Abstract
BACKGROUND AND PURPOSE
Agonists selective for the α7 nicotinic acetylcholine (nACh) receptor produce anti-hyperalgesic effects in rodent models of inflammatory pain, via direct actions on spinal pain circuits and possibly via attenuated release of peripheral pro-inflammatory mediators. Increasingly, allosteric modulation of ligand-gated receptors is recognized as a potential strategy to obtain desired efficacy in the absence of the putative adverse effects associated with agonist activation.
EXPERIMENTAL APPROACH
We compared the anti-hyperalgesic and anti-inflammatory effects of the α7 nACh receptor agonist compound B with the positive allosteric modulator (PAM) PNU-120596 and the standard non-steroidal anti-inflammatory drug (NSAID), diclofenac, in rats with hind paw inflammation induced by either formalin, carrageenan or complete Freund's adjuvant (CFA).
KEY RESULTS
When administered before carrageenan, both diclofenac (30 mg·kg−1) and PNU-120596 (30 mg·kg−1) significantly reduced mechanical hyperalgesia and weight-bearing deficits for up to 4 h. Compound B (30 mg·kg−1) also attenuated both measures of pain-like behaviour, albeit less robustly. Whereas compound B and PNU-120596 attenuated the carrageenan-induced increase in levels of TNF-α and IL-6 within the hind paw oedema, diclofenac only attenuated IL-6 levels. Established mechanical hyperalgesia induced by carrageenan or CFA was also partially reversed by compound B and PNU-120596. However, diclofenac was considerably more efficacious. Formalin-induced nocifensive behaviours were only reversed by compound B, albeit at doses which disrupted motor performance.
CONCLUSIONS AND IMPLICATIONS
α7 nACh receptor PAMs could prove to be useful in the treatment of inflammatory pain conditions, which respond poorly to NSAIDs or in situations where NSAIDs are contra-indicated.
Keywords: analgesia, inflammation, carrageenan, CFA, diclofenac, IL-6, IL-1β, NSAID, pain, TNF-α
Introduction
The standard of care non-steroidal anti-inflammatory drug (NSAID) diclofenac, is a relatively selective inhibitor of COX-2 and effectively inhibits prostaglandin production. This established mechanism of action contributes to its use in humans suffering from painful arthritic disorders and for treatment of mild to moderate pain (Gan, 2010). Although generally well tolerated, the huge number of patients exposed to NSAIDs means that safety concerns, principally resolving around cardiovascular, gastrointestinal and hepatic complications, are an important limitation in their routine usage. Thus, the development of novel compounds that possess anti-inflammatory properties, and if possible, mediate robust analgesia, is of high priority. Nicotinic acetylcholine (nACh) receptors are ligand-gated ion channels present in both the peripheral and central nervous systems. They are composed of five subunits with the various pentameric combinations differing in their kinetic and pharmacological properties (Albuquerque et al., 2009). While modulation of nACh receptor function has primarily been considered for treating various psychiatric disorders, considerable interest has increasingly been directed towards chronic pain. This latter effort has primarily focussed around attempts to develop α4β2 nACh receptor-preferring agonists as novel analgesics (Bannon et al., 1998; Rowbotham et al., 2009) but to date has been severely hindered by tolerability issues.
Thus, attention has moved towards developing different strategies in relation to obtaining nACh receptor-mediated analgesia. Accordingly, agonist-mediated activation of α7 nACh receptors has been shown to attenuate pain-like behaviours in rodents induced by carrageenan, complete Freund's adjuvant (CFA), peripheral nerve injury and glycoprotein-120 (Medhurst et al., 2008; Feuerbach et al., 2009; Gurun et al., 2009; Loram et al., 2010; Pacini et al., 2010; Bagdas et al., 2011). Importantly, reversal of CFA-induced inflammatory pain with the α7 nACh receptor agonist compound B is blocked by intrathecal application of the α7 nACh receptor antagonist methyllycaconitine (MLA), confirming a spinal site of action (Medhurst et al., 2008). However, a contributory role of peripherally localized nACh receptors in inflammatory pain cannot be easily discounted. Notably, inactivation of α7 nACh receptor function within primary macrophages, lymphocytes and dendritic cells prevents nicotine and α7 nACh receptor agonists from attenuating peripheral immune cell infiltration and secretion of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 (Wang et al., 2003; de Jonge et al., 2005; Nizri et al., 2009; Li et al., 2011). Accordingly, in rats with carrageenan-induced inflammation, intraplantar cytidine-5′-diphosphate (CDP)-choline (a precursor to choline, which is a full agonist at α7 nACh receptors) reverses hind paw mechanical hypersensitivity and production of the pro-inflammatory cytokine TNF-α (Gurun et al., 2009).
Alternatively, positive allosteric modulation of ligand-gated receptor function represents another strategy that can be pursued to obtain novel nACh receptor-mediated analgesia. Alone, this should not in itself induce intrinsic activation but should theoretically amplify the effects of agonists, which in the case of nACh receptors implies tonic ACh-mediated neurotransmission, to putatively confer a higher degree of selectivity at the target of interest (Taly et al., 2009). PNU-120596 is a positive allosteric modulator (PAM) of α7 nACh receptors that displays no detectable effect on α4β2, α3β4 or α9α10 nACh receptors and improves auditory gating deficits induced by d-amphetamine in anaesthetized rats (Hurst et al., 2005). It remains, however, to be established whether an α7 nACh receptor PAM also displays efficacy in inflammatory pain. Here, we show that PNU-120596 produces anti-hyperalgesia that is at least comparable with that obtained with compound B in rats with carrageenan and CFA-induced hind paw inflammation. Furthermore, α7 nACh receptor activation differentially affects pro-inflammatory cytokine levels compared with diclofenac.
Methods
Animals
Adult male Sprague-Dawley rats (Harlan Scandinavia, Alleroed, Denmark) were used unless indicated otherwise; the total number of rats used was 722. They were housed in Macrolon III cages (Bayer MaterialScience AG, Leverkusen, Germany; 20 × 14 × 18 cm or 20 × 40 × 18 cm; in groups of three to five per cage according to weight) containing woodchip bedding material (3 × 1 × 4 mm). The environment was kept at 20 ± 2°C, humidity was controlled (55 ± 15%) and a light–dark cycle of 13:11 h (lights on at 0600 h and off at 1900 h) was imposed. Food (Altromin®; Altromin Spezialfutter GmbH & Co., Lage, Germany) and water were available ad libitum. The rats were allowed to habituate to the housing facilities for at least 1 week before being assigned to behavioural experiments whereupon they were randomly distributed across treatment groups. At the end of each experiment, the rats were killed by cervical dislocation. All experiments were performed according to the Ethical Guidelines of the International Association for the Study of Pain (Zimmermann, 1983) and the Danish Committee for Experiments on Animals. All the studies involving animals are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010).
Formalin test
Assessment of formalin-induced flinching behaviour in normal, uninjured rats (body weight 180–300 g) was made with the use of an Automated Nociception Analyser (University of California, San Diego, CA, USA; Yaksh et al., 2001) as described previously (Munro et al., 2008; 2011). Briefly, this involved placing a small C-shaped metal band (10 mm wide × 27 mm long) around the hind paw of the rat to be tested. Each rat (four rats were included in each testing session) was administered drug or vehicle according to the experimental paradigm being followed (see Table 1) and then placed in a cylindrical acrylic observation chamber (diameter 15 cm and height 30.5 cm). Individual rats were then gently restrained, and formalin (5% in saline, 50 µL, s.c.) was injected into the dorsal surface of the hind paw using a 27G needle. They were then returned to their separate observation chambers, each of which was, in turn, situated upon an enclosed detection device composed of a pair of electromagnetic coils designed to produce an electromagnetic field in which movement of the metal band could be detected. The analogue signal was then digitized and a software algorithm applied to enable discrimination of flinching behaviour from other paw movements prior to binning into 1 min sampling intervals. On the basis of the resulting response patterns, three phases of nocifensive behaviour were identified: first phase (0–5 min), interphase (6–15 min) and second phase (16–40 min). Raw data from the 1 min sampling intervals were summed for each phase to obtain the total number of flinches occurring during that period. For purposes of statistical analysis, this value was then expressed as a % of the vehicle response according to the equation: % vehicle = (post-treatment value) / (vehicle value) × 100.
Table 1.
