Abstract
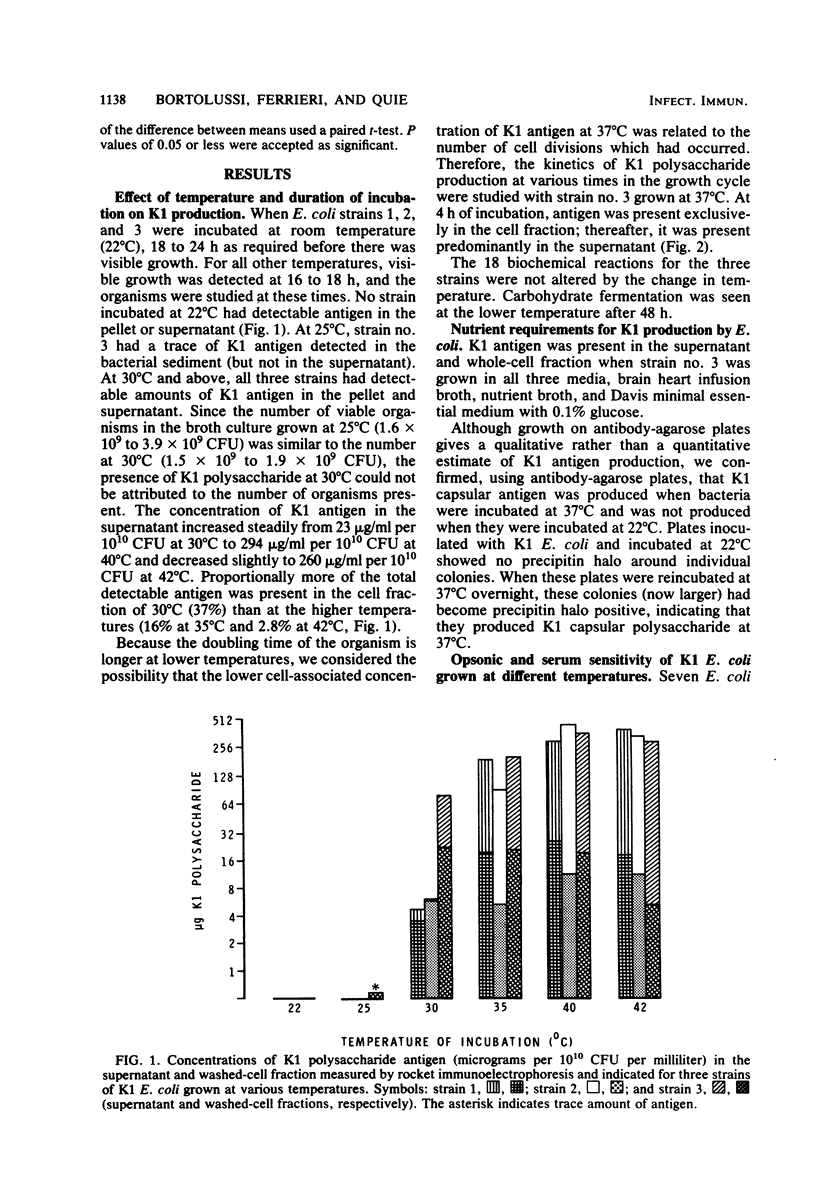
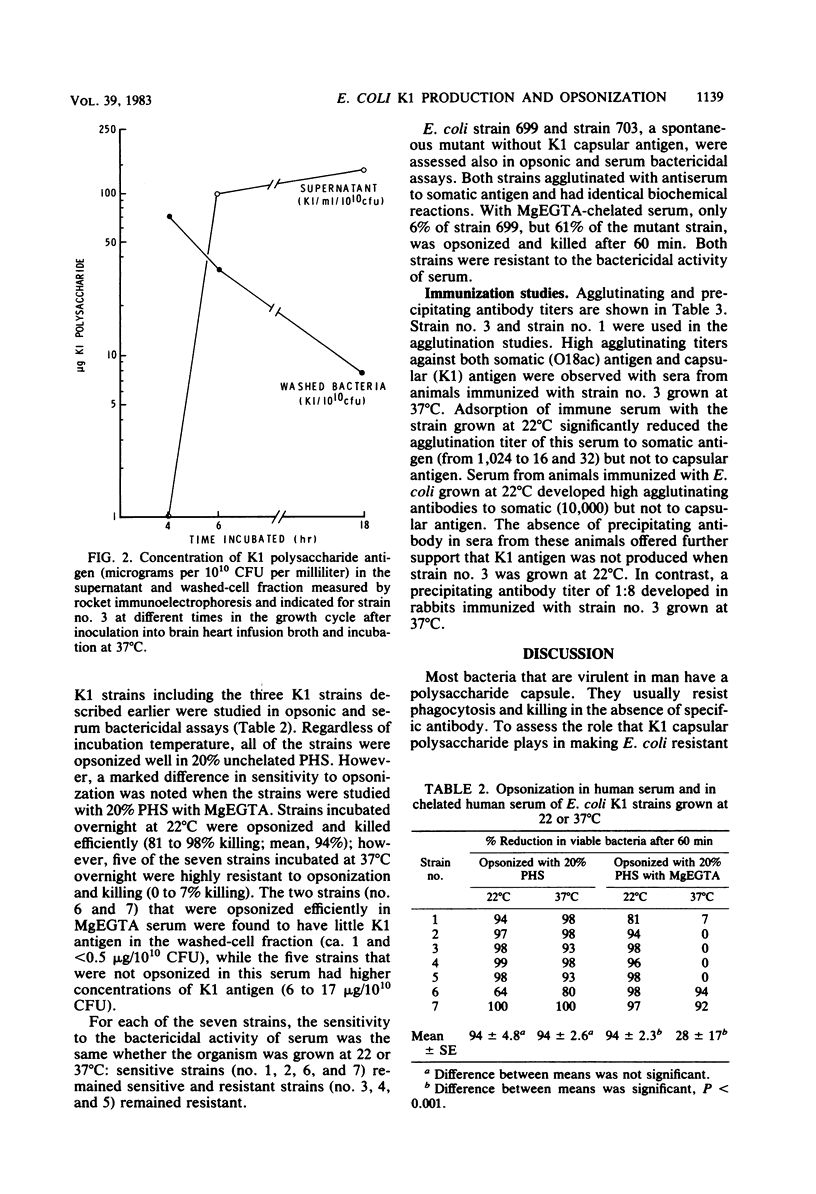
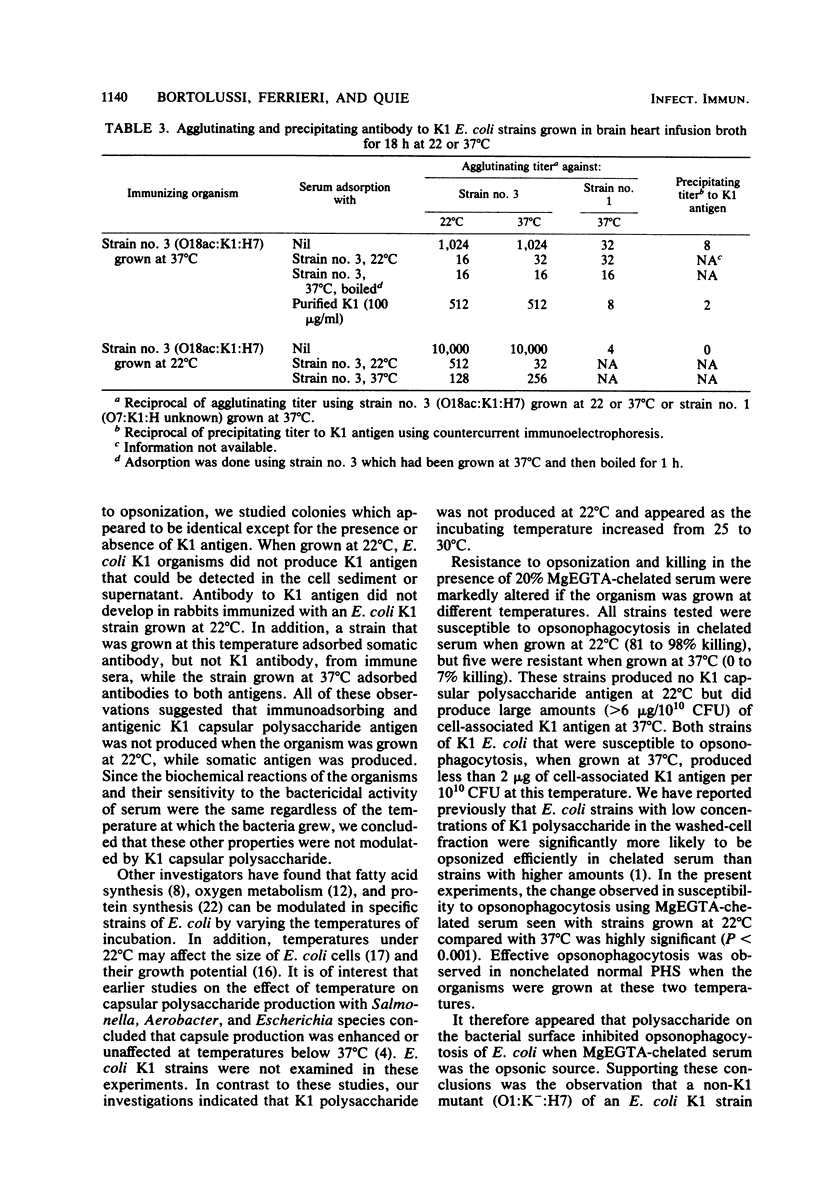
When Escherichia coli strains that produce K1 capsular polysaccharide antigen at 37 degrees C were grown at 22 degrees C, K1 antigen was not detected in the supernatant or washed-cell fraction of broth cultures. Significant amounts of K1 polysaccharide were detected only when the organism was grown at temperatures of 30 degrees C or higher. Rabbits immunized with an E. coli K1 strain (serotype O18ac:K1:H7) grown at 37 degrees C produced agglutinating antibody to somatic antigen and precipitating and agglutinating antibody to capsular K1 antigen; those immunized with this strain grown at 22 degrees C produced antibody to somatic antigen, but not to K1 antigen. Antibody to somatic antigen was markedly reduced by adsorption with the organism grown at 22 degrees C, while antibody to capsular antigen was not. E. coli K1 strains grown at 37 degrees C (K1 present) resisted phagocytosis and killing if they were opsonized solely by the alternative complement pathway (ACP) using magnesium ethylene glycol-bis(beta-aminoethyl ether)-N,N-tetraacetic acid-chelated serum. When these strains were grown at 22 degrees C (K1 absent), they were opsonized efficiently by the ACP (28 versus 94% killing, respectively; P less than 0.001). In addition, a non-K1 mutant of an E. coli K1 strain was opsonized efficiently by the ACP although its encapsulated K1 parent was not. Sensitivity of E. coli strains to the bactericidal