Abstract
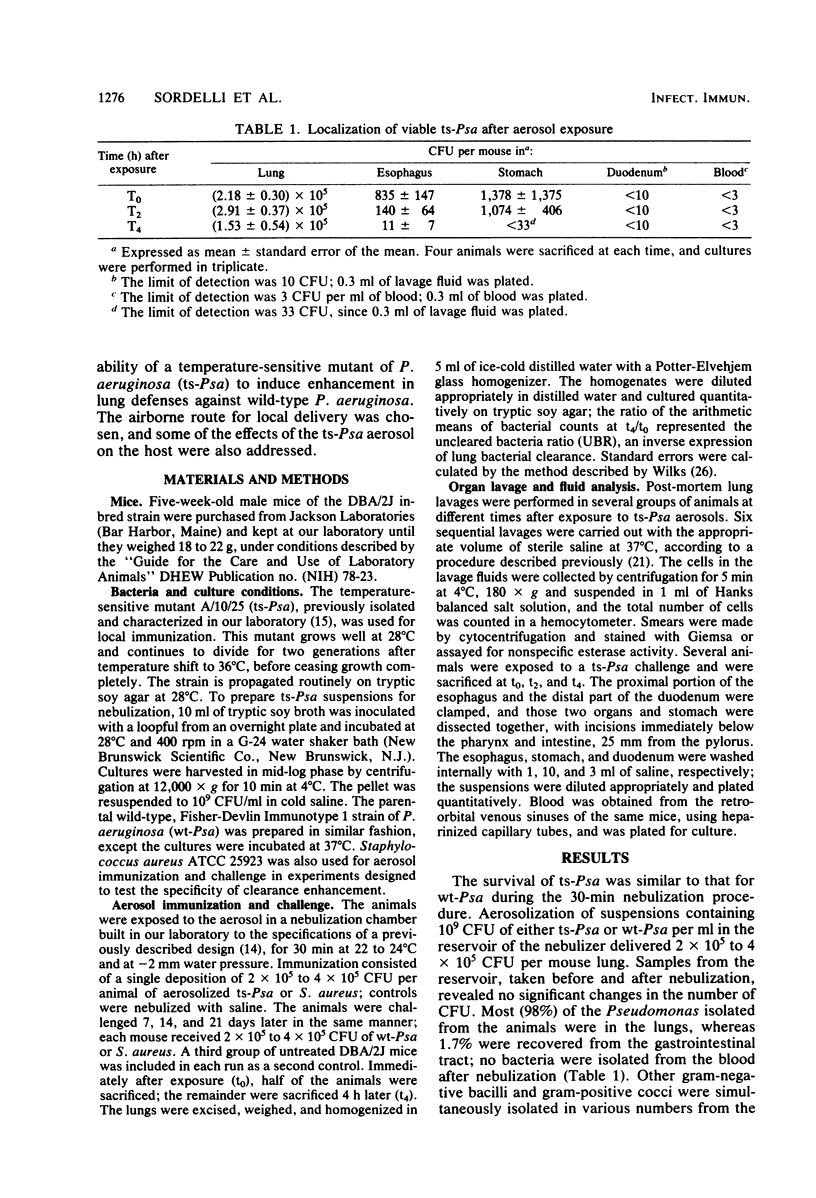
We investigated the capacity of the temperature-sensitive mutant strain A/10/25 of Pseudomonas aeruginosa (ts-Psa) to induce enhancement of lung defenses against wild type P. aeruginosa (wt-Psa). Mice of the DBA/2J inbred strain were immunized by aerosolization with a single dose of 2 x 10(5) to 4 x 10(5) CFU of ts-Psa and were challenged 7, 14, and 21 days later with wt-Psa. The uncleared bacteria ratio was determined 4 h after aerosol exposure; significant enhancement in lung clearance of wt-Psa (P less than 0.01) was evident as early as 7 days after immunization and detectable for at least 21 days. Aerosol immunization with Staphylococcus aureus did not enhance lung clearance of wt-Psa; however, slight but significant enhancement in S. aureus clearance was observed in mice immunized 7 days before with ts-Psa. No enhancement of S. aureus clearance was seen in ts-Psa immunized animals after 14 and 21 days. Analysis of the cell composition of lung lavage fluids revealed a transient cell response characterized by rapid increase in the absolute number of polymorphonuclear leukocytes, followed later by an increase in alveolar macrophages. The characteristics of lung lavages returned to base-line values 6 days after aerosol immunization, and a second exposure to a ts-Psa aerosol produced a response of similar magnitude and quality. We conclude that aerosol immunization with a temperature-sensitive mutant of P. aeruginosa enhances specific pulmonary defense mechanisms against the parental pathogen in mice.