Effects of compound B and PNU-120596 in the formalin test
| Drug | Dose (mg·kg−1) | First phase % Vehicle | Interphase % Vehicle | Second phase % Vehicle |
|---|---|---|---|---|
| Compound B | Vehicle | 100 ± 9.9 | 100 ± 21.5 | 100 ± 10.8 |
| (171.1 ± 16.9) | (167.8 ± 36.1) | (907.5 ± 98.4) | ||
| 3 | 113.4 ± 25.7 | 113.8 ± 36.7 | 109.5 ± 10.9 | |
| 10 | 97.9 ± 20.1 | 89 ± 29.7 | 102.4 ± 8.4 | |
| 30 | 92.2 ± 18.6 | 79.2 ± 23.6 | 99.9 ± 13.6 | |
| 45 | 37.6 ± 8.1* | 24.2 ± 9 | 49.5 ± 16** | |
| 60 | 16.7 ± 6.3** | 9.1 ± 1.4* | 18.8 ± 2.9*** | |
| PNU-120596 | Vehicle | 100 ± 13.9 | 100 ± 22.9 | 100 ± 4.4 |
| (169.1 ± 23.4) | (162.8 ± 37.2) | (1052.9 ± 46.3) | ||
| 10 | 75.8 ± 10.8 | 65.7 ± 13.0 | 107.7 ± 8.7 | |
| 30 | 73.5 ± 16.4 | 72.8 ± 20.7 | 97.7 ± 10.1 | |
| 60 | 97.3 ± 9.8 | 83.2 ± 14.6 | 93.9 ± 12.7 | |
| Diclofenac | Vehicle | 100 ± 21.8 | 100 ± 30.8 | 100 ± 12.2 |
| (123.1 ± 26.8) | (86.7 ± 26.7) | (962.6 ± 117.8) | ||
| 3 | 102.6 ± 15.9 | 62.3 ± 28.8 | 98.8 ± 10.3 | |
| 10 | 101.5 ± 17.5 | 118.3 ± 58.3 | 117.9 ± 11.7 | |
| 30 | 116.2 ± 23.3 | 86 ± 21.2 | 107 ± 7 | |
| Morphine | Vehicle | 100 ± 19.9 | 100 ± 24.4 | 100 ± 7.9 |
| (111.4 ± 22.19 | (117.3 ± 28.6) | (968.6 ± 76.8) | ||
| 1 | 120.4 ± 25.3 | 65.6 ± 27.8 | 65.3 ± 13.2* | |
| 3 | 98.9 ± 16.1 | 35.4 ± 12 | 43.4 ± 12.4*** | |
| 6 | 33.2 ± 11* | 16.3 ± 8.7* | 7.9 ± 3.2*** |
The rats were administered either compound B (3–60 mg·kg−1, s.c. t =−30 min), PNU-120596 (10–60 mg·kg−1, i.p., t =−30 min), diclofenac (3–30 mg·kg−1, p.o., t =−60 min), morphine (1–6 mg·kg−1, s.c., t =−30 min) or vehicle at the time (t) indicated before hind paw formalin (5% in saline, 50 µL, s.c.) injection. Subsequently, the % vehicle values for first phase (0–5 min), interphase (6–15 min) or second phase (16–40 min) were calculated; data in parentheses represent raw untransformed flinch data for vehicle-treated animals. All treatment groups: n= 8–9 rats, except compound B n= 6–8 rats. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus corresponding vehicle group (one-way anova followed by Bonferonni's t-test).
Carrageenan- and CFA-induced inflammatory pain
Four separate studies were performed in order to follow the time course of pain-like behaviours induced by carrageenan and correlate them with hind paw swelling and cytokine levels. The anti-hyperalgesic actions of compound B, PNU-120596 and diclofenac were determined in rats with established inflammatory pain induced by either carrageenan or complete Freund's adjuvant (CFA). In addition, we determined the anti-hyperalgesic and anti-inflammatory actions of the same three compounds administered before the development of carrageenan-induced inflammation.
Study 1
In this time course experiment, nine groups of rats (body weight 200–300 g) were tested in total. Eight groups of rats were gently restrained and given an s.c. injection of λ-carrageenan (2% in saline, 100 µL, Sigma-Aldrich Danmark A/S, Brondby, Denmark) into the plantar surface of the hind paw before being examined for the development of non-evoked and evoked pain-like behaviours and inflammatory activity as described later. Individual groups were killed at 60, 120, 180, 240, 300, 360, 420 or 480 min after carrageenan injection. A control group of rats received no injection and killed at time = 0 min.
At the designated time point, non-evoked pain-like behaviours were obtained by measuring hind paw weight bearing distribution using an Incapacitance tester (Linton Instrumentation, Palgrave, UK). Normally, uninjured rats distribute their weight evenly between the two hind paws. However, after tissue injury, the rat preferentially favours the non-injured hind paw such that the weight-bearing deficit can be used as a surrogate index of spontaneous nociception. The rat was placed in the supplied Perspex chamber (Linton Instrumentation), which is designed so that each hind paw must be placed on a separate transducer pad. The testing duration was set to 5 s and the digital read out for each hind paw was taken as the distributed body weight on each paw (g). Five readings were obtained to ensure that consistent responses were measured. These were averaged for each hind paw and the weight-bearing deficit calculated as the difference between them. Immediately afterwards, the rat was gently restrained by the investigator before the application of progressively increasing mechanical pressure to the mid-hind paw region using an electronic version of the Randall-Selitto device (IITC Life Science Inc., Woodland Hills, CA, USA), to obtain an index of evoked mechanical hyperalgesia (Munro et al., 2011). The point at which the rat attempted to make a reflex hind paw withdrawal, which in some instances, was followed by vocalization, was recorded as the paw pressure threshold (g). After 2–3 min, a second measurement was taken from a different region of the hind paw and an average value was calculated.
Subsequently, putative anti-inflammatory effects of compounds were assessed by measuring effects on hind paw swelling. First, paw thickness, both ipsilateral and contralateral to carrageenan injection, was measured using a pair of microcalipers after which the rat was killed by decapitation. Trunk blood was collected and stored on ice before being subjected to cytokine analyses as described later. Immediately thereafter, both hind paws were removed at the level of the ankle joint, enabling the wet paw weight to be measured. They were then stored on ice before being subjected to cytokine analyses as described later.
Study 2
Mechanical paw pressure thresholds were assessed in the rats (body weight 200–300 g) in four separate experiments as described earlier to obtain a pre-carrageenan baseline response. On the following day, each rat was again gently restrained before a s.c. injection of λ-carrageenan (2% in saline, 100 µL, Sigma) into the plantar surface of the hind paw. The rats were then returned to their home cage for 2.75–3.5 h after which time the paw pressure threshold was again measured (post-carrageenan baseline response) for each rat. In three of the experiments, the rats were randomly assigned to receive either drug (compound B, PNU-120596 or diclofenac) or vehicle before a final paw pressure threshold value was measured. In a fourth experiment, MLA was injected either before or after PNU-120596 before the measurement of the final paw pressure value. In all cases, post-drug threshold responses corresponded to 4 h after carrageenan injection.
Study 3
Mechanical paw pressure thresholds were assessed in the rats (body weight 250–300 g) as described earlier. Each rat was briefly anaesthetized with 2 % isoflurane (Baxter A/S; Alleroed, Denmark) in a mixture of oxygen (0.3 L min-1) and nitrous oxide (0.7 L min-1). The appropriate level of anaesthesia was verified by the absence of a hind paw nociceptive reflex to interdigital stimulation. Thereafter, a s.c. injection of CFA (50% in saline, 100 µL, Sigma) was given into the plantar surface of the hind paw. All the rats were then immediately returned to their home cage, whereupon they quickly recovered from anaesthesia. After 72 h, paw pressure thresholds were measured (post-CFA baseline response) and then the rats were randomly assigned to either drug or vehicle treatment. After either 30 min (compound B and PNU-120596) or 60 min (diclofenac) had elapsed, the paw pressure threshold was reassessed (post-CFA baseline response) for each rat.
Study 4
The rats were removed from their home cage to enable measurement of non-evoked and evoked pain-like behaviours using an Incapacitance tester and electronic Randall–Selitto device as described earlier. On the following day, they were randomly injected with drug or vehicle and then returned to their home cage for a period of time, which depended on the treatment to which they had been randomly assigned (compound B, 30 mg·kg−1, t = 30 min; PNU-120596, 30 mg·kg−1, t = 30 min; diclofenac, 10 mg·kg−1, 2 h). Next, they received an injection of 2% carrageenan or vehicle (referred to as sham in Figures 4 and 5). Evoked mechanical and non-evoked weight-bearing responses were then measured at 2 h and 4 h after injection of carrageenan or vehicle. Finally, the putative anti-inflammatory effects of the compounds were assessed by removing the inflamed hind paw and measuring the wet weight, as described earlier, and subjecting the samples to cytokine analyses.
Figure 4.
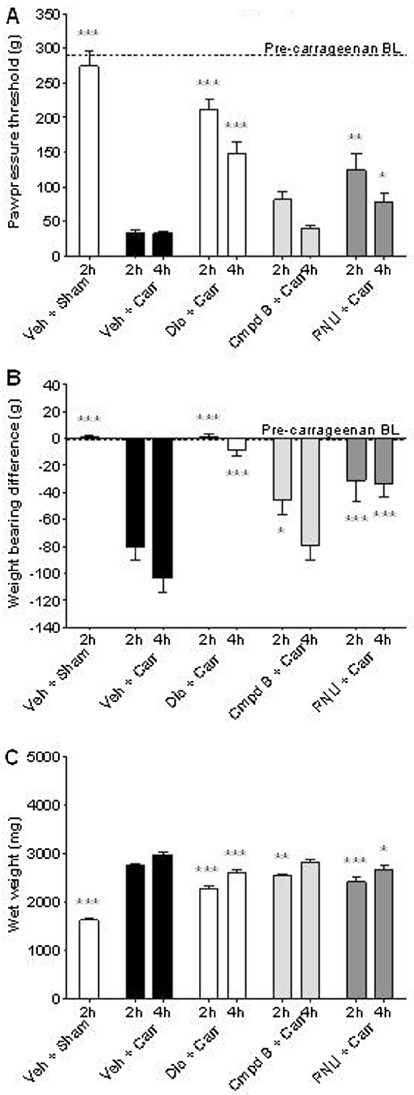
Pre-emptive α7 nACh receptor activation attenuates signs of carrageenan-induced inflammatory hypersensitivity. The rats were administered either diclofenac (10 mg·kg−1, p.o., t =−120 min), compound B (30 mg·kg−1, s.c., t =−30 min), PNU-120596 (30 mg·kg−1, i.p., t =−30 min) or vehicle (15% MBCD) at the time (t) indicated before carrageenan (2% in saline, 100 µL, s.c.) injection into the plantar surface of the hind paw. Pre- and post-inflammation baseline (BL) values are indicated with dashed lines. (A) Mechanical hyperalgesia: effects on paw withdrawal threshold (g) as an index of mechanical hyperalgesia. (B) Ongoing pain: effects on weight bearing deficits as an index of spontaneous ongoing pain. (C) Paw weight: effects on hind paw weights as an index of anti-inflammatory activity. All groups n= 7–9 rats. All data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus corresponding vehicle group (one-way anova followed by Bonferroni's t-test).
