Abstract
Soil alkalinity is a widespread environmental problem that limits agricultural productivity. The hypothesis that an auxin-regulated proton secretion by plasma membrane H+-ATPase plays an important role in root adaption to alkaline stress was studied. It was found that alkaline stress increased auxin transport and PIN2 (an auxin efflux transporter) abundance in the root tip of wild-type Arabidopsis plants (WT). Compared with WT roots, the pin2 mutant roots exhibited much reduced plasma membrane H+-ATPase activity, root elongation, auxin transport, and proton secretion under alkaline stress. More importantly, roots of the pks5 mutant (PKS5, a protein kinase) lacking PIN2 (a pks5/pin2 double mutant) lost the previous higher proton-secretion capacity and higher elongation rate of primary roots under alkaline stress. By using Arabidopsis natural accessions with a high proton-secretion capacity, it was found that their PIN2 transcription abundance is positively related to the elongation rate of the primary root and proton-secretion capacity under alkaline stress. Taken together, our results confirm that PIN2 is involved in the PKS5-mediated signalling cascade under alkaline-stress and suggest that PIN2 is required for the adaptation of roots to alkaline stress by modulating proton secretion in the root tip to maintain primary root elongation.
Key words: Alkaline stress, auxin transport, PIN2, PKS5, plasma membrane H+-ATPase, primary root growth, proton secretion, root tip
Introduction
Saline–alkaline soils (saline and alkalinesoils) are widespread in the world. Based on the soil map of the world, 831 million hectares of area are saline–alkaline soils (Jin et al., 2006; Wang et al., 2008). Of this area, saline soils underline 397 million hectares (about 47%), while alkalinized soils underline 434 million hectares (nearly 53%). In northeast China, more than 70% of land area is alkaline grassland (Kawanabe and Zhu, 1991). Because of high pH stress, only a few plants can survive on the alkalinized soil. So, soil alkalinity is a widespread environmental problem and an important factor that limits agricultural productivity.
In recent years, although some reports have shown that alkaline stress causes much stronger inhibition of plant growth and development than salt stress, little attention has been given to the effect of alkaline stress on the plant and the adaptation mechanisms of plants to alkaline stress compared with the attention given to salt stress (Hurkman, 1992; Degenhardt et al., 2000; Zhu, 2001; Yang et al., 2008). Under alkaline stress, high environmental pH is a main factor that affects root growth and development during the life cycle of the plant. Recently, some research has shown that plasma membrane H+-ATPase plays an important role in the adaptation of roots to alkaline stress by mediating proton secretion (Fuglsang et al., 2007; Yang et al., 2010). For example, protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein, and thus loss-of-function pks5 mutant Arabidopsis plants are more tolerant to high pH stress due to the extrusion of protons to the extracellular space. On the other hand, Chaperone J3 activates the plasma membrane H+-ATPase by repressing PKS5, and Arabidopsis plants lacking J3 are hypersensitive to alkaline stress and exhibit decreased plasma membrane H+-ATPase. Under abiotic stress, plasma membrane H+-ATPase is regulated by numerous factors (Palmgren, 2001). Although auxin has also been shown to play a role in regulating the activity of plasma membrane H+-ATPase (Hager et al., 1991; Rober-Kleber et al., 2003), the components that mediate the effect of auxin on plasma membrane H+-ATPase are unclear.
The plastic and adaptive responses by roots have been proposed as the major mechanism by which plants cope with fluctuating environments. In particular, primary root growth regulated by the sensory root tip plays a very important role in these adaptive responses (Darwin, 1880; Baluška et al., 2010; Li et al., 2010). Acidification of the cell wall is an important part of the growth-promoting effect by auxin, which is one of the key factors that determine the elongation of primary root (Moloney et al., 1981; Hager, 2003). The polar transport of auxin in the root tip strongly influences primary root growth (Fu and Harberd, 2003; Grieneisen et al., 2007) and is driven by PIN2, an auxin efflux transporter, which is a member of a complex network of PIN transporters (PIN1-4, and PIN7) that co-ordinate auxin flow in the root tip (Kleine-Vehn et al., 2008). Recently, some researchers reported that, among the PIN transporters, PIN2 is a general stress target and underlines the adaptive mechanism of roots to abiotic stress (Baluška et al., 2010). Thus, by mediating auxin transport, PIN2 may also be involved in the regulation of plasma membrane H+-ATPase.
Therefore, it was hypothesized that PIN2 plays an important role in mediating the regulation of auxin on plasma membrane H+-ATPase to release protons in the root tip adapting to alkaline stress. Some Arabidopsis natural accessions, relevant Arabidopsis mutants, and transgenic lines were used when the maintenance of primary root growth, the proton-secretion capacity, auxin distribution, and PIN2 transcription abundance in the root tip under alkaline stress were investigated. Our results suggest that PIN2 is required for the maintenance of primary root growth in its adaptation to alkaline stress by modulating proton secretion in root tips.
Materials and methods
Plant materials, growth conditions, and stress treatment
The wild-type Arabidopsis plants (WT) were ecotype Col-0 if not otherwise indicated. The pin2 mutant (eir1-4) was obtained from Christian Luschnig (Universität für Bodenkultur, Vienna, Austria), and the PIN2:GFP line and the DR5rev:GFP line were obtained from Rujin Chen (The Samuel Roberts Noble Foundation, Ardmore, Oklahoma, USA). The DR5rev:GFP/pin2 homozygous line (DR5rev:GFP x eir1-4) was kindly provided by Jiri Friml (Department of Plant Systems Biology, Flanders Institute for Biotechnology, Belgium). Some stocks of Arabidopsis seeds (Arabidopsis pks5 mutant: Salk_108074; Arabidopsis natural accessions: Lov-5, Uod-1, Uod-7, and Ws-2) were obtained from the Arabidopsis Biological Resource Center (ABRC, Ohio State University, Columbus, OH, USA). The homozygous pks5 mutant was identified by PCR using some primers specific to the T-DNA of Salk_108074 (see Supplementary Table S1 at JXB online). To generate the pks5/pin2 (double mutant), the homozygous pks5 mutant was crossed with the pin2 mutant (eir1-4). After that, the homozygous pks5/pin2 (double mutant) was selected based on primers specific to the T-DNA of Salk_108074 (pks5 mutant) and the agravitropic phenotype of the pin2 mutant (eir1-4). Further, the double mutant (pks5/pin2) was identified by real-time RT-PCR (see Supplementary Fig. S1 and Supplementary Table S1 at JXB online).
