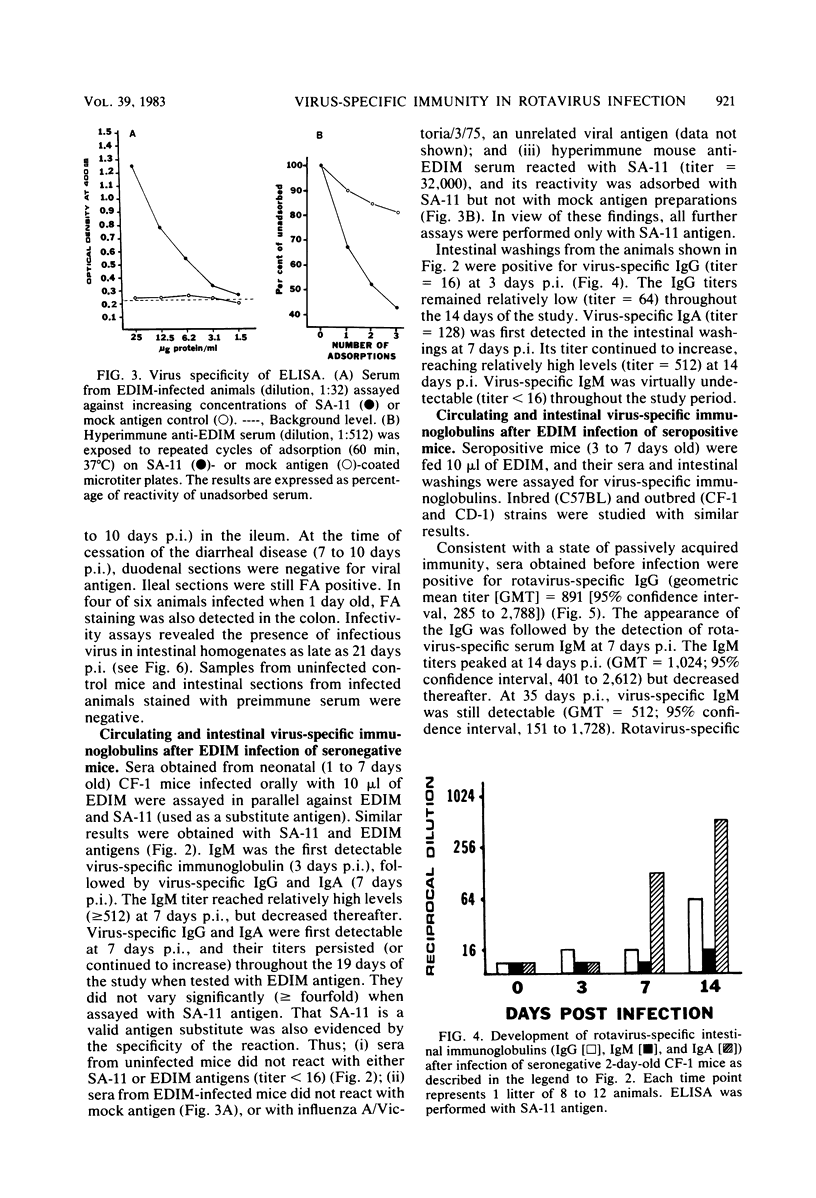
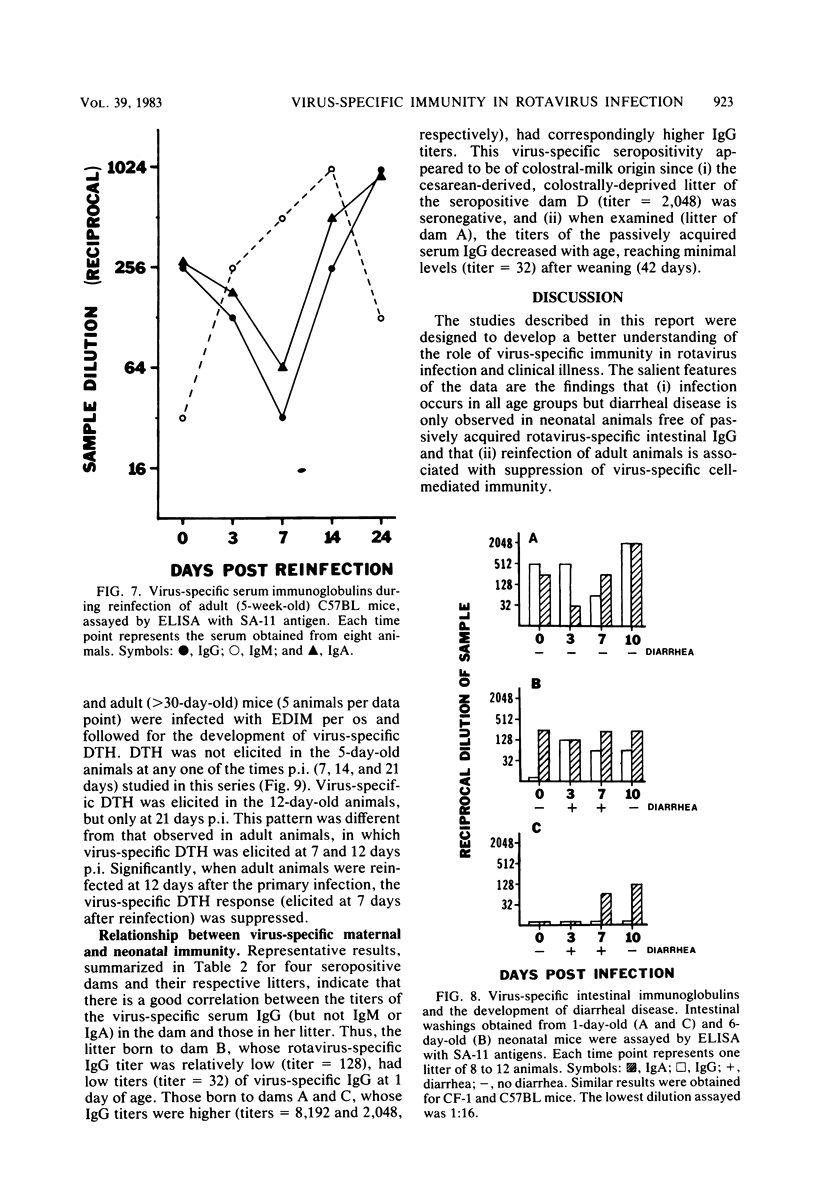
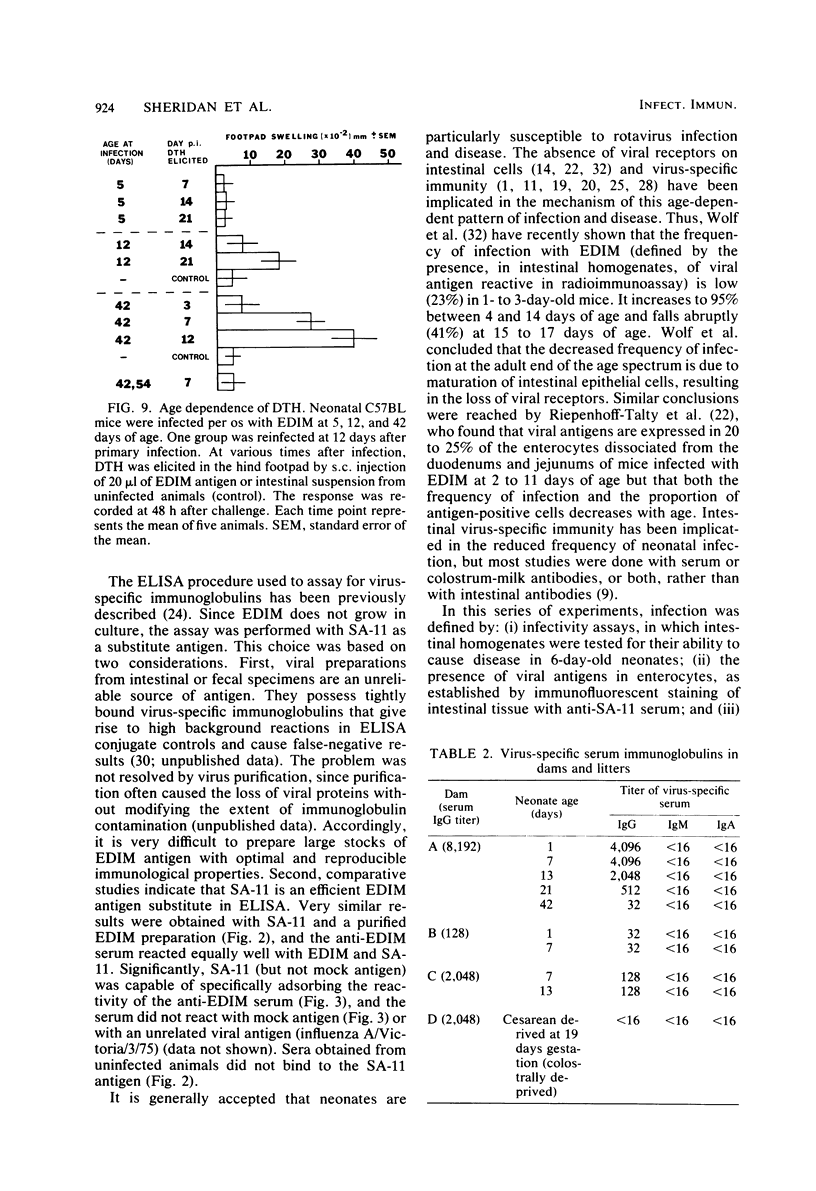
Mouse rotavirus (epizootic diarrhea of infant mice) was used as a model to study the role of virus-specific immunity in infection and diarrheal disease. The distribution of viral antigen in intestinal tissues was determined by immunofluorescent staining with anti-simian rotavirus (SA-11) serum. The location and proportion of antigen-positive cells appeared to vary as a function of time postinfection and age of the animal at the time of infection. In animals infected at 1 and 7 days of age, antigen-positive cells (5 to 25%) were first detected (1 day postinfection) in the proximal segment of the small intestine, and infection progressed to the middle and distal segments. At 10 days postinfection, virus-infected cells were no longer observed in the proximal segment. In animals infected at 21 days of age (disease-free), a significantly lower proportion of cells were antigen positive (2 to 5%), and they were restricted to the middle and distal segments of the small intestine. Infection, defined according to the presence of virus and viral antigens in intestinal tissues and by seroconversion in the immunoglobulin M (IgM) isotype as determined by enzyme-linked immunosorbent assay with SA-11 antigen, was observed for all age groups (neonatal to adult), even in the presence of virus-specific serum or intestinal immunoglobulins. On the other hand, diarrheal disease was not detected in neonatal mice (1 to 3 days old) positive for passively acquired virus-specific intestinal IgG. The presence of virus-specific IgA in the intestinal tract at the time of infection did not protect from subsequent diarrheal disease. Virus-specific, cell-mediated immunity, determined by a delayed-type hypersensitivity response, did not develop in neonatal mice infected at 5 and 12 days of age. Reinfection of