Figure 5.
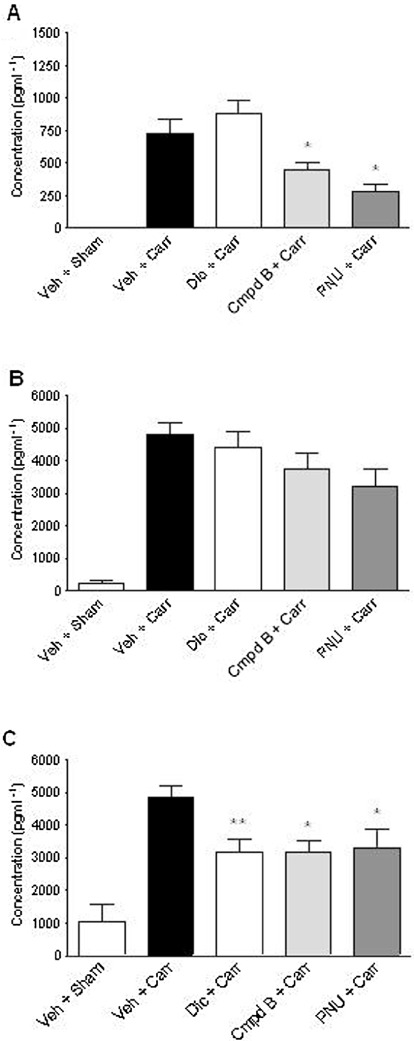
Activation of α7 nACh receptors selectively attenuates carrageenan-induced pro-inflammatory cytokine levels in the rat hind paw. The rats were administered either diclofenac (10 mg·kg−1, p.o., t =−120 min), compound B (30 mg·kg−1, s.c., t =−30 min), PNU-120596 (30 mg·kg−1, i.p., t =−30 min) or vehicle (15% MBCD) at the time (t) indicated before injection of either carrageenan or vehicle (sham) in the hind paw and determination of effects on hind paw oedema-associated levels of the pro-inflammatory cytokines (A) TNF-α, (B) IL-1β and (C) IL-6 at 2 h post-carrageenan. ###P < 0.001 versus Veh + sham; *P < 0.05, **P < 0.01 versus Veh + Carr (one-way anova followed by Student Newman–Keuls t-test).
Cytokine analyses
Immediately after measurement of hind paw wet weight, a sharp scalpel blade was used to make single 1 cm incisions through the dorsal and ventral skin surfaces of the hind paw, respectively. Extracellular (oedema) fluid samples were extracted from the whole hind paw by centrifuging at 16 000 x g (4°C) for 10 min. Corresponding trunk blood samples were centrifuged at 4000 x g (4°C) for 20 min. The clear supernatants were collected in aliquots and stored at −80°C until further use. Determination of cytokine concentrations was subsequently performed with a Luminex 200 instrument in 96-well plates after the application of 50 µL sample, 100 µL sample buffer and 50 µL mouse antibody-coated microsphere beads, according to the manufacturer's instructions (Invitrogen, Camarillo, CA, USA). For initial broad profiling of various cytokines measurable after carrageenan treatment (0–8 h post-carrageenan), a 10-plex cytokine analysis was applied using a rat 10-plex kit, which included TNF-α, IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, INF-γ and granulocyte-macrophage colony-stimulating factor (GM-CSF) (Invitrogen). This method was also used for measurement of plasma cytokine levels in study 4. Cytokine analyses in oedema samples from study 4 were performed in triplicate for the simultaneous detection of rat TNF-α, IL-1β and IL-6, respectively (Invitrogen). One hundred beads were counted per sample. A sigmoidal concentration-response curve fitting equation was applied to the individual rat cytokine standard curves using the log of standard cytokine concentrations versus median fluorescence intensity. Regression coefficients for all cytokine standard curves were >0.99. The cytokine concentrations in plasma and oedema samples were extrapolated from the corresponding standard curves and expressed as pg·mL−1.
Motor function tests
Exploratory motility
The non-habituated male rats (body weight 148–211 g) were administered either the test drug or corresponding vehicle (see Figure 3 for dosing paradigms) and returned to their home cage. They were then placed individually in transparent cages (30 × 20 × 25 cm, TSE Systems, Bad Homburg, Germany) equipped with 12 (6 × 2) infrared sensors. Locomotor activity, measured as distance travelled (m), was monitored automatically in the chambers via the interruption of two consecutive infrared sensors. Interruptions were detected by a control unit and recorded via a computer running ActiMot software (TSE Systems). Raw data obtained via 3 min sampling intervals were summed for the 30 min duration of the experiment and for the purposes of statistical analysis expressed as a percentage of the vehicle response according to the equation: % vehicle = (post-treatment value / vehicle value) × 100.
Figure 3.
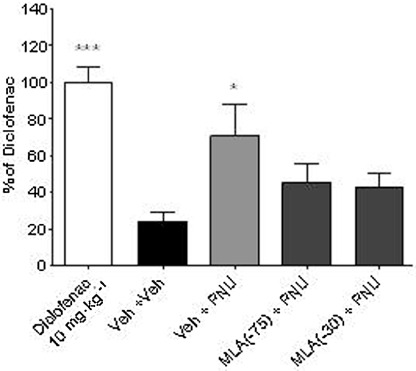
Anti-hyperalgesic actions of PNU-120596 are mediated via activation of α7 nACh receptors. The rats were injected with carrageenan (2% in saline, 100 µL, s.c.) into the plantar surface of the hind paw and then administered either drug or vehicle at the time (t) indicated before measurement of a post-treatment response. Accordingly, PNU-120596 (10 mg·kg−1, i.p., t =−60 min) or vehicle (15% MBCD) was either preceded 15 min earlier (t =−75 min) or followed 30 min later (t =−30 min) by injection of MLA (3 mg·kg−1, i.p.) or vehicle (0.9% NaCl/0.5% glucose). Diclofenac (10 mg·kg−1) was included as a positive control and data expressed as a percentage of diclofenac-mediated anti-hyperalgesia. All groups (n= 8 rats). All data are presented as mean ± SEM. *P < 0.05, ***P < 0.001 versus Veh + Veh group (one-way anova followed by Bonferroni's t-test).
Rotarod test
In normal, uninjured rats (body weight 250–350 g), the effects of test drugs on induced motor function were evaluated using an accelerating rotarod (Ugo Basile, Comero, Italy). The rotarod (6 cm diameter) speed was increased from 3–30 r.p.m. over a 180 s period, with the minimum time possible to spend on the rod designated as 0 s and the maximum cut-off time set at 180 s. The rats received two training trials (separated by 3–4 h) 1–2 days before drug testing for acclimatization purposes. On the day of drug testing, a baseline response was obtained, and the rats were subsequently administered drug or vehicle and the effects on motor performance tested 30 min later. Raw data were subsequently expressed as a percentage of the corresponding baseline response according to the equation: % baseline = (post-treatment value / baseline value) / × 100.
Drugs
Compound B was provided by Eli Lilly & Co. (Windelsham, UK). Compound B is a potent and efficacious agonist at human recombinant and rat native α7 nACh receptors, with confirmed selectivity against this receptor subtype compared with α1, α4β2 and α3 nACh receptors (Medhurst et al., 2008). PNU-120596, MLA and diclofenac sodium were purchased from Sigma. PNU-120596 is a type II α7 nACh receptor PAM that can enhance agonist-evoked peak current and produce profound temperature-dependent changes to receptor desensitization and deactivation (Hurst et al., 2005; Sitzia et al., 2011). Morphine hydrochloride and diazepam solution were purchased from Nomeco A/S (Copenhagen, Denmark). Compound B and morphine were dissolved in 0.9% NaCl/0.5% glucose, and administered s.c. MLA and diazepam were dissolved in 0.9% NaCl/0.5% glucose and administered i.p. PNU-120596 was dissolved in 15% methyl-β-cyclodextrin (15% MBCD, Sigma) and administered i.p. diclofenac was suspended in 10% hydroxypropyl-β-cyclodextrin (10% HBCD, Sigma) and administered p.o. Dosing volumes for p.o., i.p and s.c. administration were 5 mL·kg−1, 2–5 mL·kg−1 and 1–2 mL·kg−1, respectively. All doses are expressed as either mg weight salt (where indicated) kg−1 body weight or mg weight free base kg−1 body weight. All experiments with the exception of automated exploratory motility and formalin test experiments were performed with the investigator blinded to treatment.
Data analysis and statistics
Analysis of the data was performed using SigmaPlot for Windows version 11.0 (Systat Software, Inc., Hounslow, UK). All data are presented as mean ± SEM. Unless otherwise stated, one-way anova was used to analyse the overall effects of treatments. This was followed by appropriate post-treatment analysis for comparison against either vehicle treatment or baseline response obtained before drug treatment. P < 0.05 was considered to be statistically significant.