Before planting, Arabidopsis seeds were surface-sterilized with 70% ethanol for 1min, then with 1% sodium hypochlorite solution plus SDS for 9min, and subsequently rinsed with sterile deionized water six times, and then kept in the dark for 3 d at 4 °C for stratification. Subsequently, Arabidopsis seeds were grown hydroponically using a sugar-free agar medium solution culture system as described by Xu and Shi (2008). The sterilized seeds were sown on agar medium containing only full-strength Murashige and Skoog nutrients and 6g l–1-agar without sucrose. This sugar-free agar medium was filled into bottom-removed Eppendorf tubes, which were held in a plastic platform. Two to three seeds were sown in each tube and thinned to one plant after 7 d growth. The nutrient solution consisted of: 5mM KNO3, 1mM KH2PO4, 2mM MgSO4, 2mM Ca(NO3)2, 0.5mM Fe-Na-EDTA, 70 µM H3BO3, 14 µM MnCl2, 0.5 µM CuSO4, 1 µM ZnSO4, 0.2 µM Na2MoO4, 10 µM NaCl, and 0.01 µM CoCl2. The solution pH was adjusted to 5.8 every day and the solution was renewed every 2 d. For alkaline stress, the solution pH was adjusted to 8.0 every day using 0.1mM KOH. Arabidopsis plants were grown at 22±1 °C, with a light intensity of 120 µmol photons m–2 s–1, 16/8h light/dark photoperiod, and a relative humidity of 70% in the growth chamber (Sanyo Electric Co., Ltd., Kyoto, Japan). 15-d-old Arabidopsis plants were treated with control condition (pH 5.8) or alkaline stress (pH 8.0) for 12h, 24h or 48h under the hydroponic system. After that, some plants were frozen immediately into liquid nitrogen and stored at –80 °C in order to analyse mRNA level or enzyme activity, while other plants were directly used to analyse some parameters.
Real-time RT-PCR
Real-time RT-PCR was assayed according to the method of Xu and Shi (2006). Total RNA was extracted from Arabidopsis plants under control conditions (pH 5.8) and alkaline stress (pH 8.0). Gene sequences were available in at the National Center of Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov) and gene-specific primers for real-time RT-PCR were designed using Primer 5 software (see Supplementary Table S1 at JXB online). At-ACT2 is a strongly and constitutively expressed ‘house-keeping’ gene in Arabidopsis plants (Xu et al., 2012), so the quantification of mRNA levels was based on comparison with the level of mRNA for At-ACT2.
Measurement of primary root elongation
The primary root length of Arabidopsis plants was measured using a root analysis instrument (WinRHIZO; Regent Instruments Inc., Quebec, ON, Canada) according to the method of Xu and Shi (2007). The elongation rate of primary roots (µm h–1) in Arabidopsis plants was calculated from the primary root length with respect to the displacement of primary root apex after some time for control condition (pH 5.8) or alkaline stress (pH 8.0).
Measurement of DR5rev:GFP or PIN2:GFP in Arabidopsis root tip
The fluorescence of DR5rev:GFP or PIN2:GFP in Arabidopsis root tips was observed with a confocal laser scanning microscope (Olympus FV-1000 spectral type SPD mar/G/R IX81 FLUOVIEW laser confocal system) according to the method of Schlicht et al. (2008). For imaging GFP, the 488nm line of the Argon laser was used for excitation and emission was detected at 520nm. At least 10 seedlings/images were examined, and at least two independent experiments were performed, giving the same statistically significant results. Also, all images were taken under the same conditions.
Auxin transport assay
DR5rev:GFP-based auxin transport assay, as described by Lewis and Muday (2009), was used to measure basipetal auxin transport in Arabidopsis plants. In brief, to measure basipetal auxin transport, plates with control plants or treated plants were incubated in the dark for 2h. IAA treatment was conducted by placing a solidified agar block containing 1 µM IAA such that it overlapped with the root tip. Basipetal auxin transport was determined by comparing the fluorescence of DR5rev:GFP from the site of IAA application of the treated seedlings with that of the controls. At least 10 seedlings for each treatment were measured and the experiments were repeated twice independently.
Assay of plasma membrane H+-ATPase activity
Plasma membrane vesicles of Arabidopsis roots were prepared according to the method of Shen et al. (2006). The plasma membrane was stored at –80 °C until analysis. Root plasma membrane H+-ATPase activity was determined by the method of Shen et al. (2006). In a reaction volume of 0.5ml that contained 30mM BTP/MES, pH 6.5, 5mM MgSO4, 50mM KCl, and 4mM TRIS-ATP, plasma membrane H+-ATPase activity was measured. Brij 58 (0.02% w/v) was applied to obtain membrane vesicles of uniform size. Reactions were initiated by adding 3–5 µg of membrane protein. Reactions proceeded for 30min at 30 °C and were stopped with 1ml of stopping solution containing 2% (v/v) concentrated H2SO4, 5% (w/v) sodium dodecyl sulphate, 0.7% (w/v) sodium molybdate, followed by 50 µl of 10% (w/v) ascorbic acid. Colour development of the phosphomolybdate complex proceeded for 30min. Absorbance at 700nm was measured with a spectrophotometer. Plasma membrane H+-ATPase activity was calculated as the phosphorus liberated in the excess of boiled-membrane controls. To assess the purification of the H+-ATPase activity, its activity was expressed as the difference in activity in the presence and absence of 0.1mM vanadate (see Supplementary Fig. S2 at JXB online). A standard curve of phosphorus in the reaction mixture was included in each assay.
