Abstract
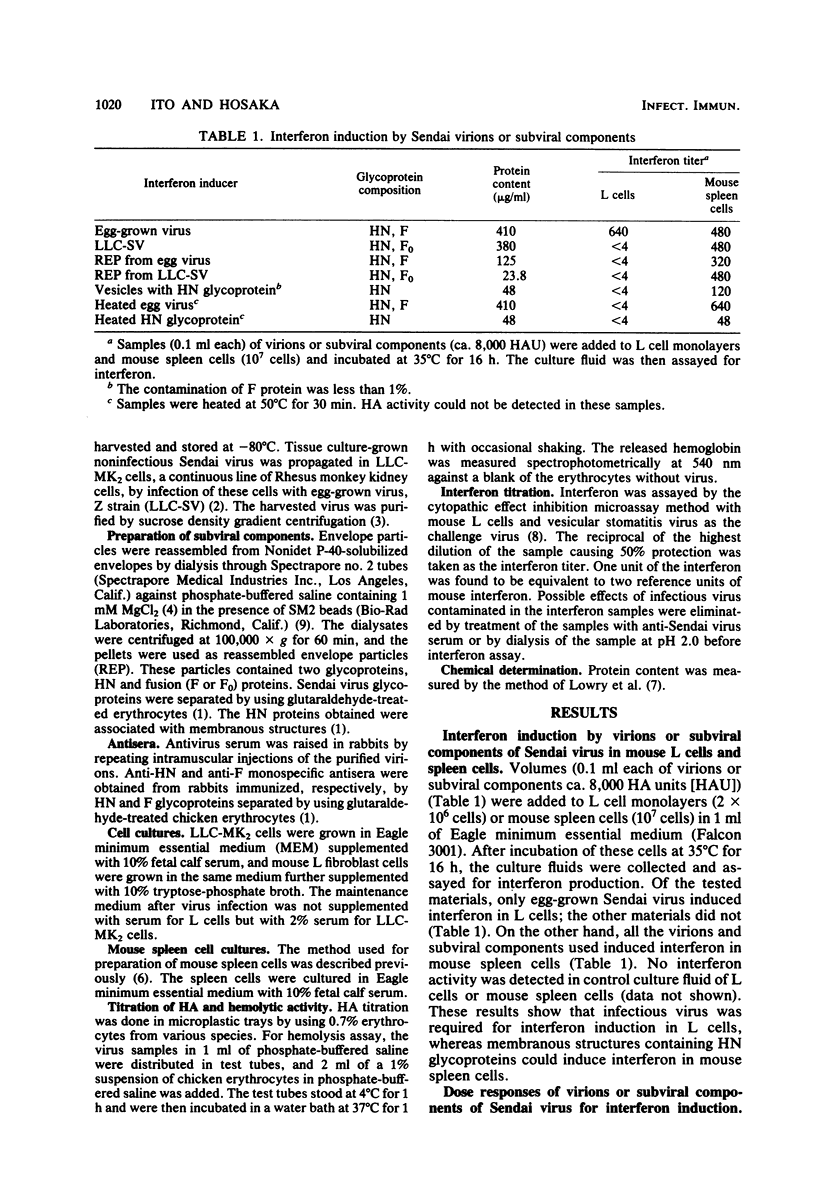
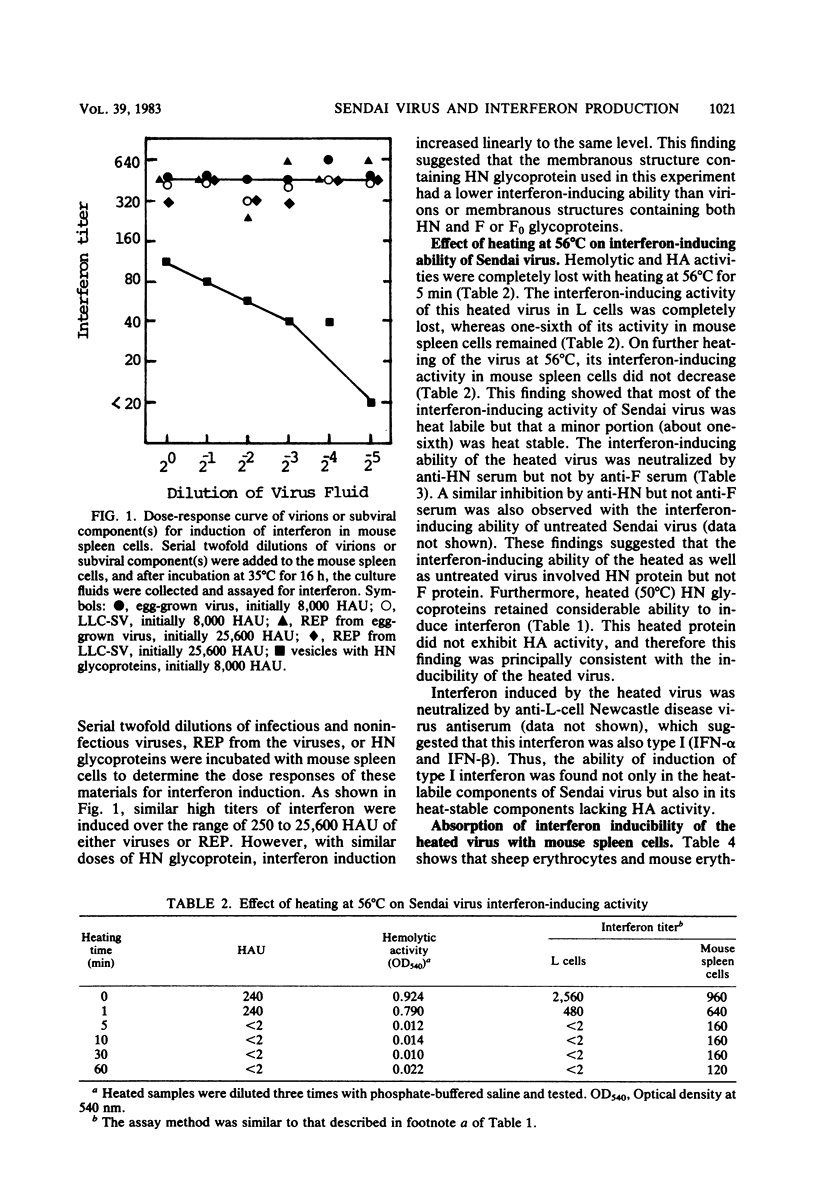
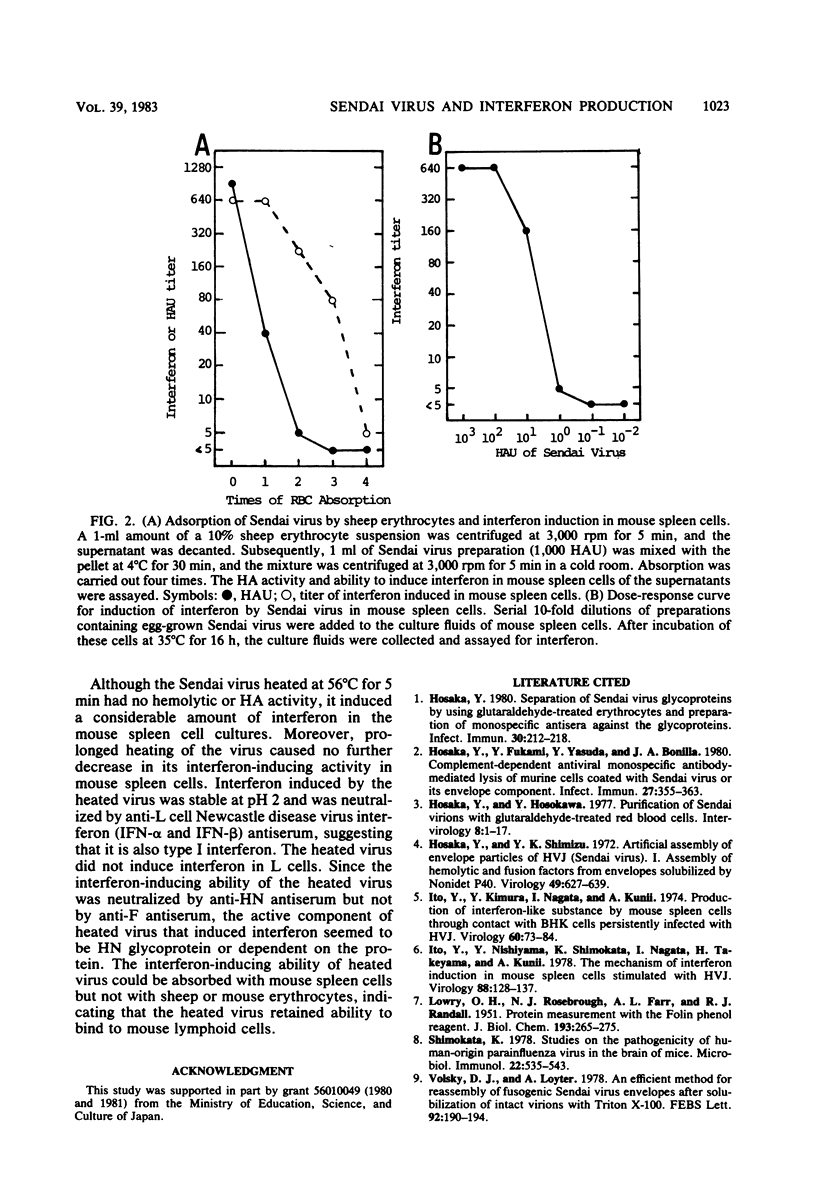
To identify the active component of Sendai virus that induces interferon in mouse spleen cells, infectious and noninfectious viruses, envelope particles derived from them, and isolated hemagglutinin-neuraminidase (HN) glycoproteins were examined for interferon induction. The interaction between membranous structures containing Sendai virus HN glycoprotein and the receptors on the cell surface was shown to be sufficient for interferon induction in mouse spleen cells, suggesting that the actual inducer of interferon in mouse spleen cells is the HN glycoprotein of Sendai virus. When mouse spleen cells were stimulated in vitro with Sendai virus grown in eggs or LLC-MK2 cells or with membranous structures containing glycoproteins obtained from these viruses, interferon could be detected in the culture fluid. Furthermore, isolated HN glycoprotein per se could induce interferon in the cells. A linear correlation was found between the titer of interferon induced and the hemagglutinating activity of the membranous structure containing the HN glycoprotein. It was concluded from these findings that HN glycoprotein was the active component of Sendai virus responsible for interferon induction in mouse spleen cells and that viral RNA and F