Results
Putative analgesia mediated by α7 nAChR activation in formalin-injected rats
Injection of formalin into the rat hind paw initiates spontaneous nocifensive behaviours composed of flinching, licking and/or biting of the injected paw. The first phase can be attributed to direct chemical stimulation of nociceptors, the interphase to activation of noxious inhibitory controls and the final phase to peripheral inflammatory processes that induce sensitization of nociceptive spinal neurones (Coderre et al., 1993; Henry et al., 1999). Administration of compound B (3–60 mg·kg−1) before the formalin injection was associated with a significant treatment effect throughout all three phases of the test [F(5,39) = 6.361, P < 0.001; F(5,39) = 3.552, P < 0.05 and F(5,39) = 11.025, P < 0.001 for the first phase, interphase and second phase, respectively], (Table 1). Notably, only doses exceeding 30 mg·kg−1 attenuated second-phase nocifensive behaviours compared with vehicle treatment. In contrast, both PNU-120596 (10–60 mg·kg−1, i.p.) and diclofenac (3–30 mg·kg−1, p.o.) had no effect on flinching behaviour throughout the entire duration of the test. Finally, and as expected, the µ-opioid receptor agonist morphine (1–6 mg·kg−1) robustly attenuated flinching during all three phases [F(3,31) = 4.647, P < 0.01; F(3,31) = 3.856, P < 0.05 and F(3,31) = 17.064, P < 0.001], with a level of analgesic efficacy and potency similar to that described previously (Munro, 2009).
Study 1 – developmental time course of nociceptive behaviours and correlation with cytokine profiles in rats with hind paw carrageenan inflammation
Intraplantar administration of carrageenan into the rat hind paw markedly reduced evoked paw pressure thresholds [F(9,77) = 52.4, P < 0.001], and non-evoked weight-bearing deficits [F(9,77) = 19.8, P < 0.001], and increased hind paw weights [F(9,77) = 65.8, P < 0.001], indicating the presence of substantial inflammatory hypersensitivity. Post-treatment analysis confirmed that these markers were not definitively time-locked with each other. In this respect, a significant reduction in the paw pressure threshold to mechanical stimulation was observed from 60 min after carrageenan injection (P < 0.001 vs. vehicle) before peaking at 4 h (Figure 1A). Reductions in the weight-bearing deficit were a little more delayed in onset and did not reach significance until nearly 2 h after carrageenan (P < 0.05 vs. vehicle), albeit they also peaked at 4 h (Figure 1B). Notably, both paw thickness and paw weight increased significantly from as soon as 30 min after carrageenan injection (P < 0.01 vs. vehicle) and reached a peak shortly thereafter (Figure 4C and D). One obvious point to mention is that whereas paw thickness and weight remained elevated throughout the 8 h duration of the experiment, the weight-bearing deficit, and to a lesser extent, the paw pressure threshold, slowly started to migrate back towards the corresponding level observed prior to carrageenan injection.
Figure 1.
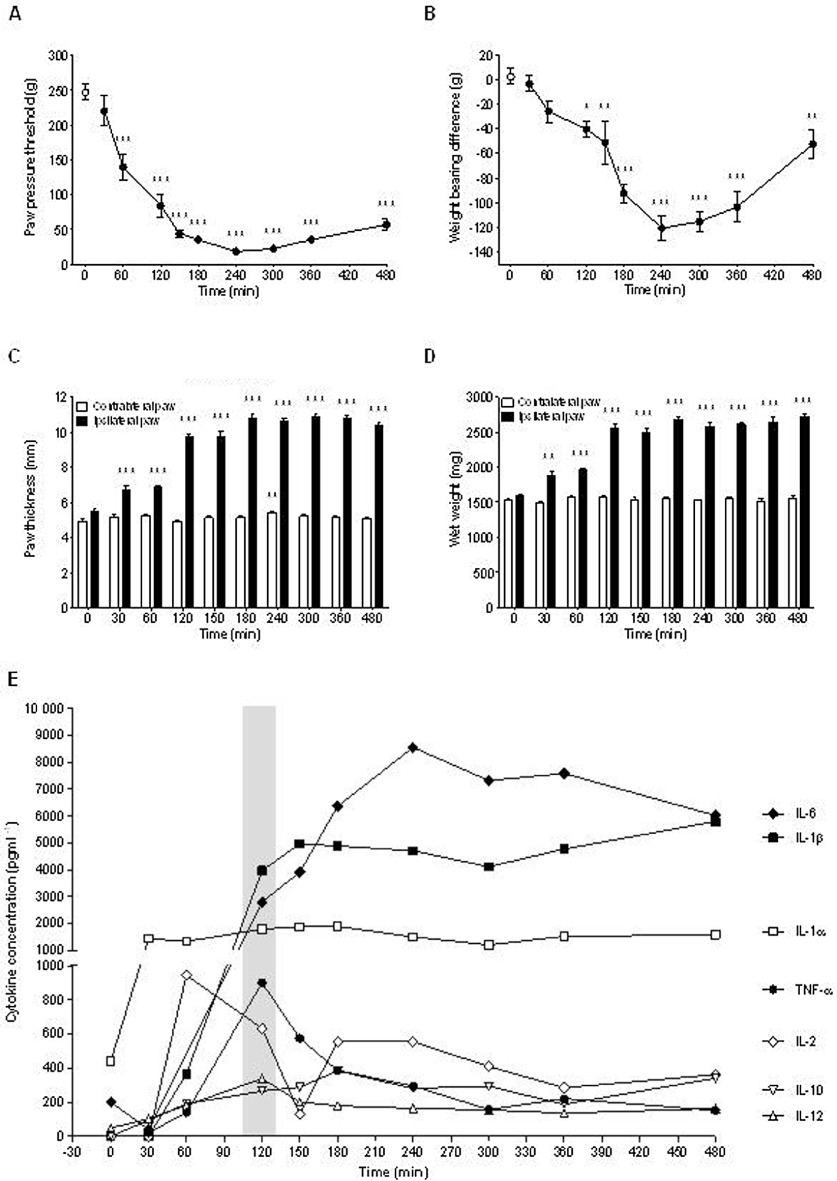
Development of pain-like behaviours and correlation with hind paw swelling and cytokine release in rats with carrageenan-induced inflammation. Effect of intraplantar carrageenan (2% in saline, 100 µL, s.c.) administration on time course (0–8 h) of (A) paw pressure threshold as an index of mechanical hyperalgesia, (B) weight-bearing deficit as an index of spontaneous pain, (C) paw thickness and (D) paw weight as an index of hind paw swelling; (E) cytokine levels within hind paw oedema. All groups (n= 7–8 rats). Shaded bar represents 2 h time point where compound effects on cytokine levels were determined in subsequent pharmacology experiments. All data are presented as mean ± SEM except in (E) where SEMs were omitted for purposes of clarity. *P < 0.05, **P < 0.01, ***P < 0.001 compared with vehicle (one-way anova followed by Bonferroni's t-test).
In keeping with previous observations, injection of carrageenan into the hind paw induced a robust increase in several oedema-associated cytokines. Baseline levels of TNF-α, IL-1β, IL-2, IL-4, IL-10, IFN-γ and GM-CSF were below detection limits. While IL-4, IFN-γ and GM-CSF remained undetectable up to 8 h after carrageenan injection, TNF-α (P < 0.0001), IL-1α (P= 0.0006), IL-1β (P < 0.0001), IL-2 (P= 0.0036), IL-6 (P < 0.0001), IL-10 (P < 0.0001) and IL-12 (P= 0.0028) showed significant and robust increases after carrageenan (Figure 1E). The time-response study of carrageenan-evoked nociceptive behaviour indicated that the subset of oedema-associated cytokines affected by carrageenan injection showed different temporal profiles in their induction pattern, peaking either within 2 h (TNF-α, IL-1α, IL-2, IL-10 and IL-12) or later (IL-1β, IL-6) with IL-1α, IL-1β and IL-6 levels remaining at high intensities. All inducible cytokines remained significantly elevated throughout the experiment.
Studies 2 and 3 – assessment of anti-hyperalgesic efficacy in carrageenan- and CFA-inflamed rats
First, we assessed the efficacy of the NSAID diclofenac (Figure 2A). Correspondingly, at 3 h after injection of carrageenan, a marked reduction in the paw pressure threshold to evoked mechanical stimulation had occurred (36.1 ± 3.4 g vs. 212.1 ± 9.1 g, P < 0.001, Student's t-test, n= 40 rats). Subsequently, diclofenac administration dose-dependently reversed this reduction [F(4,39) = 5.8, P < 0.001]. Similarly, in separate experiments, compound B and PNU-120596 dose-dependently reversed the reduction in paw pressure threshold produced by carrageenan [F(4,39) = 4.6, P < 0.01 and F(6,78) = 14.6, P < 0.001, respectively] (Figure 2B and C). Notably, the maximal level of efficacy obtained with compound B and PNU-120596 was similar to that produced with a 10 mg·kg−1 dose of diclofenac. Confirmation that the anti-hyperalgesic action of PNU-120596 in carrageenan-injected rats was mediated at least in part via α7 nACh receptors was demonstrated by MLA block of the increase in paw pressure threshold obtained with PNU-120596 (P < 0.05 vs. vehicle) (Figure 3). Appropriate target engagement for compound B being mediated via α7 nACh receptor activation in rats with hind paw inflammation has been reported previously (Medhurst et al., 2008).
