Abstract
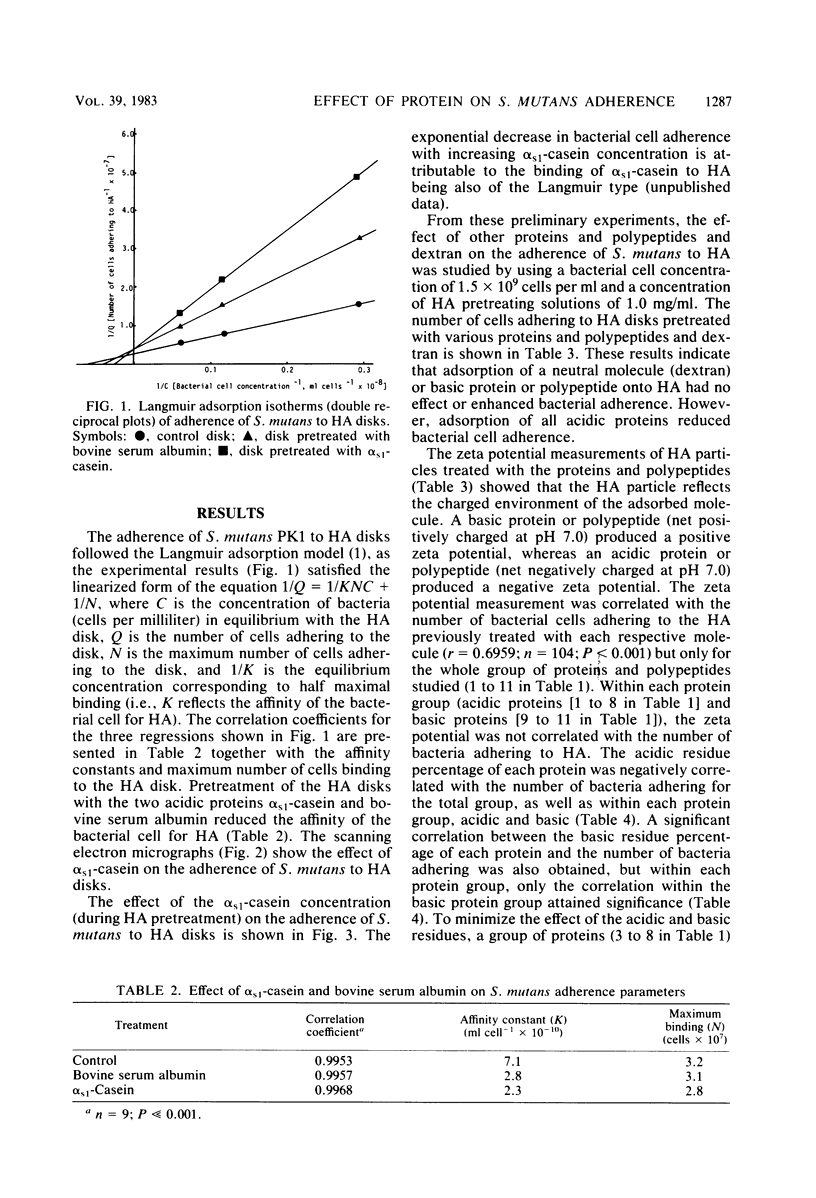
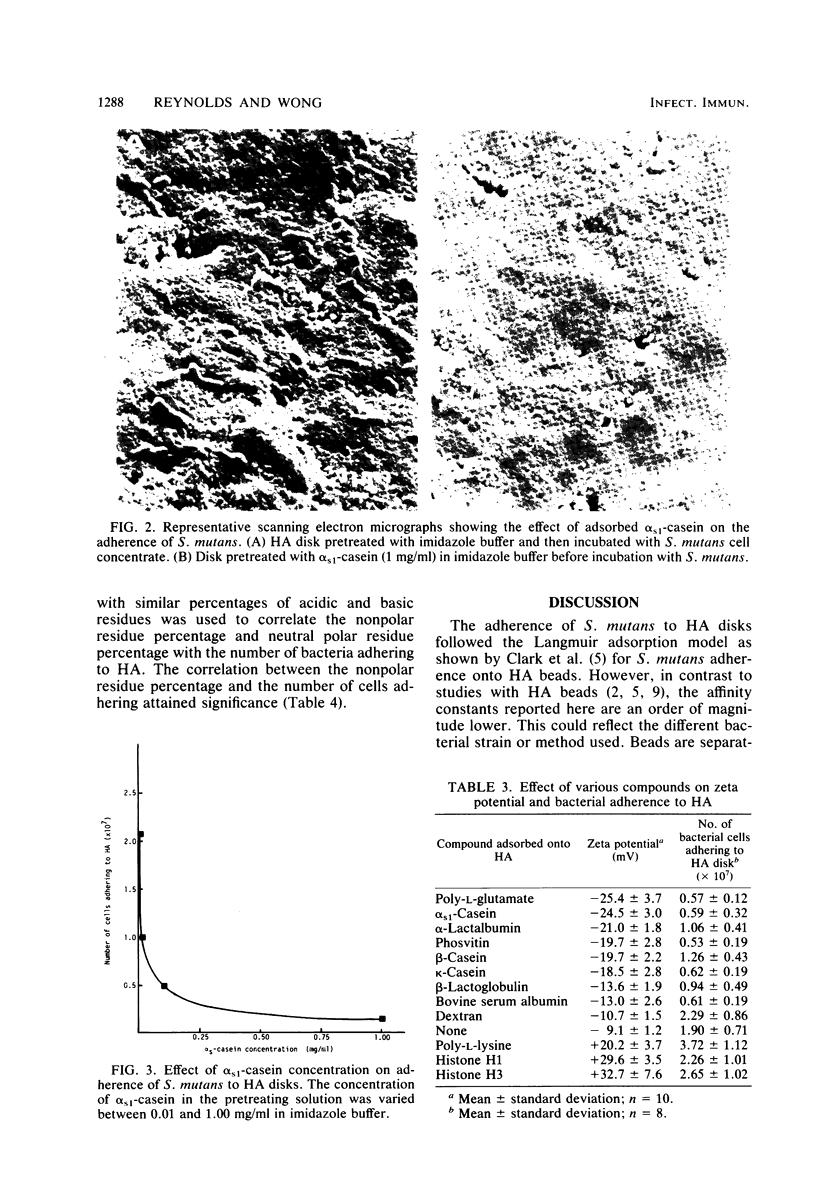
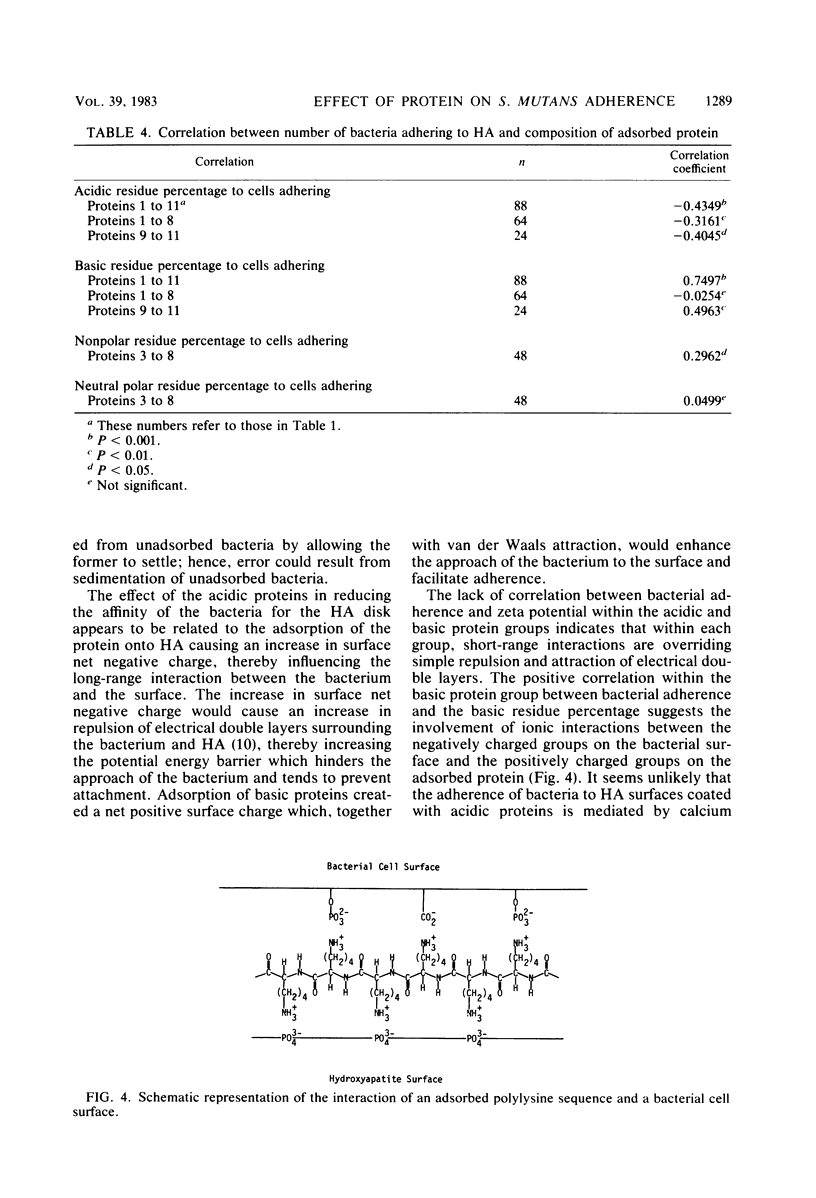
The adherence of Streptococcus mutans PK1 to hydroxyapatite disks pretreated with various acidic and basic proteins in imidazole buffer was studied. Adsorption of a basic protein onto an hydroxyapatite disk enhanced or had no effect on bacterial adherence, whereas adsorption of an acidic protein reduced adherence. The effect of adsorbed protein on bacterial adherence was of both short and long range. The long-range effect of the acidic proteins in reducing the number of bacteria adhering to hydroxyapatite was related to protein adsorption causing an increase in surface net negative charge, as shown by zeta potential measurement. Basic protein produced a net positive surface charge which facilitated adherence. Within the acidic protein group, the acidic residue percentage of the adsorbed protein was negatively correlated with the number of bacteria adhering, whereas the nonpolar residue