Abstract
A CBL-interacting protein kinase (CIPK) gene, BnCIPK6, was isolated in Brassica napus. Through yeast two-hybrid screening, 27 interaction partners (including BnCBL1) of BnCIPK6 were identified in Brassica napus. Interaction of BnCIPK6 and BnCBL1 was further confirmed by BiFC (bimolecular fluorescence complementation) in plant cells. Expressions of BnCIPK6 and BnCBL1 were significantly up-regulated by salt and osmotic stresses, phosphorous starvation, and abscisic acid (ABA). Furthermore, BnCIPK6 promoter activity was intensively induced in cotyledons and roots under NaCl, mannitol, and ABA treatments. Transgenic Arabidopsis plants with over-expressing BnCIPK6, its activated form BnCIPK6M, and BnCBL1 enhanced high salinity and low phosphate tolerance, suggesting that the functional interaction of BnCBL1 and BnCIPK6 may be important for the high salinity and phosphorous deficiency signalling pathways. In addition, activation of BnCIPK6 confers Arabidopsis plants hypersensitive to ABA. On the other hand, over-expression of BnCIPK6 in Arabidopsis cipk6 mutant completely rescued the low-phosphate-sensitive and ABA-insensitive phenotypes of this mutant, further suggesting that BnCIPK6 is involved in the plant response to high-salinity, phosphorous deficiency, and ABA signalling.
Key words: Abiotic stress tolerance, abscisic acid (ABA), Brassica napus, BnCBL1, BnCIPK6, interaction, regulation of gene expression
Introduction
As an essential second messenger, calcium regulates diverse cellular processes in plants. Several Ca2+-sensor protein families, including calmodulin (CaM), the superfamily of calcium-dependent protein kinases (CDPK), and calcineurin B-like (CBL) proteins, have been characterized and implicated in a variety of physiological functions in plants (Albrecht et al., 2001; Kim et al., 2003; Pandey et al., 2004). Ca2+ sensors can be classified into sensor responders and sensor relays (Sanders et al., 2002). Upon binding of Ca2+, sensor responders change their conformation and modulate their own enzymatic activity or function through intramolecular interactions. By contrast, sensor relays must interact with their target proteins (such as protein kinases) to regulate their activity after binding Ca2+. CDPKs act as sensor responders (Sanders et al., 2002; Kim et al., 2003), while CaM and CBL proteins are sensor relays (Luan et al., 2002; Sanders et al., 2002). However, unlike CaMs targeting a large variety of target proteins, CBLs specifically interact with a family of protein kinases referred to as CBL-interacting protein kinases (CIPKs) or SnRK3s (Luan et al., 2002). 10 CBLs and 25 CIPKs in Arabidopsis and 10 CBLs and 30 CIPKs in rice were identified, respectively (Kolukisaoglu et al., 2004; Batistic and Kudla, 2004).
CIPK family proteins have an N-terminal kinase catalytic domain, related to sucrose non-fermenting kinase (SNF1) and AMP-activated protein kinase (AMPK), via a junction domain, fused to a highly variable C-terminal regulatory domain. The N-terminal kinase domain contains a putative activation loop located between the conserved DFG and APE sequences (Batistic and Kudla, 2004). Mutational analysis revealed that the constitutively active forms of CIPK activity might be generated through substitution of one of three conserved residues (serine, threonine, and tyrosine) to aspartate within the activation loop (Guo et al., 2001; Gong et al., 2002a ). C-terminal regulatory domains of all CIPKs possess a highly conserved FISL motif, also named a NAF domain, which consists of a unique 24 amino-acid residues, required and sufficient for interacting with CBL proteins (Albrecht et al., 2001). It was revealed that the intramolecular interaction between NAF domain and regulatory domain exerts an inhibitory effect towards the kinase activity. Both the binding of the FISL motif with CBLs and deletion of the NAF domain can relieve auto-inhibition (Guo et al., 2001; Gong et al., 2002b ). Superactive kinase SOS2 is generated when the threonine to aspartate mutation is combined with the NAF domain deletion (Guo et al., 2001; Gong et al., 2002b ). However, little is known about how CIPKs are activated in vivo. It was assumed that activation of AtCIPKs take place in vivo through phosphorylation of the activation loop and binding of CBL proteins working simultaneously (Gong et al., 2004; Batistic and Kudla, 2004). Previous study indicated that a motif, PPI (protein phosphatase interaction), adjacent to the FISL motif is necessary for interaction with ABI (Abscisic acid-Insensitive) protein phosphatases (Ohta et al., 2003). It is proposed that CIPK kinases and a 2C-type protein phosphatase control the phosphorylation status of each other and/or downstream target proteins (Rodriguez, 1998; Ohta et al., 2003; Lee et al., 2007).