Figure 2.
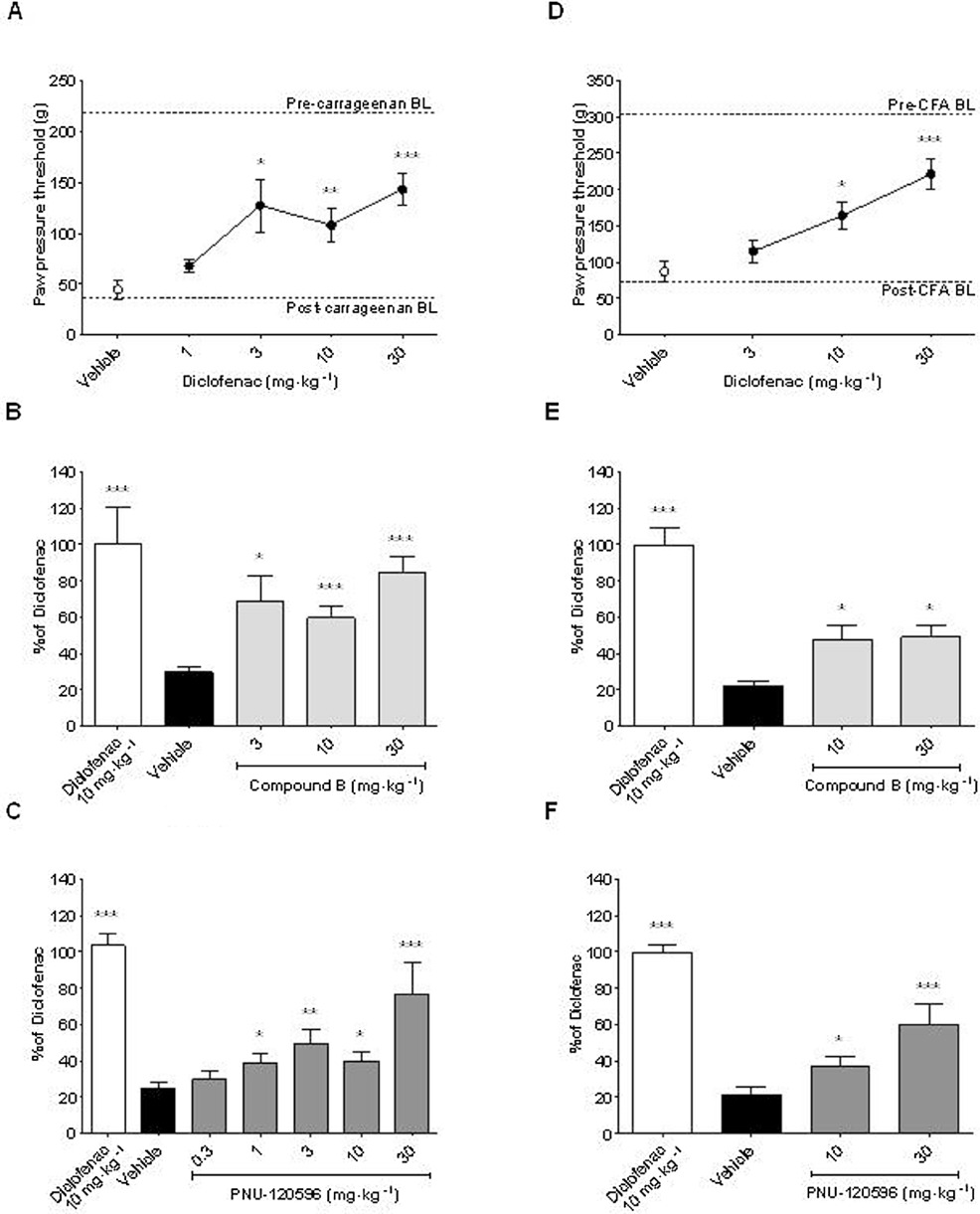
Activation of α7 nACh receptors attenuates signs of established inflammatory hypersensitivity. The rats were injected with either (A, B, C) carrageenan (2% in saline, 100 µL, s.c.) or (D, E, F) CFA (50% in saline, 100 µL, s.c.) into the plantar surface of the hind paw. They were then administered either drug or vehicle at the time (t) indicated before measurement of a post-treatment response. (A, D) Effects of diclofenac (1–30 mg·kg−1 and 3–30 mg·kg−1 p.o., t =−60 min) and vehicle (10% HBCD) on paw pressure threshold (g) in response to mechanical stimulation of the ipsilateral hind paw as an index of mechanical hyperalgesia. Pre- and post-inflammation baseline (BL) values are indicated with dashed lines. In subsequent experiments, diclofenac (10 mg·kg−1) was included as a positive control and data expressed as a % of diclofenac-mediated anti-hyperalgesia. Effects of (B, E) compound B (3–30 mg·kg−1 and 10, 30 mg·kg−1, s.c., t =−30 min) and vehicle (0.9% NaCl/0.5% glucose) and (C, F) PNU-120596 (0.3–30 mg·kg−1 and 10, 30 mg·kg−1 i.p., t =−30 min) and vehicle (15% MBCD) on mechanical hyperalgesia. All groups n= 8–16 rats. All data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus corresponding vehicle group (one-way anova followed by Bonferroni's t-test, and where appropriate, sub-analysis by Student's t-test).
Next, we proceeded to assess the efficacy of the same compounds in a model of longer term inflammatory pain produced by hind paw injection of CFA. To this end, we started by assessing the efficacy of diclofenac in the CFA-inflamed rats. Seventy-two hours after CFA injection, a marked reduction in the paw pressure threshold to evoked mechanical stimulation occurred (73.7 ± 4.9 g vs. 302.7 ± 6.9 g, P < 0.001, Student's t test, n= 39–40 rats), and this effect was dose-dependently reversed by diclofenac [F(3,38) = 11.0, P < 0.001] (Figure 2D). Moreover, compound B and PNU-120596 dose-dependently reversed the reduction in paw pressure threshold produced by CFA injection [F(3,38) = 22.2, P < 0.001 and F(3,38) = 23.6, P < 0.001, respectively] (Figure 2E and F). However, in the CFA-inflamed rats, the maximal level of efficacy obtained with compound B and PNU-120596 was approximately half that produced with a 10 mg·kg−1 dose of diclofenac.
Study 4 – assessment of anti-inflammatory efficacy in carrageenan-inflamed rats
Next, we assessed the efficacy of the same compounds administered before the carrageenan injection to determine if they possess putative anti-inflammatory actions in addition to the anti-hyperalgesic efficacy described above. Administration of diclofenac 2 h before carrageenan blunted the development of hind paw mechanical hyperalgesia and ongoing pain at both 2 h and 4 h post carrageenan injection (Figure 4A and B). Compound B only attenuated ongoing pain, albeit less markedly than diclofenac with significant effects observed only at 2 h post-carrageenan injection (Figure 4B). Similar to diclofenac, the attenuation of pain-like behaviours by pretreatment with PNU-120596 was significant at 2 h and 4 h post-carrageenan injection (Figure 4A and B).
Compound B, PNU-120596 and diclofenac all significantly attenuated hind paw swelling at 2 h post-carrageenan injection [F(4,65) = 80.4, P < 0.001] (Figure 4C). The magnitude of this reduction in hind paw swelling matched the anti-hyperalgesic profiles at 2 h post-carrageenan injection; more specifically, compound B, PNU-120596 and diclofenac reduced hind paw swelling by 19%, 30% and 42%, respectively, when compared with vehicle-treated animals. However, by 4 h post-carrageenan, only PNU-120596 and diclofenac continued to significantly affect hind paw swelling with a commensurate reduction in efficacy to 23% and 28% of vehicle-treated animals at the corresponding time point. In contrast to their capacity to affect hind paw swelling, the three compounds differed in respect to their effect on hind paw cytokine profiles. Correspondingly, diclofenac only attenuated the carrageenan-induced increase in IL-6 levels (P < 0.01 vs. vehicle), whereas compound B and PNU-120596 both attenuated levels of IL-6 and TNF-α (both P < 0.05 vs. vehicle for each cytokine) (Figure 5), with the effects of PNU-120596 on TNF-α levels being particularly robust. Although compound B and particularly PNU-120596 appeared to reduce IL-1β levels, in contrast to diclofenac, this trend was not significant. Measurement of 10 different cytokine levels in plasma revealed that none were significantly elevated 2 h after carrageenan injection in the hind paw (Table 2). Accordingly, pretreatment with either diclofenac or compound B had no effect on plasma levels of TNF-α, IL-1β or IL-6.
Table 2.