adult mice was associated with suppression of virus-specific delayed-type hypersensitivity and a significant decrease in the titers of the virus-specific serum IgG and IgA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acres S. D., Babiuk L. A. Studies on rotaviral antibody in bovine serum and lacteal secretions, using radioimmunoassay. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):555–559. [PubMed] [Google Scholar]
- Bartz C. R., Conklin R. H., Tunstall C. B., Steele J. H. Prevention of murine rotavirus infection with chicken egg yolk immunoglobulins. J Infect Dis. 1980 Sep;142(3):439–441. doi: 10.1093/infdis/142.3.439. [DOI] [PubMed] [Google Scholar]
- Bishop R. F., Cameron D. J., Veenstra A. A., Barnes G. L. Diarrhea and rotavirus infection associated with differing regimens for postnatal care of newborn babies. J Clin Microbiol. 1979 Apr;9(4):525–529. doi: 10.1128/jcm.9.4.525-529.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop R. F., Davidson G. P., Holmes I. H., Ruck B. J. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973 Dec 8;2(7841):1281–1283. doi: 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- Bohl E. H., Gupta R. K., Olquin M. V., Saif L. J. Antibody responses in serum, colostrum, and milk of swine after infection or vaccination with transmissible gastroenteritis virus. Infect Immun. 1972 Sep;6(3):289–301. doi: 10.1128/iai.6.3.289-301.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohl E. H., Saif L. J. Passive immunity in transmissible gastroenteritis of swine: immunoglobulin characteristics of antibodies in milk after inoculating virus by different routes. Infect Immun. 1975 Jan;11(1):23–32. doi: 10.1128/iai.11.1.23-32.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar R., Conklin R. H., Vollet J. J., Pickering L. K., DuPont H. L., Walters D. L., Kohl S. Rotavirus in travelers' diarrhea: study of an adult student population in Mexico. J Infect Dis. 1978 Mar;137(3):324–327. doi: 10.1093/infdis/137.3.324. [DOI] [PubMed] [Google Scholar]