CBL/CIPK signal components are involved in many kinds of signalling pathways. SOS is one of the CBL/CIPK signalling pathways conferring plant salt tolerance (Zhu et al., 1998). Under salt stress, the calcium sensor SOS3 (CBL4) activates the kinase SOS2 (CIPK24) that positively regulates SOS1, a plasma membrane sodium/proton antiporter (Shi et al., 2000; Qiu et al., 2002). Moreover, it was demonstrated that CBL10 can also interact with CIPK24 to form the CBL10/CIPK24 complex that regulates the downstream protein SOS1. The CBL10/CIPK24/SOS1 pathway mainly regulates Na+-homeostasis in shoots and leaves, revealing that CBL4 and CBL10 are required for salt tolerance (Quan et al., 2007). Previous study showed that CIPK3 transcripts were induced by cold, salt, drought, and abscisic acid (ABA), and the cipk3 loss-of-function mutant is hypersensitive to ABA. Disruption of CIPK3 altered the expression pattern of a number of stress gene markers in response to ABA, cold and high salt, but did not affect transcription of low temperature-inducible transcription factors (e.g. CBF3), suggesting CIPK3 act as a cross-talk component between cold and ABA signalling (Kim et al., 2003). Furthermore, CBL9 and CIPK3 physically and functionally interact with each other and form a specific complex that functions in ABA response in seed germination (Pandey et al., 2004, 2008). Over-expression of CIPK20/PKS18 (T169D) rendered the transgenic plants hypersensitive to ABA, whereas RNAi plants showed insensitivity to ABA (Gong et al., 2002b ). CIPK23, activated by the binding of CBL1 and CBL9, directly phosphorylates the K+ transporter AKT1, and enhance K+ uptake under low-K+ conditions (Li et al., 2006; Xu et al., 2006). A later study revealed that CIPK23 also phosphorylates the nitrate transporter CHL1 to maintain a low-level primary response to low nitrate concentration (Ho et al., 2009).
Brassica napus, as an important oilseed plant, often encounters abiotic stresses, such as high salinity, drought, cold, and nutrient deficiency (such as P and K limitation), resulting in plant growth retardation and reduced agricultural productivity. It is both of biological and agricultural importance to understand the molecular mechanism of Brassica napus in response to abiotic stresses. Although Arabidopsis CBLs/CIPKs participating in various calcium-signalling pathways are well characterized, little is known about the CBL/CIPK signalling pathway in Brassica napus. In this study, a gene in Brassica napus, BnCIPK6, was isolated. Through yeast two-hybrid screening, 27 novel interaction partners (including BnCBL1) of BnCIPK6 were identified in Brassica napus. Interaction of BnCIPK6 and BnCBL1 was further confirmed by BiFC in plant cells. Over-expression of both BnCIPK6 and BnCBL1 in Arabidopsis enhance the plant’s tolerance to salt and low phosphate stresses, suggesting that BnCBL1 and BnCIPK6 may functionally interact with each other to be involved in the plant response to salt and low phosphate stresses. The data also revealed that BnCIPK6M (T182D) transgenic plants were hypersensitive to abscisic acid (ABA). To our knowledge, this is the first report that Brassica napus CBL/CIPK is functionally involved in the response to abiotic stress and ABA signalling. More importantly, no CBLs/CIPKs have been identified as signalling components involved in phosphorus starvation signalling so far.