Measurement of net H+ flux with the SIET system
Net fluxes of H+ were measured non-invasively using SIET (scanning ion-selective electrode technique, SIET system BIO-003A; Younger USA Science and Technology Corporation; Applicable Electronics Inc.; Science Wares Inc., Falmouth, MA, USA). The principle of this method and instrument are detailed in Xu et al. (2012). Measurements were performed at room temperature (24–26 °C). After the roots of Arabidopsis plants were equilibrated in measuring solution for 20–30min, these equilibrated Arabidopsis plants were transferred to the measuring chamber, a small plastic dish (3cm in diameter) containing 2–3ml of fresh measuring solution. When the root became immobilized at the bottom of the dish, the microelectrode was vibrated in the measuring solution between two positions, 5 µm and 35 µm from the root surface, along an axis perpendicular to the root. The background was recorded by vibrating the electrode in the measuring solution not containing roots. Microelectrodes were made by Xuyue Science and Technology Co., Ltd. After backfilling, the electrode tip was filled with a commercially available ionophore H+ cocktail (95297 from Fluka). During the entire measurement process, the shoot was not in contact with the measuring solution. H+ fluxes were measured along the root tip, concentrating on the following zones: 50, 100, 150, 200, 250, 300, 400, 520, 600, 700, 750, 850, 900, 1000, 1100, 1200, and 1500 µm from the root cap junction. Each plant was measured once. The final flux values at each zone were the mean of six individual plants from each treatment. All measurements of H+ fluxes were carried out at Xuyue Science and Technology Co., Ltd (Beijing, China).
Statistical analyses
Data were subjected to analysis of variance and post hoc comparisons were done with Duncan’s Multiple Range Test at the P <0.05 level. The statistical software program used was SPSS version 13.0. The values are the means and SD of six replicates from two independent experiments. A rank test was performed for a, b and c in the figures, and different letters are used to indicate means that are significantly different.
Results
Root growth responses to alkaline stress under hydroponic system
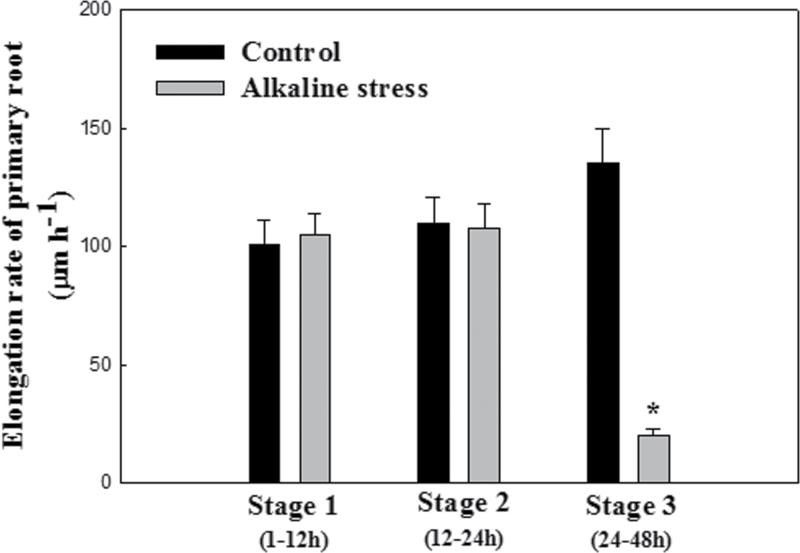
In this study, the hydroponic system of Xu and Shi (2008) was used and primary root elongation under alkaline stress was studied (Fig. 1). Although the elongation rate of primary roots in the WT under Stage 3 (24–48h) alkaline stress was significantly lower than under control conditions, no significant difference was found in the elongation rate of primary roots in the WT under Stage 1 (1–12h) or Stage 2 (12–24h) alkaline stress compared with control conditions. The results indicate that, compared with control conditions, primary root elongation in Arabidopsis plants was maintained during Stage 1 (1–12h) or Stage2 (12–24h) alkaline stress.