activity of serum was observed in strains with and without K1 capsular antigen. These studies demonstrated that production of K1 polysaccharide antigen was regulated by environmental temperature and that K1 capsule plays an essential role in rendering the organism resistant to opsonization by the ACP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bortolussi R., Ferrieri P., Björkstén B., Quie P. G. Capsular K1 polysaccharide of Escherichia coli: relationship to virulence in newborn rats and resistance to phagocytosis. Infect Immun. 1979 Jul;25(1):293–298. doi: 10.1128/iai.25.1.293-298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolussi R., Ferrieri P., Wannamaker L. W. Dynamics of Escherichia coli infection and meningitis in infant rats. Infect Immun. 1978 Nov;22(2):480–485. doi: 10.1128/iai.22.2.480-485.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGUID J. P., WILKINSON J. F. The influence of cultural conditions on polysaccharide production by Aerobacter aerogenes. J Gen Microbiol. 1953 Oct;9(2):174–189. doi: 10.1099/00221287-9-2-174. [DOI] [PubMed] [Google Scholar]
- Edwards M. S., Nicholson-Weller A., Baker C. J., Kasper D. L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980 May 1;151(5):1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Current concepts in immunology: the alternative pathway of complement--a system for host resistance to microbial infection. N Engl J Med. 1980 Jul 31;303(5):259–263. doi: 10.1056/NEJM198007313030505. [DOI] [PubMed] [Google Scholar]