Plasma cytokine levels in carrageenan-inflamed rats
| Veh + sham | Veh + Carr | Dic + Carr | Cmpd B + Carr | |
|---|---|---|---|---|
| TNF-α | <LOD | 9.1 ± 3.6 | 2.7 ± 2.7 | <LOD |
| IL-1α | 73.4 ± 3.9 | 83.2 ± 5.5 | 79.5 ± 6.1 | 76.1 ± 0.5 |
| IL1-β | <LOD | <LOD | <LOD | <LOD |
| IL-2 | 462.7 ± 15.9 | 414.9 ± 57.4 | 452.4 ± 56.5 | 467.9 ± 107 |
| IL-4 | <LOD | <LOD | <LOD | <LOD |
| IL-6 | 36.2 ± 0.7 | 44.1 ± 6.5 | 37.1 ± 8.8 | 40.9 ± 12.5 |
| IL-10 | <LOD | <LOD | <LOD | <LOD |
| IL-12 | 928.4 ± 186.2 | 751.6 ± 149.3 | 969.7 ± 164.7 | 703.2 ± 144.6 |
| INF-γ | <LOD | <LOD | <LOD | <LOD |
| GM-CSF | <LOD | <LOD | <LOD | <LOD |
The rats were administered either diclofenac (Dic; 10 mg·kg−1, p.o., t =−120 min), compound B (Cmpd B; 30 mg·kg−1, s.c., t =−30 min) or vehicle (Veh, 15% MBCD) at the time (t) indicated before the injection of either carrageenan (Carr) or vehicle (sham) in the hind paw. Terminal plasma samples were collected at 2 h post-carrageenan treatment (n= 4 per group) and cytokine levels (pg·mL−1) measured. The lower detection limits for the individual cytokines were as follows: TNF-α (1.7 pg·mL−1), IL-1α (43.4 pg·mL−1), IL-1β (23.1 pg·mL−1), IL-2 (46.6 pg·mL−1), IL-4 (2.8 pg·mL−1), IL-6 (11.4 pg·mL−1), IL-10 (49.8 pg·mL−1), IL-12 (14.2 pg·mL−1), INF-γ (5.8 pg·mL−1), GM-CSF (7.3 pg·mL−1).
Assessment of motor function and coordination
In order to better understand the putative analgesia mediated by compound B in the formalin test and to be able to confidently attribute the anti-hyperalgesic effects of compound B and PNU-120596, measured in carrageenan- and CFA-inflamed rats, to direct actions on nociceptive circuits rather than via indirect effects on motor circuits, both compounds were tested for possible impairment of motor function (Munro et al., 2008; 2011).
In exploratory motility experiments, administration of compound B or the µ-opioid receptor agonist morphine (included as a positive control) attenuated the distance travelled throughout the 30 min duration of the experiment [F(3,27) = 3.860, P < 0.05 and F(3,27) = 51.703, P < 0.001, respectively] (Figure 6) In contrast, neither PNU-120596 nor diclofenac had any effect on locomotor behaviour. Based on these exploratory motility experiments, we were able to choose appropriate doses for the assessment of motor coordination in the rotarod test. When tested at 30 mg·kg−1, and 30 mg·kg−1 and 60 mg·kg−1, respectively, motor function was completely unaffected by compound B and PNU-120596 as compared with vehicle (Table 3). However, when the dose of compound B was increased to 60 mg·kg−1, a marked deficit in motor function and coordination was observed (P < 0.001 vs. vehicle). As expected, motor function was clearly disrupted by administration of the GABAA receptor agonist diazepam (P < 0.001 vs. vehicle) (Munro et al., 2008).
Figure 6.
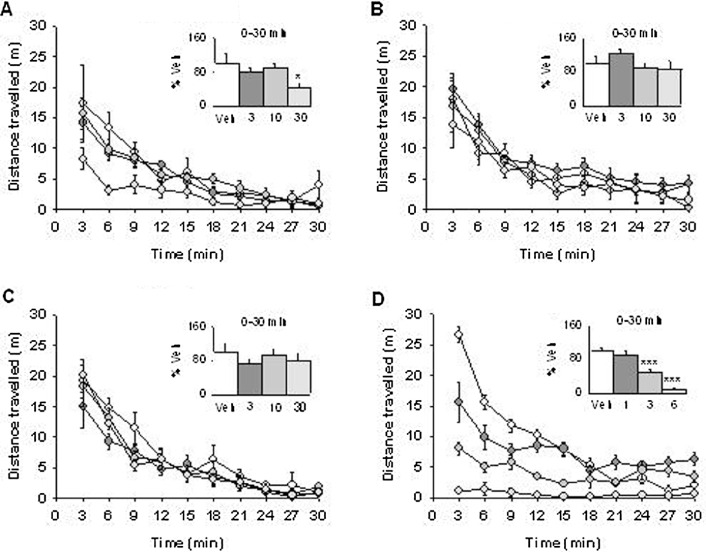
Effects of compound B and PNU-120596 on exploratory motility. The normal, uninjured rats were administered either (A) compound B (3–30 mg·kg−1, s.c., t =−30 min) or vehicle (0.9% NaCl/0.5% glucose), (B) PNU-120596 (3–30 mg·kg−1, i.p., t =−30 min) or vehicle (15% MCBD), (C) diclofenac (3–30 mg·kg−1, p.o. t =−60 min) or vehicle (10% HBCD), (D) morphine (1–6 mg·kg−1, s.c. t =−30 min) or vehicle (0.9% NaCl/0.5% glucose), where t = pre-administration time of drug or vehicle, prior to being individually placed in motility cages. Automated recording of activity was measured as distance travelled (m) enabling the time course to be expressed in 3 min bins for the 30 min duration of the test. Inset: total distance travelled represented as percentage of vehicle recorded from 0–30 min. All groups n= 7 rats. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus corresponding vehicle group (one-way anova followed by Bonferonni's t-test).
Table 3.
Effects of compound B and PNU-120596 on motor function and coordination in the rotarod test
| Drug | Dose (mg·kg−1) | % Baseline |
|---|---|---|
| Vehicle | 100 ± 0 (180 ± 0 s) | |
| Compound B | 30 | 89.9 ± 6.7 |
| 60 | 52.6 ± 5.2* | |
| PNU-120596 | 60 | 85.3 ± 9.7 |
| Diazepam | 5 | 30.8 ± 14.1* |
Normal, uninjured rats were administered either drug or vehicle (15% MBCD) immediately after a baseline response had been obtained and the maximal effects on activity-induced motor performance (represented as % baseline) determined 30 min later. Numbers in parentheses represent raw untransformed data for vehicle-treated animals. All groups n= 8 rats. Data are presented as mean ± SEM.
P < 0.001 versus vehicle (one way anova followed by Bonferroni's t-test).
Discussion
This study is the first to compare the anti-hyperalgesic actions of a systemically administered α7 nACh receptor agonist with that of an α7 nACh receptor PAM in rats with persistent hind paw inflammation. In the formalin test, none of the compounds tested, including diclofenac, reversed nocifensive behaviours either per se or at doses that did not impair motor function and coordination. Moreover, whilst the anti-hyperalgesic efficacies of compound B and PNU-120596 against established inflammation induced by either carrageenan or CFA were similar, only PNU-120596 was found to be completely devoid of motor-related issues. Subsequently, when each compound was administered before the initiation of inflammation, both the level and duration of anti-hyperalgesic efficacy obtained with PNU-120596 was superior to that obtained with compound B, albeit it did not quite match that provided by diclofenac. Nevertheless, while all three compounds attenuated paw swelling and levels of the pro-inflammatory cytokine IL-6 within the hind paw oedema after carrageenan, only compound B and PNU-120596 significantly attenuated levels of TNF-α. Thus, the modulation of α7 nACh receptor function appears to confer a means of relieving pain-like behaviours induced by inflammation, and in addition, might also reduce the sensitizing effect of pro-inflammatory mediators on peripheral sensory neurones after injury, thereby differentiating these compounds from standard of care medications such as diclofenac.
Carrageenan-induced pain-like behaviours correlate with hind paw swelling and pro-inflammatory cytokine levels
Following trauma or injury to cutaneous tissues, tissue acidification, combined with the local release of peptides, cytokines and prostanoids, initiates and contributes to neurogenic inflammatory processes, which act to lower the threshold of activation of peripheral nociceptors (Julius and Basbaum, 2001). We carefully simulated this process by injecting carrageenan into the plantar hind paw and then monitored the rapid development of mechanical hyperalgesia and weight-bearing deficits (Hedo et al., 1999); these peaked at 4 h and persisted throughout the 8 h duration of the time course study (Loram et al., 2007b). The occurrence of these pain-like behaviours was, in fact, preceded by an increase in paw swelling, albeit this in itself did not manifest fully until 2–3 h after the carrageenan injection. We also noted early and sustained increases in IL-1α and IL-2, followed later by elevations in TNF-α, IL-6 and IL-1β. Notably, consistent with previous findings, plasma levels of TNF-α, IL-6 and IL-1β were not elevated by carrageenan injection (Loram et al., 2007a,b). However, others have noted increased levels of either serum IL-1β (Vajja et al., 2004) or plasma IL-6 (Oka et al., 2007), presumably due to methodological differences. Importantly, the robust elevation in TNF-α, IL-6 and IL-1β within the hind paw was confirmed in a later experiment where we assessed the various modulatory effects of diclofenac, compound B and PNU-120596. Thus, primary mechanical hyperalgesia does not necessarily temporally associate with elevations in the local concentration of these cytokines.
This contrasts with the slower onset of hind paw weight-bearing deficits, which correlated more closely with increased cytokine production and the peak in hind paw swelling. TNF-α activates and sensitizes cutaneous nociceptors to mechanical and thermal stimulation (Cunha et al., 1992; Woolf et al., 1997). Similarly, IL-6 and IL-1β sensitize nociceptors to mechanical and thermal stimulation (Cunha et al., 1992; Binshtok et al., 2008). Notably, intraplantar TNF-α can stimulate the local release of IL-1β and IL-6, evoking subsequent release of nerve growth factor and prostaglandins to further trigger sensitization in carrageenan-treated mice (Cunha et al., 1992; 2005). The sequential increase of these cytokines is in general agreement with the temporal profile of individual cytokine levels observed in the current study. Overall, cytokine-mediated enhanced primary afferent drive, together with IL-1β induction of central COX-2 expression (Samad et al., 2001), would be expected to contribute to central sensitization within spinal pain circuits. Importantly, such central changes contribute to secondary hyperalgesia and might provide a mechanistic basis for the delayed onset of weight-bearing deficits associated with carrageenan-induced inflammation.