Fig. 1.
Elongation rate of primary roots in wild-type Arabidopsis plants (WT: Col-0) under alkaline stress for 2 d (Stage 1: 1–12h; Stage 2: 12–24h; Stage 3: 24–48h).
Abundance and distribution of auxin or PIN2 in root under alkaline stress
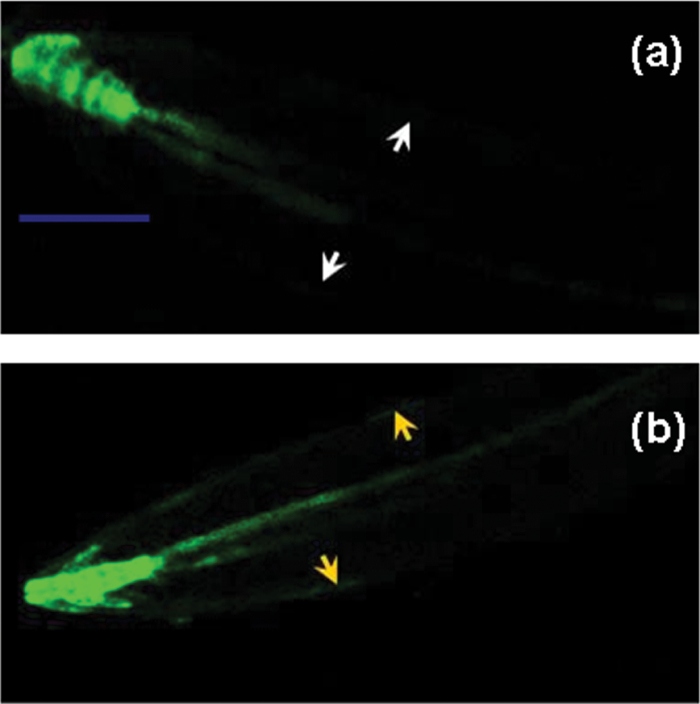
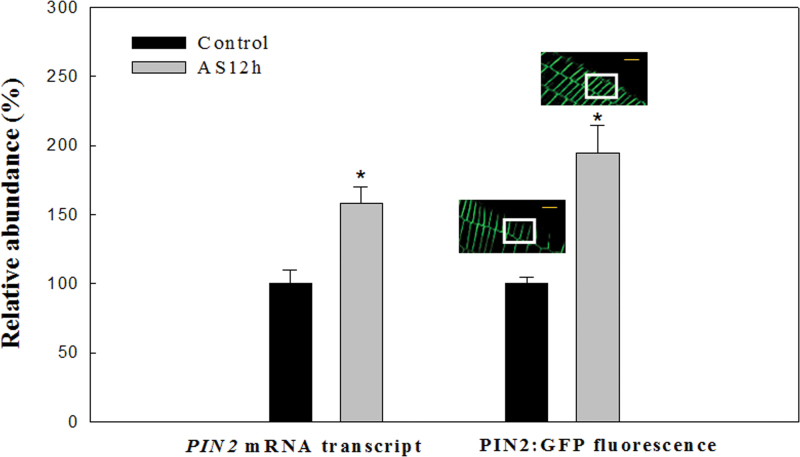
The abundance and distribution of auxin and PIN2 in Arabidopsis root tips under control conditions (pH 5.8) or alkaline stress (pH 8.0) for 12h was investigated. The auxin-responsive report DR5rev:GFP (Fig. 2) was used to study the abundance and distribution of auxin in the root tips of wild-type (WT) Arabidopsis plants (DR5rev:GFP). Alkaline stress significantly increased auxin distribution in the epidermal cells of root tips compared with the control conditions (Fig. 2a, 2b). Figure 3 showed that alkaline stress significantly increased the PIN2 transcript abundance in WT root compared with control conditions. Also, alkaline stress significantly increased the PIN2:GFP abundance in the epidermal cells of WT root tips compared witho control conditions (Fig. 3). Further, using DR5rev:GFP/pin2 homozygous line (DR5rev:GFP x eir1-4), the link between the effects of alkaline stress on the role of PIN2 and auxin subcellular distribution in the root tips of the pin2 mutant (eir1-4) was explored (see Supplementary Fig. S3 at JXB online). According to the results shown in Fig. 2 (and in Supplementary Fig. S3 and Supplementary Fig. S4a at JXB online), auxin abundance in the root tips of pin2 mutant (0–200 µm) was significantly higher than that of the WT (P <0.05) whether under control conditions or under alkaline stress. As shown in Supplementary Fig. S4b at JXB online, alkaline stress significantly increased basipetal auxin transport in the root tip of the WT. Further, whether under control conditions or under alkaline stress, compared with the WT, basipetal auxin transport in the root apices of the pin2 mutant line was weak.
Fig. 2.
Auxin distribution and abundance in the root tips of wild-type Arabidopsis plants (WT) (DR5rev:GFP) under alkaline stress for 12h. 15-d-old Arabidopsis plants were treated with control condition (pH 5.8) (a) and alkaline stress (pH 8.0) for 12h (b) under a hydroponic system. Yellow arrowheads indicated increased expression of GFP in the epidermal cells, and white arrowheads indicated no GFP expression in the epidermal cells. Bar=100 µm (blue line).
Fig. 3.
Transcript abundance of PIN2 in the whole roots of wild-type Arabidopsis plants (WT) or PIN2:GFP abundance in the epidermal cells of root tip in WT (PIN2:GFP) under alkaline stress for 12h. ‘PIN2 mRNA transcript abundance in control’ is plotted as ‘100%’. Quantification of green fluorescent protein (GFP) fluorescence is shown, and ‘GFP fluorescence abundance in control’ is also plotted as ‘100%’. Bar=25 µm (yellow line).