Full text
PDF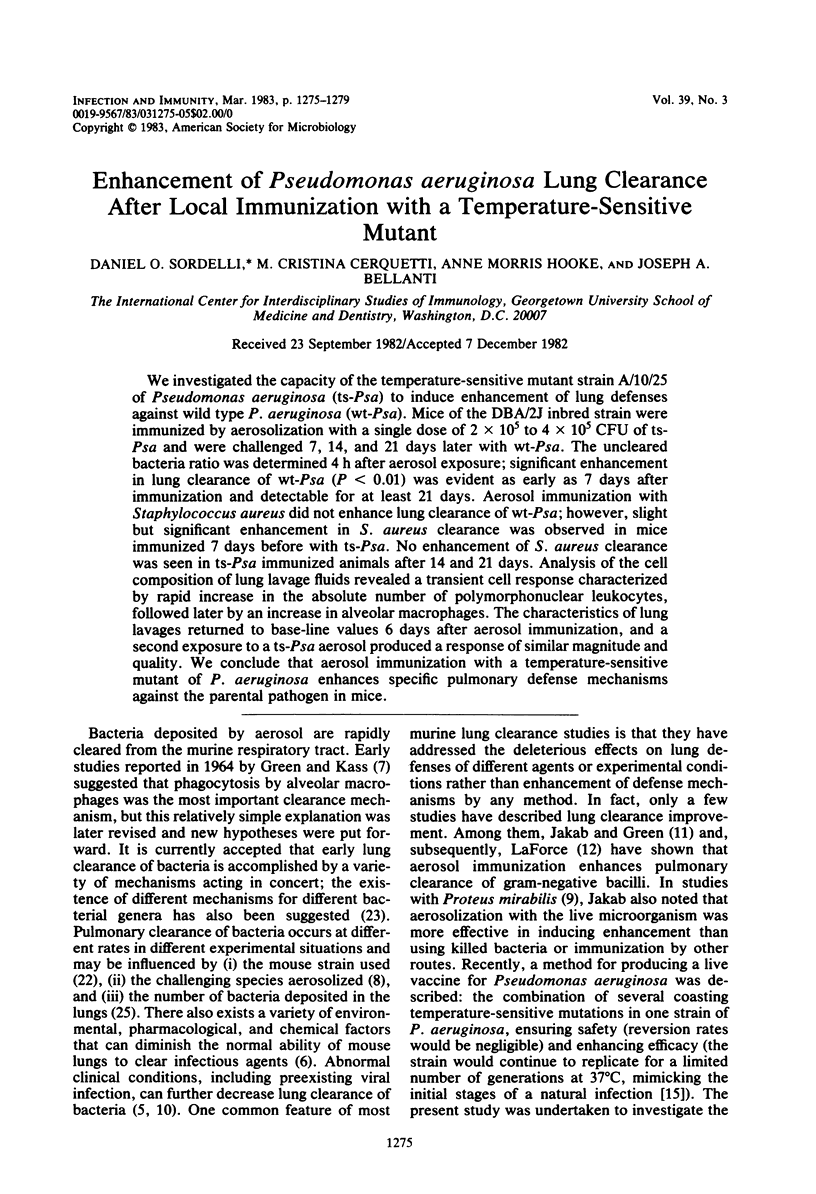


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W. Serum and leukocyte lysosomal enzymes. Derangements following severe thermal injury. Arch Surg. 1967 Sep;95(3):482–491. doi: 10.1001/archsurg.1967.01330150158020. [DOI] [PubMed] [Google Scholar]
- Bryant R. E., Hood A. F., Hood C. E., Koenig M. G. Factors affecting mortality of gram-negative rod bacteremia. Arch Intern Med. 1971 Jan;127(1):120–128. [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Matthay R. A., Reynolds H. Y. Cystic fibrosis pseudomonas opsonins. Inhibitory nature in an in vitro phagocytic assay. J Clin Invest. 1981 Oct;68(4):899–914. doi: 10.1172/JCI110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freid M. A., Vosti K. L. The importance of underlying disease in patients with gram-negative bacteremia. Arch Intern Med. 1968 May;121(5):418–423. [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. FACTORS INFLUENCING THE CLEARANCE OF BACTERIA BY THE LUNG. J Clin Invest. 1964 Apr;43:769–776. doi: 10.1172/JCI104961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E., Green G. M. The effect of acute renal failure on the bacterial clearance mechanisms of the lung. J Lab Clin Med. 1966 Oct;68(4):531–542. [PubMed] [Google Scholar]
- Green G. M., Jakab G. J., Low R. B., Davis G. S. Defense mechanisms of the respiratory membrane. Am Rev Respir Dis. 1977 Mar;115(3):479–514. doi: 10.1164/arrd.1977.115.3.479. [DOI] [PubMed] [Google Scholar]
- Jakab G. J. Factors influencing the immune enhancement of intrapulmonary bactericidal mechanisms. Infect Immun. 1976 Aug;14(2):389–398. doi: 10.1128/iai.14.2.389-398.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. Immune enhancement of pulmonary bactericidal activity in murine virus pneumonia. J Clin Invest. 1973 Nov;52(11):2878–2884. doi: 10.1172/JCI107484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. The effect of Sendai virus infection on bactericidal and transport mechanisms of the murine lung. J Clin Invest. 1972 Aug;51(8):1989–1998. doi: 10.1172/JCI107005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURENZI G. A., BERMAN L., FIRST M., KASS E. H. A QUANTITATIVE STUDY OF THE DEPOSITION AND CLEARANCE OF BACTERIA IN THE MURINE LUNG. J Clin Invest. 1964 Apr;43:759–768. doi: 10.1172/JCI104960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaForce F. M., Boose D. Immune stimulation of lung bactericidal activity: evidence for both cellular and humoral participation after immunization with Serratia marcescens. Am Rev Respir Dis. 1980 Jun;121(6):921–929. doi: 10.1164/arrd.1980.121.6.921. [DOI] [PubMed] [Google Scholar]