glycoprotein were not required. The results also showed that the interaction between HN glycoprotein and receptors on the cell surface triggered production of type I interferon (IFN-alpha and IFN-beta). Although when Sendai virus was incubated at 56 degrees C for 5 min it lost its hemolytic and hemagglutinating activities, it induced a considerable amount of interferon in the culture fluid of mouse spleen cells. The interferon-inducing ability of heat-inactivated virus could be absorbed with mouse spleen cells but not with sheep erythrocytes or mouse erythrocytes, indicating that the inactivated virus retained ability to bind to mouse lymphoid cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hosaka Y., Fukami Y., Yasuda Y., Bonilla J. A. Complement-dependent antiviral monospecific antibody-mediated lysis of murine cells coated with Sendai virus or its envelope component. Infect Immun. 1980 Feb;27(2):355–363. doi: 10.1128/iai.27.2.355-363.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka Y., Hosokawa Y. Purification of Sendai virions with glutaraldehyde-treated red blood cells. Intervirology. 1977;8(1):1–17. doi: 10.1159/000148872. [DOI] [PubMed] [Google Scholar]
- Hosaka Y. Separation of Sendai virus glycoproteins by using glutaraldehyde-treated erythrocytes and preparation of monospecific antisera against the glycoproteins. Infect Immun. 1980 Oct;30(1):212–218. doi: 10.1128/iai.30.1.212-218.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka Y., Shimizu Y. K. Artificial assembly of envelope particles of HVJ (Sendai virus). I. Assembly of hemolytic and fusion factors from envelopes solubilized by Nonidet P40. Virology. 1972 Sep;49(3):627–639. doi: 10.1016/0042-6822(72)90519-3. [DOI] [PubMed] [Google Scholar]
- Ito Y., Kimura Y., Nagata I., Kunii A. Production of interferon-like substance by mouse spleen cells through contact with BHK cells persistently infected with HVJ. Virology. 1974 Jul;60(1):73–84. doi: 10.1016/0042-6822(74)90367-5. [DOI] [PubMed] [Google Scholar]
- Ito Y., Nishiyama Y., Shimokata K., Nagata I., Takeyama H., Kunii A. The mechanism of interferon induction in mouse spleen cells stimulated with HVJ. Virology. 1978 Jul 1;88(1):128–137. doi: 10.1016/0042-6822(78)90116-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Shimokata K. Studies on the pathogenicity of human-origin parainfluenza virus in the brain of mice. Microbiol Immunol. 1978;22(9):535–543. doi: 10.1111/j.1348-0421.1978.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Volsky D. J., Loyter A. An efficient method for reassembly of fusogenic Sendai virus envelopes after solubilization of intact virions with Triton X-100. FEBS Lett. 1978 Aug 15;92(2):190–194. doi: 10.1016/0014-5793(78)80751-0. [DOI] [PubMed] [Google Scholar]