PM H+-ATPase, proton flux or primary root elongation in root under alkaline stress
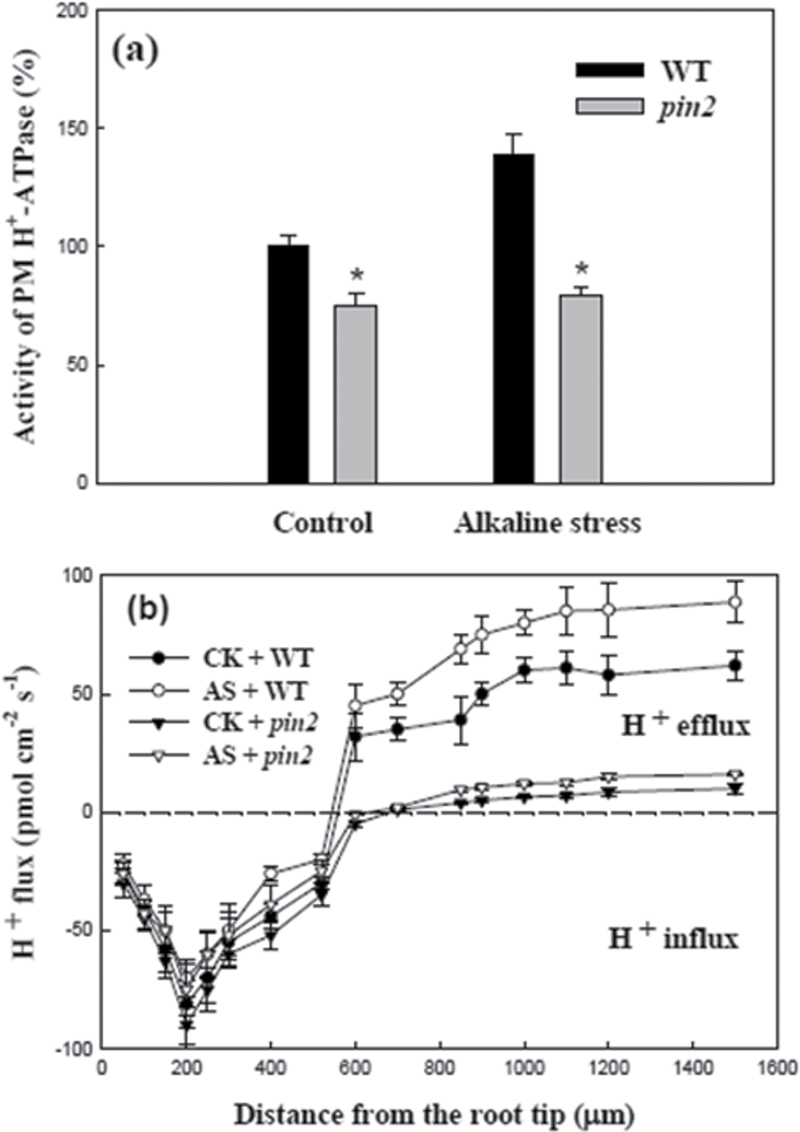
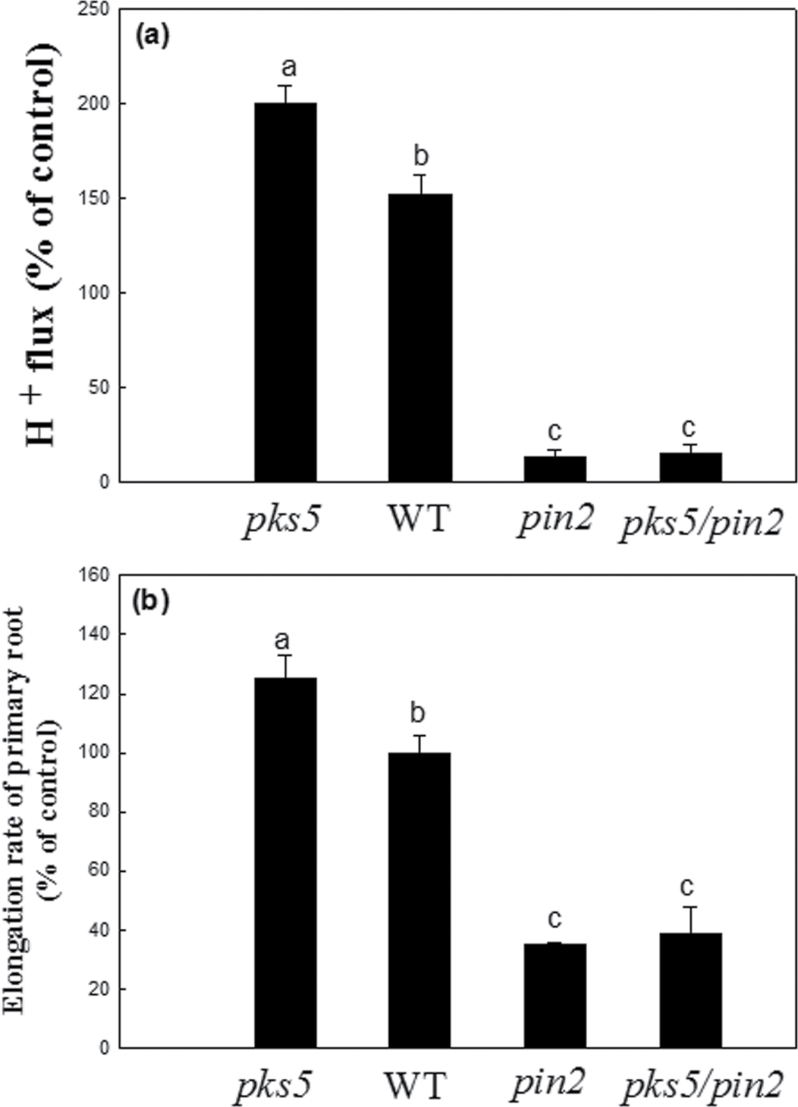
PM H+-ATPase activity of the whole root, the elongation rate of primary roots or proton flux along the root tip in Arabidopsis plants were analysed under control conditions (pH 5.8) or alkaline stress (pH 8.0) for 12h (Figs 4, 5). Firstly, in order to evaluate the purity of the plasma membrane, the activity of various inhibitor-sensitive ATPases in both the microsome and plasma membrane fractions was analysed (see Supplementary Fig. S2 at JXB online), and the results suggest that the isolation techniques used for the plasma membrane of roots are practicable. Either under control conditions or under alkaline stress, PM H+-ATPase activity of whole roots in the pin2 mutant was significantly lower than that of the WT (Fig. 4a). The changes of proton (H+) fluxes along the root tips of WT and pin2 mutant plants was then investigated, concentrating on the following zones: 50, 100, 150, 200, 250, 300, 400, 520, 600, 700, 850, 900, 1000, 1100, 1200, and 1500 µm from the root cap junction under control conditions or alkaline stress (Fig. 4b). No significant difference in the H+ influx was observed between the WT and pin2 mutants at the meristem zone (0–200 µm from the RCJ: root cap junction) or the transition zone (200–520 µm from the RCJ) under control conditions or alkaline stress (P <0.05). However, under both control conditions or under alkaline stress, the H+ efflux in the WT was significantly higher than that of the pin2 mutant at the elongation zone (520–850 µm from the RCJ) or the growth terminating zone (850–1500 µm from the RCJ) (P <0.05). In addition, the H+ efflux at the elongation zone (750 µm from the RCJ) or hte elongation rate of the primary root in the WT, the pks5 mutant, the pin2 mutant, and in the double mutant (pks5/pin2) under alkaline stress were also analysed (Fig. 5). Our results show that, among them (WT, pks5 mutant, pin2 mutant, and pks5/pin2), under alkaline stress, H+ efflux or primary root elongation was the highest in the pks5 mutant; H+ efflux or primary root elongation in the WT was moderate, whereas the H+ efflux and primary root elongation was the lowest in the pin2 mutant and the pks5/pin2 double mutant.
Fig. 4.
PM H+-ATPase activity in root (a) or proton flux along root tip (b) in wild-type Arabidopsis plants (WT: Col-0) or pin2 mutant Arabidopsis plants (eir1-4) under alkaline stress for 12h. Note: CK (control) and AS (alkaline stress).
Fig. 5.
Proton flux (a) at the root tip elongation zone (750 µm distance from the root cap junction) or the elongation rate of primary roots (b) in wild-type Arabidopsis plants (WT: Col-0) and mutant Arabidopsis plants (pks5, pin2, pks5/pin2) under alkaline stress for 12h. ‘Proton flux or primary root elongation in WT under control condition’ is plotted as ‘100%’.