Materials and methods
Plant growth conditions
Seeds of Brassica napus (cv. Zhongyou 821) were surface-sterilized and germinated on half-strength Murashige and Skoog (MS) medium (pH 5.8) containing 0.8% agar under a 16/8h light/dark cycle at 25 °C for 7 d. Roots, cotyledons, and hypocotyls were collected from sterile seedlings for RNA isolation. Other tissues, such as stems, leaves, and flowers, were derived from B. napus plants grown in the field.
In stress experiments, 1-week-old seedlings of B. napus were transferred to MS medium containing 150mM NaCl, 200mM mannitol, 100 µM ABA (abscisic acid), or low phosphate (10 µM phosphate) for certain durations, respectively.
BiFC analyses of interaction between BnCIPK6 and BnCBL1
pUC-SPYNE-BnCBL1, pUC-SPYNE-BnCIPK6, pUC-SPYCE-BnCBL1, and pUC-SPYCE-BnCIPK6 vectors were constructed using gene-specific primers, respectively (see Supplementary Table S1 at JXB online). The constructs were then introduced into onion epidermal cells by DNA particle bombardment according to the manufacturer’s instructions (Biolistic PDS-1000/He Particle Delivery System, Bio-Rad, USA), respectively. Fluorescence microscopy was performed on a SP5 Meta confocal laser microscope (Leica, Germany). YFP fluorescence in transformed cells was deteceted, using bZIP63 dimerization as the positive control and pUC-SPYNE-BnCBL1+pUC-SPYCE and pUC-SPYNE+pUC-SPYCE-BnCIPK6 as the negative controls.
Quantitative RT-PCR and Northern blot analyses
The expression of the BnCIPK6 and BnCBL1 genes in B. napus tissues was analysed by quantitative reverse transcriptase (RT)-PCR as described earlier (Li et al., 2005), and using the BnACT2 gene as a quantitative control. To assay the expression of stress- and ABA-responsive genes, RT-PCR analysis was performed with the RNA samples isolated from 2-week-old seedlings treated with or without 100 µM ABA for 6h, using the ACTIN2 gene as an internal control. All the quantitative RT-PCR analyses were repeated three times along with three independent repetitions of the biological experiments, and means of three biological experiments were calculated for estimating gene expression levels. Primer sequences for real-time PCR are listed in Supplementary Table S1 at JXB online.
RNA Northern-blot hybridization was performed as described previously by Li et al.(2002).
Yeast two-hybrid analysis
The coding sequences of BnCIPK6 and 10 AtCBL genes were cloned into the yeast two-hybrid vectors pGBKT7 (bait vector) and pGADT7 (prey vector), respectively. Afterwards, the pGBKT7-BnCIPK6 construct was introduced singly into the yeast strain Y187 using the high-efficiency lithium acetate transformation procedure (Gietz et al., 1992), and each pGADT7-AtCBL construct was transferred into the yeast strain AH109. The mating reactions and further interaction assays between the two haploid strains containing pGBKT7-BnCIPK6 and pGADT7-AtCBL constructs were performed by the method described previously by Zhang et al. (2010).
A yeast two-hybrid library of B. napus cDNAs from mRNAs of different tissues (roots, hypocotyls, cotyledons, stems, leaves, and flowers) was constructed, using the Clontech BD Matchmaker Library Construction and Screening Kits according to the manufacturer’s instruction (BD Biosciences Clontech). For screening target proteins of BnCIPK6 from the B. napus cDNA library, yeast two-hybrid analysis was performed using the BnCIPK6 proteins as a bait to screen the two-hybrid library of B. napus cDNAs constructed on the prey vector, by the method described previously by Zhang et al. (2010). Primer sequences for pGBKT7-BnCIPK6 and pGADT7-AtCBL constructs were listed in Supplementary Table S1 at JXB online.
Construction of BnCIPK6p:GUS chimeric genes and Arabidopsis transformation
A SalI site and BamHI site were introduced at the 5’-end and the 3’-end of the BnCIPK6 5’-upstream region, respectively. The SalI/BamHI fragments (0.8kb and 1.2kb, respectively) were then subcloned into the pBI101 vector, replacing the CaMV 35S promoter to generate chimeric BnCIPK6p:GUS constructs. The constructs were introduced into Arabidopsis by the floral dip method. Transformed seeds were selected on MS medium containing 50mg l–1 kanamycin. Homozygous lines of the T3 and T4 generations were used for phenotypic analysis of transgenic plants. Corresponding primer sequences were listed in Supplementary Table S1 at JXB online.