α7 nACh receptor activation mediates anti-hyperalgesic and anti-inflammatory actions in carrageenan-inflamed rats
Our initial comparative pharmacology experiments were designed to enable possible anti-hyperalgesic effects of the various drugs tested to coincide with the peak of the inflammatory hypersensitivity induced by carrageenan. As expected, diclofenac reversed mechanical hyperalgesia irrespective of whether it was administered before or after carrageenan (Jett et al., 1999). This said, it appeared more efficacious when administered before the establishment of inflammation, especially against weight-bearing deficits, which, as already suggested, are probably associated with both peripheral and central hyperexcitability changes induced by changes in the level of COX-2 (Samad et al., 2001). The reversal of established carrageenan- and CFA-induced pain-like behaviours by compound B is consistent with results from other studies that have reported efficacious effects of α7 nACh receptor agonists in models of inflammatory pain (Medhurst et al., 2008; Feuerbach et al., 2009; Gurun et al., 2009). However, our present data are the first to demonstrate that a compound with an α7 nACh receptor PAM mechanism has a similar efficacy to an α7 nACh receptor agonist in a model of inflammatory pain. Importantly, partial block of the antihyperalgesic effects of PNU-120596 in carrageenan-treated rats by systemic administration of MLA indicates that PNU-120596's actions are likely to be mediated at least in part via α7 nACh receptors. The finding that the effects of PNU-120596 and compound B on paw weights were temporally matched to effects on inflammatory hyperalgesia, suggests, albeit indirectly, that stimulation of α7 nACh receptors at the primary inflammatory site might contribute to both these actions. Although, systemic MLA has been reported to be ineffective at blocking compound B reversal of CFA-induced inflammatory hyperalgesia, a low dose (0.5 mg·kg−1, s.c.) was used in order to minimize CNS penetration (Medhurst et al., 2008). Notably, we did not set out here to define whether the effects of PNU-120596 on pain-like behaviours are mediated peripherally or centrally. This said, we have obtained central target engagement with an intrathecally administered proprietary type II α7 nACh receptor PAM in carrageenan-inflamed rats (unpublished observations).
Cobratoxin possesses MLA-reversible analgesic actions in arthritic rats that are accompanied by reductions in joint swelling, pro-inflammatory cytokine levels and altered pathohistological changes (Liu et al., 2009). Moreover, administration of the α7 nACh receptor-selective agonist AR-R17779 delays the onset of collagen-induced arthritis in mice and protects against joint destruction (van Maanen et al., 2009). Additionally, several pro-inflammatory immune cell types, including macrophages and neutrophils, migrate to the inflammatory site upon intraplantar carrageenan administration (Lazzarini et al., 2006), while a growing body of evidence indicates that activation of α7 nACh receptors on peripheral immune cells suppresses peripheral inflammatory activity by inhibiting the production and secretion of cytokines (Tracey, 2009). Together, these studies suggest that modulation of α7 nACh receptor function might also mediate anti-inflammatory actions in carrageenan-injected rats. In keeping with these observations, in addition to compound B and PNU-120596 being able to partially prevent the development of pain-like behaviours in carrageenan-inflamed rats, we were able to show that both compounds attenuated paw swelling and local production of TNF-α and IL-6. This facet of anti-inflammatory activity agrees with a recent report showing that intraplantar CDP-choline attenuates mechanical hypersensitivity and reduces hind paw skin TNF-α production in carrageenan-inflamed rats (Gurun et al., 2009).
Involvement of α7 nACh receptor-evoked anti-hyperalgesic mechanisms requires a minimal level of inflammatory insult
PAMs such as PNU-120596 have low intrinsic activity and require coordinated binding of an agonist to exert their effects on α7 nACh receptor function (Barron et al., 2009; Taly et al., 2009). In the context of endogenous agonists, PNU-120596 markedly increases the maximal efficacy and prolongation of α7 nACh receptor-evoked currents and intracellular calcium responses induced by a subsequent challenge with ACh in vitro (Hurst et al., 2005; Dickinson et al., 2007). Moreover, co-application of PNU-120596 enables sub-threshold physiological concentrations of choline to activate native α7 nACh receptor-evoked currents in rat hypothalamic slices (Gusev and Uteshev, 2010). Although this modulation is greatly attenuated when conditions are raised to near physiological levels (Sitzia et al., 2011), it is unlikely to be completely ablated given that PNU-120596 must have enhanced endogenous cholinergic activation of α7 nACh receptors to produce its anti-hyperalgesic and anti-inflammatory effects described here. Notably, peripheral inflammation can induce the synthesis and release of ACh from T cells, via increased vagal sympathetic tone, to target α7 nACh receptors expressed in immune cells and suppress pro-inflammatory activity, a mechanism that may potentially apply to both splenic and peripheral lymph node-associated T cells (Tracey, 2009; Rosas-Ballina et al., 2011). Interestingly, an increased sympathetic drive is also associated with the carrageenan model as blockade of catecholamine release by guanethidine partially inhibits carrageenan-induced hyperalgesia (Cunha et al., 2005).
Balancing some of the above with reports that choline possesses dose-dependent analgesic actions after i.t. and i.c.v. injection in uninjured mice (Damaj et al., 2000), we had presumed (incorrectly as events transpired) that central α7 nACh receptor activation would mediate analgesia in all rodent models of persistent sensitization irrespective of the level of inflammatory insult. However, multiple α7 nACh receptor agonists with varying brain/plasma ratios fail to produce analgesia in the rat formalin test at doses that correspondingly impair locomotor function in open field experiments (Gao et al., 2010). Similarly, our rotarod data indicate that compound B-mediated reversal of formalin-induced nocifensive behaviours probably occurred as a result of actions on motor circuits rather than effects on pain circuits. Although we noted a small reduction in exploratory motility behaviour after administration of compound B, at a dose that maximally reversed carrageenan-induced mechanical hyperalgesia, lower doses were also efficacious in carrageenan-injected rats. Moreover, activity-induced motor function in the rotarod test was completely unaffected by either compound B or PNU-120596 at doses up to 30 mg·kg−1 (Table 1), indicating that the anti-hyperalgesic actions obtained in carrageenan- and CFA-inflamed rats did not occur by virtue of indiscriminative actions on motor circuits (Gao et al., 2010).
It is of course possible that the formalin test involves a relatively limited inflammatory pathology that might not sufficiently engage an α7 nACh receptor-mediated mechanism to enable the efficacy of an agonist or PAM to be determined. This notwithstanding, Gao et al., (2010) also tested the effects of SSR-180711, compound Q and two other α7 nACh receptor agonists on pro-inflammatory cytokine levels in response to various antigen challenges. Surprisingly, none of the compounds tested affected levels of TNF-α, IL1β, IL-6, IL-2 or IFNγ despite being tested at concentrations reported to activate α7 nACh receptors in cultured HEK293 cells (Gao et al., 2010). This is at odds with our current findings showing attenuating effects of both compound B and PNU-120596 on carrageenan-induced increases in TNF-α and IL-6 levels within hind paw oedema.
Conclusions
Our data clearly show that an α7 nACh receptor PAM mediates anti-hyperalgesic efficacy in response to inflammatory injury, which is at least comparable with that obtained with a full nACh receptor agonist. Importantly, PNU-120596 was well tolerated in contrast to compound B, which impaired motor performance at high doses, albeit at non-motor impairing doses, it was clearly efficacious in carrageenan- and CFA-inflamed rats. Of course, how, and even if, this narrower therapeutic index for an α7 nACh receptor agonist versus PAM would translate into treating patients remains to be determined and might be of little consequence. Moreover, the differential effects of PNU-120596 and, to a lesser extent, compound B on pro-inflammatory cytokine release compared with diclofenac indicate that their anti-inflammatory actions are probably mediated via distinct mechanisms of action. Thus, α7 nACh receptor PAMs could prove to be especially useful in the treatment of inflammatory pain conditions that respond poorly to NSAIDs or in situations where NSAIDs are contra-indicated due to their side effects.
Acknowledgments
We would like to thank Helene Dyhr, Sanne Lauland and Nina Borkovic for expert technical help in performing behavioural experiments and Pernille Hulgaard for expertly performing cytokine analyses.
Glossary
- CDP
cytidine-5′-diphosphate
- CFA
complete Freund's adjuvant
- compound B
(R)-N-[1-azabicyclo(2.2.2)oct-3-yl][5-(2-pyridyl)thiopene-2-carboxamide]
- GM-CSF
granulocyte-macrophage colony stimulating factor
- HBCD
hydroxypropyl-β-cyclodextrin
- MLA
methyllycaconitine
- MBCD
methyl-β-cyclodextrin
- nACh
nicotinic ACh
- NSAID
non-steroidal anti-inflammatory drug
- PAM
positive allosteric modulator
- PNU-120596
1-(5-chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl)-urea
- <LOD
below lower detection limit
Conflicts of interest
None.