Root response of different natural accessions to alkaline stress
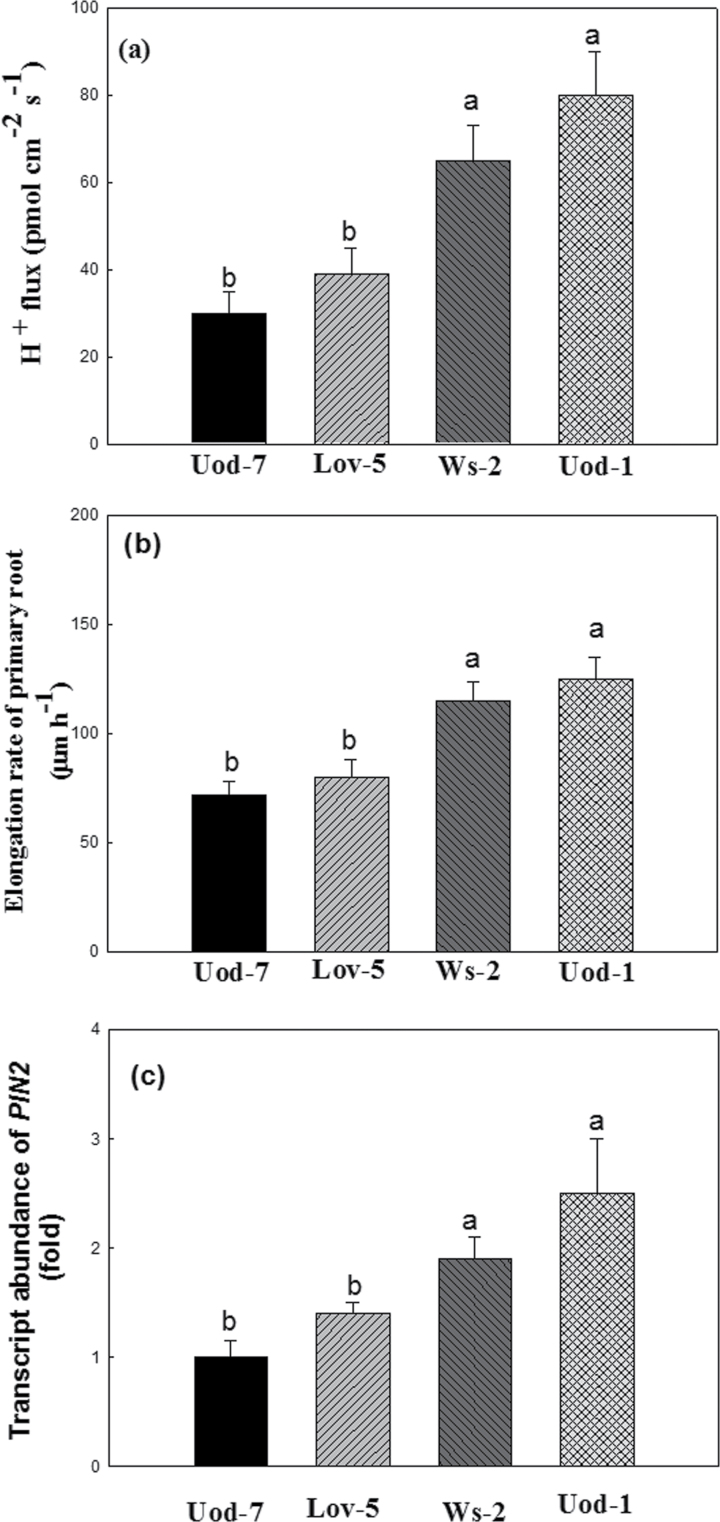
By screening a collection of 96 natural Arabidopsis accessions, Santi and Schmidt (2009) found that the two accessions (Uod-1 and Ws-2) have the highest acidification capacity in roots, while two other accessions (Uod-7 and Lov-5) have the lowest acidification capacity in roots. Our results also showed that, under alkaline stress for 12h, compared with Uod-7 or Lov-5, H+ efflux in the root tips of Uod-1 or Ws-2 was significantly higher (Fig. 6a). Further, the elongation rate of primary root or transcript abundance of PIN2 of total root in Uod-1 or Ws-2 was significantly higher than that of Uod-7 or Lov-5 under alkaline stress for 12h (Fig. 6b, 6c).
Fig. 6.
Response of different Arabidopsis natural accessions under alkaline stress for 12h. 15-d-old Arabidopsis plants were treated with alkaline stress (pH 8.0) for 12h under a hydroponic system. Note: (a) H+ efflux at the 750 µm distance from the root cap junction was analysed in Arabidopsis roots; (b) primary root elongation; (c) ‘Transcript abundance of PIN2 in the whole roots of Uod-7 under alkaline stress’ are plotted as ‘1-fold’.
Discussion
Alkaline stress can cause a much stronger inhibitory effect on plants growth than salt stress due to its external high pH (Shi and Wang, 2005; Yang et al., 2008). Such inhibition is related to the loss of cell wall acidification under alkaline stress (Liu and Guo, 2011). Our results confirm this and show that, under alkaline stress for 12h, natural Arabidopsis accessions with the higher rhizosphere acidification capacity can maintain a relatively high rate of primary root elongation (Fig. 6).
Primary root elongation, directed by sensory root tip, is important for adaptive responses to diverse abiotic stresses (Baluška et al., 2010; Yamaguchi and Sharp, 2010). Expanding and elongating root cells are characterized by their ability to undergo cell-wall extension in acidic apoplastic conditions (Hager, 2003; Staal et al., 2011). This suggests that an active proton secretion at the root apex plays a key role in response to alkaline stress by maintaining the elongation of the primary root. Our results confirm exactly such a mechanism. Mutant pin2 exhibits much reduced plasma membrane H+-ATPase activity and therefore much reduced root elongation under the stress (Fig. 4b).
PM H+-ATPase is important for the root proton-secretion adaptation to alkaline stress (Palmgren, 2001; Liu and Guo, 2011). Mutant pks5 with high activity of the PM H+-ATPase and high proton secretion is shown to be tolerant to alkaline stress (Fuglsang et al., 2007), while mutant J3 with lower activity of PM H+-ATPase and lower proton secretion is sensitive to alkaline stress (Yang et al., 2010). PM H+-ATPase is a component of the auxin-mediated cascade that may affect the proton secretion of root (Rober-Kleber et al., 2003; Staal et al., 2011). In the root epidermis, PIN2 plays a crucial role in basipetal auxin transport and distribution (Kleine-Vehn et al., 2008). According to our results, alkaline stress increases basipetal auxin transport and PIN2 abundance in the root apex of the WT (Figs 2, 23; see Supplementary Fig. S4 at JXB online). Using natural Arabidopsis accessions, it was also found that natural Arabidopsis accessions with the higher H+ efflux also have the higher primary root elongation or higher PIN2 transcription. Further, the PIN2 transcription shows a close and positive correlation to H+ efflux or primary root elongation under alkaline stress (see Supplementary Fig. S5 at JXB online). Thus, these results indicate that PIN2 plays an important role in the root proton-secretion adaptation to alkaline stress.