- Forsgren A., Mclean R. H., Michael A. F., Quie P. G. Studies of the alternate pathway in chelated serum. J Lab Clin Med. 1975 Jun;85(6):904–912. [PubMed] [Google Scholar]
- Garwin J. L., Cronan J. E., Jr Thermal modulation of fatty acid synthesis in Escherichia coli does not involve de novo enzyme synthesis. J Bacteriol. 1980 Mar;141(3):1457–1459. doi: 10.1128/jb.141.3.1457-1459.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glode M. P., Sutton A., Moxon E. R., Robbins J. B. Pathogenesis of neonatal Escherichia coli meningitis: induction of bacteremia and meningitis in infant rats fed E. coli K1. Infect Immun. 1977 Apr;16(1):75–80. doi: 10.1128/iai.16.1.75-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980 Jan;65(1):82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D. L., Winkelhake J. L., Zollinger W. D., Brandt B. L., Artenstein M. S. Immunochemical similarity between polysaccharide antigens of Escherichia coli 07: K1(L):NM and group B Neisseria meningitidis. J Immunol. 1973 Jan;110(1):262–268. [PubMed] [Google Scholar]
- Mainzer S. E., Hempfling W. P. Effects of growth temperature on yield and maintenance during glucose-limited continuous culture of Escherichia coli. J Bacteriol. 1976 Apr;126(1):251–256. doi: 10.1128/jb.126.1.251-256.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov F., Orskov I., Sutton A., Schneerson R., Lin W., Egan W., Hoff G. E., Robbins J. B. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J Exp Med. 1979 Mar 1;149(3):669–685. doi: 10.1084/jem.149.