- Bridger J. C., Woode G. N. Neonatal calf diarrhoea: identification of a reovirus-like (rotavirus) agent in faeces by immunofluorescence and immune electron microscopy. Br Vet J. 1975 Sep-Oct;131(5):528–535. [PubMed] [Google Scholar]
- Chanock R. M., Wyatt R. G., Kapikian A. Z. Immunization of infants and young children against rotaviral gastroenteritis--prospects and problems. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):570–572. [PubMed] [Google Scholar]
- Davidson G. P., Bishop R. F., Townley R. R., Holmes I. H. Importance of a new virus in acute sporadic enteritis in children. Lancet. 1975 Feb 1;1(7901):242–246. doi: 10.1016/s0140-6736(75)91140-x. [DOI] [PubMed] [Google Scholar]
- Ellens D. J., de Leeuw P. W., Straver P. J. The detection of rotavirus specific antibody in colostrum and milk by ELISA. Ann Rech Vet. 1978;9(2):337–342. [PubMed] [Google Scholar]
- Flewett T. H., Woode G. N. The rotaviruses. Arch Virol. 1978;57(1):1–23. doi: 10.1007/BF01315633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes I. H., Rodger S. M., Schnagl R. D., Ruck B. J., Gust I. D., Bishop R. F., Barnes G. L. Is lactase the receptor and uncoating enzyme for infantile enteritis (rota) viruses? Lancet. 1976 Jun 26;1(7974):1387–1388. doi: 10.1016/s0140-6736(76)93032-4. [DOI] [PubMed] [Google Scholar]
- Holmes I. H. Viral gastroenteritis. Prog Med Virol. 1979;25:1–36. [PubMed] [Google Scholar]
- KRAFT L. M. Studies on the etiology and transmission of epidemic diarrhea of infant mice. J Exp Med. 1957 Nov 1;106(5):743–755. doi: 10.1084/jem.106.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Kim H. W., Wyatt R. G., Cline W. L., Arrobio J. O., Brandt C. D., Rodriguez W. J., Sack D. A., Chanock R. M., Parrott R. H. Human reovirus-like agent as the major pathogen associated with "winter" gastroenteritis in hospitalized infants and young children. N Engl J Med. 1976 Apr 29;294(18):965–972. doi: 10.1056/NEJM197604292941801. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McLean B. S., Holmes I. H. Effects of antibodies, trypsin, and trypsin inhibitors on susceptibility of neonates to rotavirus infection. J Clin Microbiol. 1981 Jan;13(1):22–29. doi: 10.1128/jcm.13.1.22-29.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean B., Holmes I. H. Transfer of antirotaviral antibodies from mothers to their infants. J Clin Microbiol. 1980 Sep;12(3):320–325. doi: 10.1128/jcm.12.3.320-325.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebus C. A., Wyatt R. G., Kapikian A. Z. Intestinal lesions induced in gnotobiotic calves by the virus of human infantile gastroenteritis. Vet Pathol. 1977 May;14(3):273–282. doi: 10.1177/030098587701400310. [DOI] [PubMed] [Google Scholar]
- Riepenhoff-Talty M., Lee P. C., Carmody P. J., Barrett H. J., Ogra P. L. Age-dependent rotavirus-enterocyte interactions. Proc Soc Exp Biol Med. 1982 Jun;170(2):146–154. doi: 10.3181/00379727-170-41410. [DOI] [PubMed] [Google Scholar]
- Sheridan J. F., Aurelian L., Barbour G., Santosham M., Sack R. B., Ryder R. W. Traveler's diarrhea associated with rotavirus infection: analysis of virus-specific immunoglobulin classes. Infect Immun. 1981 Jan;31(1):419–429. doi: 10.1128/iai.31.1.419-429.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Wells P. W. The immunoprophylaxis of of rotavirus infections in lambs. Vet Rec. 1978 Feb 18;102(7):146–148. doi: 10.1136/vr.102.7.146. [DOI] [PubMed] [Google Scholar]
- Svennerholm A., Lange S., Holmgren J. Correlation between intestinal synthesis of specific immunoglobulin A and protection against experimental cholera in mice. Infect Immun. 1978 Jul;21(1):1–6. doi: 10.1128/iai.21.1.1-6.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderfecht S. L., Osburn B. I. Detection of anti-rotavirus antibody-producing cells in paraffin-embedded tissue sections. Am J Vet Res. 1982 Feb;43(2):356–359. [PubMed] [Google Scholar]