In Arabidopsis plants, the root apex may be considered to represent a sensory organ consisting of four distinct zones according to distance from the root cap junction (Baluška et al., 2010). They are the meristematic zone: 0–200 µm, the transition zone: 200–520 µm, the elongation zone: 520–850 µm, and the growth terminating zone: 850–1500 µm (Verbelen et al., 2006). PIN2 is responsible for the basipetal (shoot-ward) auxin flow in the root tip and plays an important role in the root tip transition zone which is a signalling-response nexus in the root apex (Baluška et al., 2010). Our results show that, under alkaline stress, the plasma membrane H+-ATPase activity of the whole root, primary root elongation, basipetal auxin transport, and proton secretion of the root tip in pin2 Arabidopsis mutants is lower than the WT (Figs 4, 45, 46; see Supplementary Fig. S3 and Supplementary Fig. S4 at JXB online). In the root apices of pin2 mutants under alkaline stress, auxin strongly accumulated in the meristematic zone (0–200 µm) as it is not able to accomplish basipetal (shoot-ward) transport efficiently in the transition zone, the elongation zone, and the growth terminating zone. Correlatively, the H+ efflux in roots of pin2 mutants was lower than that of the WT at the elongation zone (520–850 µm) or the growth terminating zone (850–1500 µm) under alkaline stress. Thus, under alkaline stress, transition zone PIN2 might also play an important role in basipetal auxin transport. Furthermore, epidermal PM H+-ATPase activated by the PIN2-mediated basipetally transported auxin might be implicated in proton release in the elongation and growth terminating zones to maintain root elongation or to acidify the rhizosphere. Taken together, these results suggest that PIN2 is required for the adaptation of Arabidopsis roots to alkaline stress by modulating proton secretion in the root apex (mainly in the elongation zone or the growth terminating zone) to maintain primary root elongation.
PKS5 inhibits PM H+-ATPase activity by phosphorylating its C-terminal domain and this phosphorylation prevents the interaction between PM H+-ATPase and 14-3-3 protein (Guo et al., 2001; Fuglsang et al., 2007). Under alkaline stress, chaperone J3 interacts with and suppresses PKS5 activity to release PM H+-ATPase. In addition, the alkaline tolerance in Arabidopsis requires a stabilizing microfilament partially through the inactivation of PKS5 activity (Liu and Guo, 2011). In the present study, the pks5 mutant, also lacking PIN2 (double mutant: pks5/pin2), lost the previous higher proton-secretion capacity in the root apex and the higher elongation rate of the primary root under alkaline stress (Fig. 5). These results indicate that PIN2 is also involved in the PKS5-mediated signalling cascade under alkaline-stress.
In conclusion, our results suggest that PIN2 is required for the adaptation of roots to alkaline stress by modulating proton secretion in root tips to maintaining primary root elongation. Firstly, alkaline stress promotes the auxin transport in root tips, and PIN2 plays animportant role in this process. After that, PIN2-transported auxin activates the plasma membrane H+-ATPase to release protons in the root tip under alkaline stress. Proton secretion in root tips (mainly in the elongation zone or the growth terminating zone) plays a key role in response to alkaline stress by maintaining primary root elongation or acidifying rhizosphere environment. Our results also indicate that PIN2 is involved in the PKS5-mediated signalling cascade underlying the alkaline-stress adaptation.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Table S1. Gene-specific primers used for PCR.
Supplementary Fig. S1. The gene expression of PKS5 or PIN2 in the wild-type Arabidopsis plant (WT) or the double mutant (pks5/pin2).
Supplementary Fig. S2. Specific activities of plasma membrane and microsome vesicles isolated from Arabidopsis roots.
Supplementary Fig. S3b. Auxin distribution and abundance in the root tip of pin2 mutant Arabidopsis plants (pin2) (DR5rev:GFP/pin2) under alkaline stress for 12h.
Supplementary Fig. S4. Auxin abundance and basipetal auxin transport in the root tips of wild-type Arabidopsis plants (WT) (DR5rev:GFP) or pin2 mutant Arabidopsis plants (pin2) (DR5rev:GFP/pin2) under alkaline stress for 12h.
Supplementary Fig. S5. The correlation between PIN2 transcript abundance and proton flux or primary root elongation in different Arabidopsis natural accessions.
Acknowledgements
This investigation is supported by grants from the National Natural Science Foundation of China (31272229), National Basic Research Program of China (2012CB114300 and 2013CB127402), the National Natural Science Foundation of China (41171234) and Hong Kong Research Grants Council (CUHK 262809 and HKBU1/CRF/10).