Histochemical assay of GUS activity
Histochemical assays for GUS activity in transgenic Arabidopsis plants were conducted according to the protocol described byLi et al. (2002).
To test the induction of GUS expression by salt, osmotic stresses, and ABA treatments, transgenic seedlings were transferred to MS liquid medium containing 150mM NaCl, 300mM mannitol, or 100 µM ABA (abscisic acid), for 6h. GUS staining patterns were confirmed by observing at least five different transgenic lines. Moreover, GUS activity in the transgenic seedlings with expressing BnCIPK6p:GUS was quantitatively measured by the fluorometrical method using 4-MUG (Sigma-Aldrich) as substrate (Jefferson et al., 1987).
Over-expression of BnCIPK6, BnCIPK6M, and BnCBL1 genes in Arabidopsis and phenotypic analysis of transgenic plants
To introduce the Thr182-to-Asp (T182D) mutation for constitutively activated BnCIPK6, the primer-based site-directed mutation BnCIPK6M (5’-acgggcttctccacGACacttgtggaactcc-3’, 5’-ggagttccacaagtGTCgtggagaagcccgt-3’) was generated. The coding sequences of BnCIPK6 and BnCIPK6M were cloned into PBI121 vector. Similarly, the coding sequence of BnCBL1 was also cloned into the PBI121 vector (see Supplementary Table S1 at JXB online). The constructs were then transferred into Arabidopsis by the floral dip method. Seeds of wild type and independent lines of BnCIPK6, BnCIPK6M, and BnCBL1 transgenic plants were germinated on MS medium with different concentrations of ABA. Seeds were considered germinated when radicles completely penetrated the seed coat; germination and seedling growth were scored at the indicated times.
Seeds of wild-type and independent lines of BnCIPK6, BnCIPK6M, and BnCBL1 transgenic plants were germinated on MS medium. Seedlings were then transferred to MS medium containing different salt concentrations (0, 75, 150, 170, 200, and 250mM) in the vertical position. The status of seedling growth was recorded a few days after the transfer. The chlorophyll content in leaves was determined. In brief, chlorophylls in leaves were extracted with 80% acetone, and the extract was incubated at 4 °C for 2h in darkness. Chlorophyll content was assayed by measuring absorbance at 645, 652, and 663nm with a spectrophotometer. Proline content in both control and transgenic plants was determined using the protocol as described by Gong et al. (2012).
Seeds of wild-type and transgenic lines germinated and grew on MS medium with or without ABA for 2 weeks, and the primary root length was measured. For seedling growth under low phosphate (LP) treatments, 6-day -old seedlings were transferred and vertically cultured at 50 µM low phosphate (LP) medium for a few days, then the status of seedling growth was recorded, including lateral root growth, and fresh and dry weight assays.
In vitro phosphorylation assay of recombinant MBP fusion proteins
The coding regions of BnCIPK6 and BnCIPK6M (T182D) were cloned into the pMAL vector. Afterwards, the vectors including recombinant and empty vectors were separately transformed into Escherichia coli strain BL21. After induction for 3.5h, the recombinant proteins were purified from the bacterial lysates by NEB according to the manufacturer’s instructions. In vitro protein phosphorylation assays were performed in the reaction mixture comprising purified recombinant MBP proteins incubated in kinase buffer (10 µCi [r32P]ATP, 25mM TRIS-HCl pH 8.0, 0.5mM DTT, 10mM MgCl2, 0.1mM CaCl2) for 30min at 30 °C. Reactions were terminated by adding 5× LSB buffer and were boiled for 5min at 95 °C. To detect autophosphorylation, the reaction mixture was separated by 12% (w/v) SDS-PAGE, and then the gel was dried and exposed to a Kodark X-ray film.