percentage was positively correlated with bacterial adherence. Within the basic protein group, the basic residue percentage was correlated with the number of cells adhering. These results indicate the involvement of short-range hydrophobic and ionic interactions in bacterial adherence to protein-coated hydroxyapatite.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum B., Golub E., Holt S. C., Rosan B. In vitro studies of dental plaque formation: adsorption of oral streptococci to hydroxyaptite. Infect Immun. 1979 Aug;25(2):717–728. doi: 10.1128/iai.25.2.717-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt W. F., von Holt C. The determination of the primary structure of histone F3 from chicken erythrocytes by automatic Edman degradation. 2. Sequence analysis of histone F3. Eur J Biochem. 1974 Jul 15;46(2):419–429. doi: 10.1111/j.1432-1033.1974.tb03635.x. [DOI] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod A. R., Wong N. C., Dixon G. H. The amino-acid sequence of trout-testis histone H1. Eur J Biochem. 1977 Aug 15;78(1):281–291. doi: 10.1111/j.1432-1033.1977.tb11739.x. [DOI] [PubMed] [Google Scholar]
- Olsson J., Glantz P. O., Krasse B. Electrophoretic mobility of oral streptococci. Arch Oral Biol. 1976;21(10):605–609. doi: 10.1016/0003-9969(76)90030-3. [DOI] [PubMed] [Google Scholar]
- Rölla G. Formation of dental integuments--some basic chemical considerations. Swed Dent J. 1977;1(6):241–251. [PubMed] [Google Scholar]
- Simonson L. G., Jackola D. Effects of dextranases on attachment of Streptococcus mutans to hydroxyapatite. Antimicrob Agents Chemother. 1979 Jul;16(1):9–12. doi: 10.1128/aac.16.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson L. G., Reiher D. A. Effect of human saliva and various compounds on the adsorption of the bacterium Streptococcus mutans to hydroxyapatite. Arch Oral Biol. 1981;26(2):143–146. doi: 10.1016/0003-9969(81)90085-6. [DOI] [PubMed] [Google Scholar]
- Taborsky G. Phosphoproteins. Adv Protein Chem. 1974;28:1–210. doi: 10.1016/s0065-3233(08)60230-2. [DOI] [PubMed] [Google Scholar]