References
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Sonat FA, Hamurtekin E, Sonal S, Gurun MS. The antihyperalgesic effect of cytidine-5′-diphosphate-choline in neuropathic and inflammatory pain models. Behav Pharmacol. 2011;22:589–598. doi: 10.1097/FBP.0b013e32834a1efb. [DOI] [PubMed] [Google Scholar]
- Bannon AW, Decker MW, Holladay MW, Curzon P, Donnelly-Roberts D, Puttfarcken PS, et al. Broad-spectrum, non-opioid analgesic activity by selective modulation of neuronal nicotinic acetylcholine receptors. Science. 1998;279:77–81. doi: 10.1126/science.279.5347.77. [DOI] [PubMed] [Google Scholar]
- Barron SC, McLaughlin JT, See JA, Richards VL, Rosenberg RL. An allosteric modulator of alpha7 nicotinic receptors, N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea (PNU-120596), causes conformational changes in the extracellular ligand binding domain similar to those caused by acetylcholine. Mol Pharmacol. 2009;76:253–263. doi: 10.1124/mol.109.056226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, et al. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci U S A. 2005;102:1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Meyer EM, Martin BR. The antinociceptive effects of alpha7 nicotinic agonists in an acute pain model. Neuropharmacology. 2000;39:2785–2791. doi: 10.1016/s0028-3908(00)00139-8. [DOI] [PubMed] [Google Scholar]
- Dickinson JA, Hanrott KE, Mok MH, Kew JN, Wonnacott S. Differential coupling of alpha7 and non-alpha7 nicotinic acetylcholine receptors to calcium-induced calcium release and voltage-operated calcium channels in PC12 cells. J Neurochem. 2007;100:1089–1096. doi: 10.1111/j.1471-4159.2006.04273.x. [DOI] [PubMed] [Google Scholar]
- Feuerbach D, Lingenhoehl K, Olpe HR, Vassout A, Gentsch C, Chaperon F, et al. The selective nicotinic acetylcholine receptor alpha7 agonist JN403 is active in animal models of cognition, sensory gating, epilepsy and pain. Neuropharmacology. 2009;56:254–263. doi: 10.1016/j.neuropharm.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Gan TJ. Diclofenac: an update on its mechanism of action and safety profile. Curr Med Res Opin. 2010;26:1715–1731. doi: 10.1185/03007995.2010.486301. [DOI] [PubMed] [Google Scholar]
- Gao B, Hierl M, Clarkin K, Juan T, Nguyen H, Valk M, et al. Pharmacological effects of nonselective and subtype-selective nicotinic acetylcholine receptor agonists in animal models of persistent pain. Pain. 2010;149:33–49. doi: 10.1016/j.pain.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Gurun MS, Parker R, Eisenach JC, Vincler M. The effect of peripherally administered CDP-choline in an acute inflammatory pain model: the role of alpha7 nicotinic acetylcholine receptor. Anesth Analg. 2009;108:1680–1687. doi: 10.1213/ane.0b013e31819dcd08. [DOI] [PubMed] [Google Scholar]
- Gusev AG, Uteshev VV. Physiological concentrations of choline activate native alpha7-containing nicotinic acetylcholine receptors in the presence of PNU-120596 [1-(5-chloro-2,4-dimethoxyphenyl)-3-(5-methylisoxazol-3-yl)-urea] J Pharmacol Exp Ther. 2010;332:588–598. doi: 10.1124/jpet.109.162099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedo G, Laird JM, Lopez-Garcia JA. Time-course of spinal sensitization following carrageenan-induced inflammation in the young rat: a comparative electrophysiological and behavioural study in vitro and in vivo. Neuroscience. 1999;92:309–318. doi: 10.1016/s0306-4522(98)00734-9. [DOI] [PubMed] [Google Scholar]
- Henry JL, Yashpal K, Pitcher GM, Coderre TJ. Physiological evidence that the ‘interphase’ in the formalin test is due to active inhibition. Pain. 1999;82:57–63. doi: 10.1016/S0304-3959(99)00033-0. [DOI] [PubMed] [Google Scholar]
- Hurst RS, Hajós M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, et al. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett MF, Ramesha CS, Brown CD, Chiu S, Emmett C, Voronin T, et al. Characterization of the analgesic and anti-inflammatory activities of ketorolac and its enantiomers in the rat. J Pharmacol Exp Ther. 1999;288:1288–1297. [PubMed] [Google Scholar]
- de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R, Maiorka PC, Liu J, Papadopoulos V, Palermo-Neto J. Diazepam effects on carrageenan-induced inflammatory paw edema in rats: role of nitric oxide. Life Sci. 2006;78:3027–3034. doi: 10.1016/j.lfs.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Li J, Mathieu SL, Harris R, Ji J, Anderson DJ, Malysz J, et al. Role of α7 nicotinic acetylcholine receptors in regulating tumor necrosis factor-α (TNF-α) as revealed by subtype selective agonists. J Neuroimmunol. 2011;239:37–43. doi: 10.1016/j.jneuroim.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Liu YL, Lin HM, Zou R, Wu JC, Han R, Raymond LN, et al. Suppression of complete Freund's adjuvant-induced adjuvant arthritis by cobratoxin. Acta Pharmacol Sin. 2009;30:219–227. doi: 10.1038/aps.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram LC, Fuller A, Cartmell T, Mitchell B, Mitchell D. Behavioural, histological and cytokine responses during hyperalgesia induced by carrageenan injection in the rat tail. Physiol Behav. 2007a;92:873–880. doi: 10.1016/j.physbeh.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Loram LC, Fuller A, Fick LG, Cartmell T, Poole S, Mitchell D. Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J Pain. 2007b;8:127–136. doi: 10.1016/j.jpain.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Loram LC, Harrison JA, Chao L, Taylor FR, Reddy A, Travis CL, et al. Intrathecal injection of an alpha seven nicotinic acetylcholine receptor agonist attenuates gp120-induced mechanical allodynia and spinal pro-inflammatory cytokine profiles in rats. Brain Behav Immun. 2010;24:959–967. doi: 10.1016/j.bbi.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Maanen MA, Lebre MC, van der Poll T, LaRosa GJ, Elbaum D, Vervoordeldonk MJ, et al. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009;60:114–122. doi: 10.1002/art.24177. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhurst SJ, Hatcher JP, Hille CJ, Bingham S, Clayton NM, Billinton A, et al. Activation of the alpha7-nicotinic acetylcholine receptor reverses complete Freund adjuvant-induced mechanical hyperalgesia in the rat via a central site of action. J Pain. 2008;9:580–587. doi: 10.1016/j.jpain.2008.01.336. [DOI] [PubMed] [Google Scholar]
- Munro G. Pharmacological assessment of the rat formalin test utilizing the clinically used analgesic drugs gabapentin, lamotrigine, morphine, duloxetine, tramadol and ibuprofen: influence of low and high formalin concentrations. Eur J Pharmacol. 2009;605:95–102. doi: 10.1016/j.ejphar.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Munro G, Lopez-Garcia JA, Rivera-Arconada I, Erichsen HK, Nielsen EØ, Larsen JS, et al. Comparison of the novel subtype selective GABAA receptor positive allosteric modulator NS11394 with diazepam, zolpidem, bretazenil and gaboxadol in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2008;327:969–981. doi: 10.1124/jpet.108.144568. [DOI] [PubMed] [Google Scholar]
- Munro G, Erichsen HK, Rae MG, Mirza NR. A question of balance – positive versus negative allosteric modulation of GABA(A) receptor subtypes as a driver of analgesic efficacy in rat models of inflammatory and neuropathic pain. Neuropharmacology. 2011;61:121–132. doi: 10.1016/j.neuropharm.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Nizri E, Irony-Tur-Sinai M, Lory O, Orr-Urtreger A, Lavi E, Brenner T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J Immunol. 2009;183:6681–6688. doi: 10.4049/jimmunol.0902212. [DOI] [PubMed] [Google Scholar]
- Oka Y, Ibuki T, Matsumura K, Namba M, Yamazaki Y, Poole S, et al. Interleukin-6 is a candidate molecule that transmits inflammatory information to the CNS. Neuroscience. 2007;145:530–538. doi: 10.1016/j.neuroscience.2006.10.055. [DOI] [PubMed] [Google Scholar]
- Pacini A, Di Cesare Mannelli L, Bonaccini L, Ronzoni S, Bartolini A, Ghelardini A. Protective effect of alpha7 nAChR: behavioural and morphological features on neuropathy. Pain. 2010;150:542–549. doi: 10.1016/j.pain.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham MC, Duan WR, Thomas J, Nothaft W, Backonja MM. A randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of ABT-594 in patients with diabetic peripheral neuropathic pain. Pain. 2009;146:245–252. doi: 10.1016/j.pain.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, et al. Interleukin-1beta-mediated induction of COX-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Sitzia F, Brown JT, Randall AD, Dunlop J. Voltage- and temperature-dependent allosteric modulation of α7 nicotinic receptors by PNU120596. Front Pharmacol. 2011;81:1–9. doi: 10.3389/fphar.2011.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8:733–750. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajja BN, Juluri S, Kumari M, Kole L, Chakrabarti R, Joshi VD. Lipopolysaccharide-induced paw edema model for detection of cytokine modulating anti-inflammatory agents. Int Immunopharmacol. 2004;4:901–909. doi: 10.1016/j.intimp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, et al. An automated flinch detecting system for use in the formalin nociceptive bioassay. J Appl Physiol. 2001;90:2386–2402. doi: 10.1152/jappl.2001.90.6.2386. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