Results
Characterization of the BnCIPK6 gene
To investigate the genes involved in response to abiotic stress, 154 salt- and drought-induced genes were identified (Chen et al., 2010), including BnCIPK6 (accession number in GenBank: JF751063) in the Brassica napus cDNA library. BnCIPK6 encodes a CBL-interacting protein kinase (CIPK). Subsequently, the complete DNA sequence of the BnCIPK6 gene in the B. napus genome was isolated, including its 5’-flanking sequence. Compared with its cDNA sequence, it was found that the BnCIPK6 gene contains no intron in its open reading frame. In addition, BnCIPK6 protein contains an N-terminal SNF1-like kinase catalytic domain and a C-Terminal regulatory domain with a CBL-interacting NAF/FISL module (see Supplementary Fig. S1 at JXB online).
Identification of BnCIPK6-interacting proteins
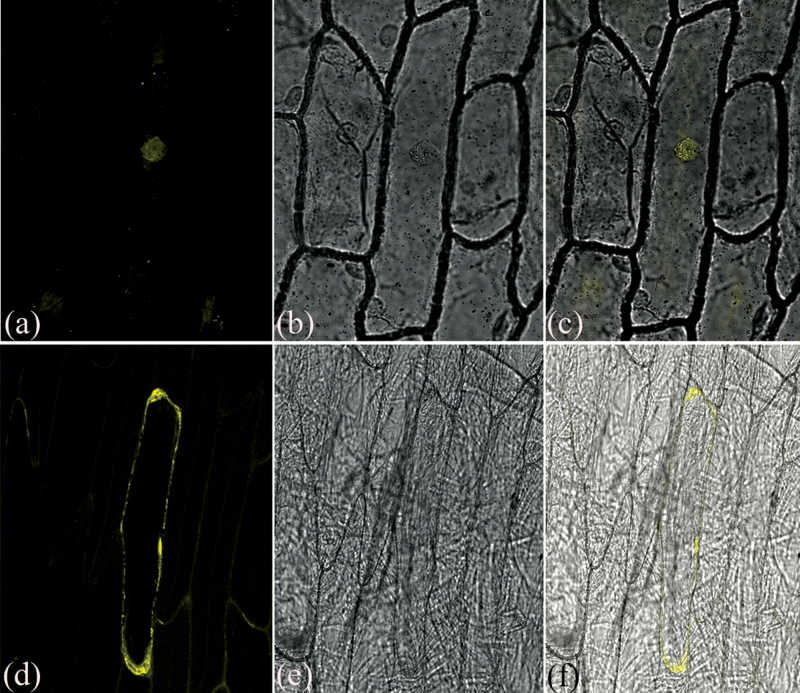
To identify interaction partners of BnCIPK6 in Brassica napus, the yeast two-hybrid library of Brassica napus cDNAs was screened using BnCIPK6 as bait. 27 unique proteins were identified as positive clones (see Supplementary Table S2 at JXB online), and all positive clones were checked for the presence of a cDNA–AD fusion and further confirmed in the one-to-one interaction analysis. The BnCIPK6-interacting proteins identified were related to various aspects of plant development, metabolism, and signal transduction. BLASTP analysis revealed that an isolated calcineurin B-like (CBL) protein (No. 27) by yeast two-hybrid screening shares high homology to AtCBL1, and consequently designated as BnCBL1 (accession number in GenBank: JF751064). To confirm the interaction between BnCIPK6 and BnCBL1 further, the BiFC method was employed. The results demonstrated that BnCIPK6 interacted with BnCBL1 in vivo (Fig. 1; see Supplementary Fig. S2 at JXB online). In addition, the experimental results indicated that BnCIPK6 protein showed no self-activation of transcription, and also interacted strongly and specifically with AtCBL1, AtCBL2, AtCBL3, and AtCBL9, suggesting that CBL-CIPK binding specificity can cross the species barrier (see Supplementary Fig. S3 at JXB online).
Fig. 1.
BiFC assays of BnCIPK6 interaction with BnCBL1 in vivo. (a–c) BiFC visualization of bZIP63 dimerization in vivo; (d–f) BiFC visualization of BnCIPK6 interaction with BnCBL1 in vivo. (a, d) YFP fluorescent images; (b, e) bright field images of images (a) and (d); (c, f) fluorescent images merged with their bright-field images.