- Watanabe H., Gust I. D., Holmes I. H. Human rotavirus and its antibody: their coexistence in feces of infants. J Clin Microbiol. 1978 May;7(5):405–409. doi: 10.1128/jcm.7.5.405-409.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsnack R. E., Blackwell J. H., Parker J. C. Identification of an agent of epizootic diarrhea of infant mice by immunofluorescent and complement-fixation tests. Am J Vet Res. 1969 Jul;30(7):1195–1204. [PubMed] [Google Scholar]
- Wolf J. L., Cukor G., Blacklow N. R., Dambrauskas R., Trier J. S. Susceptibility of mice to rotavirus infection: effects of age and administration of corticosteroids. Infect Immun. 1981 Aug;33(2):565–574. doi: 10.1128/iai.33.2.565-574.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Bridger J. C., Jones J. M., Flewett T. H., Davies H. A., Davis H. A., White G. B. Morphological and antigenic relationships between viruses (rotaviruses) from acute gastroenteritis of children, calves, piglets, mice, and foals. Infect Immun. 1976 Sep;14(3):804–810. doi: 10.1128/iai.14.3.804-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Bridger J. C. Rotavirus in calves... Vet Rec. 1976 Oct 16;99(16):322–321. doi: 10.1136/vr.99.16.322-a. [DOI] [PubMed] [Google Scholar]
- Woode G. N., Bridger J. C. Viral enteritis of calves. Vet Rec. 1975 Jan 25;96(4):85–88. doi: 10.1136/vr.96.4.85. [DOI] [PubMed] [Google Scholar]
- Woode G. N., Jones J., Bridger J. Levels of colostral antibodies against neonatal calf diaahoea virus. Vet Rec. 1975 Aug 23;97(8):148–149. doi: 10.1136/vr.97.8.148. [DOI] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Hovi T., Mákelá P., Hovi T., Tevalvoto-Aarnio M. Rotavirus associated with acute gastroenteritis in adults. Lancet. 1976 Aug 21;2(7982):423–423. doi: 10.1016/s0140-6736(76)92445-4. [DOI] [PubMed] [Google Scholar]