References
- Baluška F, Mancuso S, Volkmann D, Barlow PW. 2010. Root apex transition zone: a signaling-response nexus in the root Trends in Plant Science 15 402–408 [DOI] [PubMed] [Google Scholar]
- Darwin CR. (assisted by Darwin F). 1880. The power of movement in plants John Murray; (http://darwin-online.org.uk/) [Google Scholar]
- Degenhardt B, Gimmler H, Hose E, Hartung W. 2000. Effect of alkaline and saline substrate on ABA content, distribution and transport in plant roots Plant and Soil 225 83–94 [Google Scholar]
- Fu X, Harberd NP. 2003. Auxin promotes Arabidopsis root growth by modulating gibberellin response Nature 421 740–743 [DOI] [PubMed] [Google Scholar]
- Fuglsang AT, Guo Y, Cuin TA, et al. 2007. Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein The Plant Cell 19 1617–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieneisen VA, Xu J, Maree AFM, Hogeweg P, Scheres B. 2007. Auxin transport is sufficient to generate a maximum and gradient guiding root growth Nature 449 1008–1013 [DOI] [PubMed] [Google Scholar]
- Guo Y, Halfter U, Ishitani M, Zhu JK. 2001. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance The Plant Cell 13 731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A, Debus G, Edel HG, Stransky H, Serrano R. 1991. Auxin induces exocytosis and the rapid synthesis of a high-turnover pool of plasma-membrane H+-ATPase Planta 185 527–537 [DOI] [PubMed] [Google Scholar]
- Hager A. 2003. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects Journal of Plant Research 116 483–505 [DOI] [PubMed] [Google Scholar]
- Hurkman WJ. 1992. Effect of salt stress on plant gene expression: a review Plant and Soil 146 145–151 [Google Scholar]
- Jin H, Plaha P, Park JY, et al. 2006. Comparative EST profiles of leaf and root of Leymus chinensis, a xerophilous grass adapted to high pH sodic soil Plant Science 170 1081–1086 [Google Scholar]
- Kawanabe S, Zhu TC. 1991. Degeneration and conservation of Aneurolepisium chinense grassland in northern China Journal of Japan Grassland Science 37 91–99 [Google Scholar]
- Kleine–Vehn J, Leitner J, Zwiewka M, Sauer M, Abas L, Luschnig C, Friml J. 2008. Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting Proceedings of the National Academy of Sciences, USA 105 17812–17817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DR, Muday GK. 2001. Measurement of auxin transport in Arabidopsis thaliana Nature Protocols 4 437–451 [DOI] [PubMed] [Google Scholar]
- Li Q, Li BH, Kronzucker HJ, Shi WM. 2010. Root growth inhibition by NH4 + in Arabidopsis is mediated by the root tip and is linked to NH4 + efflux and GMPase activity Plant, Cell and Environment 33 1529–1542 [DOI] [PubMed] [Google Scholar]
- Liu J, Guo Y. 2011. The alkaline tolerance in Arabidopsis requires microfilament partially through inactivation of PKS5 kinase Journal of Genetics and Genomics 38 307–313 [DOI] [PubMed] [Google Scholar]
- Moloney MM, Elliott MC, Cleland RE. 1981. Acid growth effects in maize roots: evidence for a link between auxin-economy and proton extrusion in the control of root growth Planta 152 285–291 [DOI] [PubMed] [Google Scholar]
- Palmgren MG. 2001. Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake Annual Review of Plant Physiology and Plant Molecular Biology 52 817–845 [DOI] [PubMed] [Google Scholar]
- Rober–Kleber N, Albrechtova JTP, Fleig S, Huck N, Michalke W, Wagner E, Speth V, Neuhaus G, Fischer–Iglesias C. 2003. Plasma membrane of H+-ATPases is involved in auxin-mediated cell elongation during wheat embryo development Plant Physiology 131 1302–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi S, Schmidt W. 2009. Dissecting iron deficiency-induced proton extrusion in Arabidopsis root New Phytologist 183 1072–1084 [DOI] [PubMed] [Google Scholar]
- Schlicht M, Samajova O, Schachtschabel D, Mancuso S, Menzel D, Boland W, Baluška F. 2008. D’orenone blocks polarized tip growth of root hairs by interfering with the PIN2-mediated auxin transport network in the root apex The Plant Journal 55 709–717 [DOI] [PubMed] [Google Scholar]
- Shen H, Chen J, Wang Z, Yang C, Sasaki T, Yamamoto Y, Matsumoto H, Yan X. 2006. Root plasma membrane H+-ATPase activity is involved in the adaptation of soybean to phosphorus starvation Journal of Experimental Botany 57 1353–1362 [DOI] [PubMed] [Google Scholar]
- Shi D, Wang D. 2005. Effect of various salt-alkaline mixed stresses on Aneurolepidium chinense (Trin.) Kitag Plant and Soil 271 15–26 [Google Scholar]
- Staal M, Cnodder TD, Simon D, Vandenbussche F, Straeten DVD, Verbelen JP, Elzenga T, Vissenberg K. 2011. Apoplastic alkalinization is instrumental for the inhibition of cell elongation in Arabidopsis root by the ethylene precursor 1-aminocyclopropane-1-carboxylic acid Plant Physiology 155 2049–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbelen JP, Cnodder TD, Le J, Vissenberg K, Baluška F. 2006. The root apex of Arabidopsis thaliana consists of four distinct zones of growth activities Plant Signaling and Behavior 1 296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ma H, Liu G, Xu C, Zhang D, Ban Q. 2008. Analysis of gene expression profile of Limonium bicolor under NaHCO3 stress using cDNA microarray Plant Molecular Biology Reporter 26 241–254 [Google Scholar]
- Xu WF, Shi WM. 2006. . Expression profiling of the 14-3-3 gene family in response to salt stress and potassium and iron deficiencies in young tomato (Solanum lycopersicum) roots: analysis by real-time RT-PCR Annals of Botany 98 965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WF, Shi WM. 2007. Mechanisms of salt tolerance in transgenic Arabidopsis thaliana constitutively overexpressing the tomato 14-3-3 protein TFT7 Plant and Soil 301 17–28 [Google Scholar]
- Xu WF, Shi WM. 2008. A ‘nonsterile’ method for selecting and growing Arabidopsis thaliana transformants (T2 Transgenic Lines) resistant to kanamycin Plant Molecular Biology Reporter 26 350–357 [Google Scholar]
- Xu WF, Shi WM, Jia LG, Liang JS, Zhang JH. 2012. TFT6 and TFT7, two different members of tomato 14-3-3 gene family, play distinct roles in plant adaption to low phosphorus stress Plant, Cell and Environment 35 1393–1406 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Sharp RE. 2010. Complexity and coordination of root growth at low water potencials: recent advances from transcriptomic and proteomic analyses Plant, Cell and Environment 33 590–603 [DOI] [PubMed] [Google Scholar]
- Yang C, Wang P, Li C, Shi D, Wang D. 2008. Comparison of effect of salt and alkali stresses on the growth and photosynthesis of wheat Photosynthetica 46 107–114 [Google Scholar]
- Yang Y, Qin Y, Xie C, et al. 2010. The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase The Plant Cell 22 1313–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. 2001. Plant salt tolerance Trends in Plant Science 6 66–71 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.