- LaForce F. M. Effect of aerosol immunization with RE 595 Salmonella minnesota on lung bactericidal activity against Serratia marcescens, Enterobacter cloacae, and Pseudomonas aeruginosa. Am Rev Respir Dis. 1977 Aug;116(2):241–249. doi: 10.1164/arrd.1977.116.2.241. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Edman D. C., Leppla S. H., Wretlind B., Lewis L. R., Martin K. E. Protection against experimental Pseudomonas aeruginosa infection in mice by active immunization with exotoxin A toxoids. Infect Immun. 1981 May;32(2):681–689. doi: 10.1128/iai.32.2.681-689.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E., Hickey W. F., Blackwood L. L., Arnaut M. A. Active immunization with lipopolysaccharide Pseudomonas antigen for chronic Pseudomonas bronchopneumonia in guinea pigs. J Clin Invest. 1981 Nov;68(5):1140–1148. doi: 10.1172/JCI110358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E., Kuchmy D. Mechanism for pulmonary protection by lipopolysaccharide pseudomonas vaccine. J Infect Dis. 1980 Aug;142(2):191–198. doi: 10.1093/infdis/142.2.191. [DOI] [PubMed] [Google Scholar]
- Pier G. B. Safety and immunogenicity of high molecular weight polysaccharide vaccine from immunotype 1 Pseudomonas aeruginosa. J Clin Invest. 1982 Feb;69(2):303–308. doi: 10.1172/JCI110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Sadoff J. C. Protective immunity induced in mice by immunization with high-molecular-weight polysaccharide from Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):919–925. doi: 10.1128/iai.22.3.919-925.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivetta O. H., Cassino R. J., Sordelli D. O. Pulmonary cell response to bacterial challenge in mutant mice with some hereditary alterations resembling cystic fibrosis. Life Sci. 1980 Apr 21;26(16):1349–1357. doi: 10.1016/0024-3205(80)90096-x. [DOI] [PubMed] [Google Scholar]
- Pivetta O. H., Sordelli D. O., Labal M. L. Pulmonary clearance of Staphylococcus aureus in mutant mice with some hereditary alterations resembling cystic fibrosis. Pediatr Res. 1977 Nov;11(11):1133–1136. doi: 10.1203/00006450-197711000-00003. [DOI] [PubMed] [Google Scholar]
- Rehm S. R., Gross G. N., Pierce A. K. Early bacterial clearance from murine lungs. Species-dependent phagocyte response. J Clin Invest. 1980 Aug;66(2):194–199. doi: 10.1172/JCI109844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Thompson R. E., Devlin H. B. Development of cellular and humoral immunity in the respiratory tract of rabbits to Pseudomonas lipopolysaccharide. J Clin Invest. 1974 May;53(5):1351–1358. doi: 10.1172/JCI107683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews G. B., Gross G. N., Pierce A. K. The relationship of inoculum size to lung bacterial clearance and phagocytic cell response in mice. Am Rev Respir Dis. 1979 Sep;120(3):559–566. doi: 10.1164/arrd.1979.120.3.559. [DOI] [PubMed] [Google Scholar]