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Sarff L. D., McCracken G. H., Schiffer M. S., Glode M. P., Robbins J. B., Orskov I., Orskov F. Epidemiology of Escherichia coli K1 in healthy and diseased newborns. Lancet. 1975 May 17;1(7916):1099–1104. doi: 10.1016/s0140-6736(75)92496-4. [DOI] [PubMed] [Google Scholar]
- Shaw M. K., Marr A. G., Ingraham J. L. Determination of the minimal temperature for growth of Escherichia coli. J Bacteriol. 1971 Feb;105(2):683–684. doi: 10.1128/jb.105.2.683-684.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata T. E., Marr A. G. Effect of temperature on the size of Escherichia coli cells. J Bacteriol. 1975 Nov;124(2):857–862. doi: 10.1128/jb.124.2.857-862.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens P., Chu C. L., Young L. S. K-1 antigen content and the presence of an additional sialic acid-containing antigen among bacteremic K-1 Escherichia coli: correlation with susceptibility to opsonophagocytosis. Infect Immun. 1980 Sep;29(3):1055–1061. doi: 10.1128/iai.29.3.1055-1061.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk W. C., Verbrugh H. A., van der Tol M. E., Peters R., Verhoef J. Role of Escherichia coli K capsular antigens during complement activation, C3 fixation, and opsonization. Infect Immun. 1979 Aug;25(2):603–609. doi: 10.1128/iai.25.2.603-609.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Temperature-induced synthesis of specific proteins in Escherichia coli: evidence for transcriptional control. J Bacteriol. 1980 Jun;142(3):843–851. doi: 10.1128/jb.142.3.843-851.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