Transcripts of BnCIPK6 and BnCBL1 genes were induced by abiotic stresses and abscisic acid (ABA)
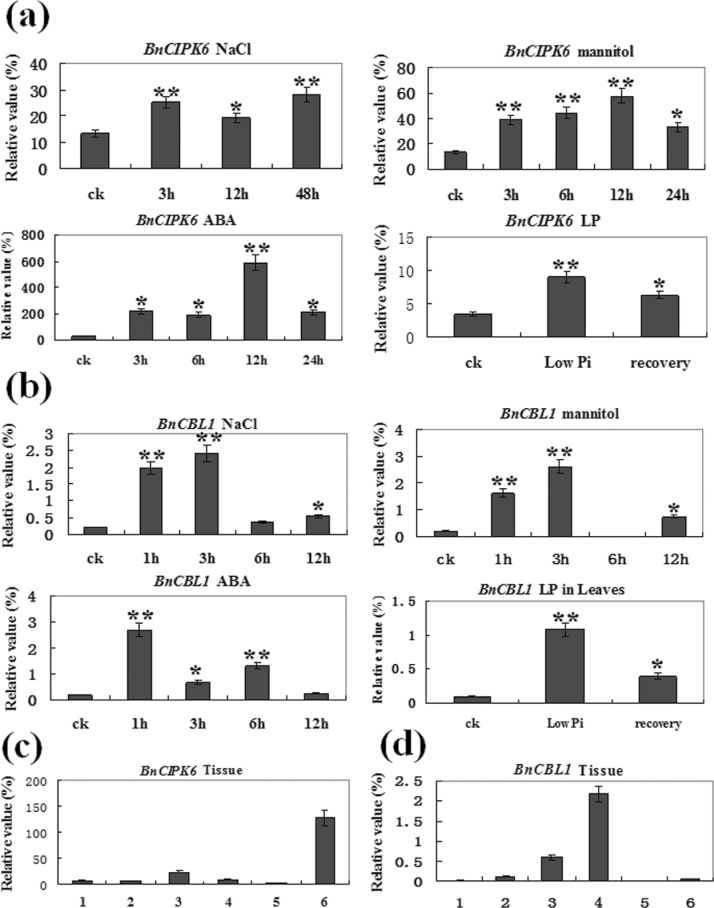
To investigate whether expressions of BnCIPK6 and BnCBL1 genes are regulated by abiotic stresses and plant hormones, one-week-old seedlings of B. napus were subjected to different abiotic stresses and exogenous plant hormone treatments. As shown in Fig. 2a, the expression level of BnCIPK6 was significantly up-regulated by high-salinity, osmotic stress, low phosphate and ABA in roots. BnCIPK6 mRNAs were largely accumulated in roots at 3h after NaCl treatment, and reached its highest level at 48h. By contrast, BnCIPK6 transcripts reached its highest level at 12h and then declined at 24h under mannitol and ABA treatments. It was also found that the BnCIPK6 gene is strongly induced by low phosphate stress, but its expression was decreased when plants were transferred from Pi starvation to normal Pi condition (1.25mM). Similarly, the expression level of the BnCBL1 gene in roots was remarkably enhanced by NaCl, mannitol, and ABA, and reached its peak values at 1–3h after the treatments. In addition, our experimental data revealed that BnCBL1 was induced by low phosphate stress in leaves, but not in roots of B. napus (Fig. 2b). Moreover, the expression patterns of BnCIPK6 and BnCBL1 genes in different tissues of B. napus were also analysed by quantitative RT-PCR. The results revealed that transcripts of BnCIPK6 were largely accumulated in flowers, at moderate or low levels in other tissues, whereas BnCBL1 expression was at relatively high levels in stems of B. napus (Fig. 2c, 2d).
Fig. 2.
Quantitative RT-PCR analysis of BnCIPK6 and BnCBL1 in Brassica napus. (a) Expression of the BnCIPK6 gene in roots of B. napus under NaCl, mannitol, ABA, and low phosphate stress treatments. Total RNA was isolated from roots of 7-d-old seedlings treated with 150mM NaCl for 3, 12, and 48h, 200mM mannitol for 3, 6, 12, and 24h, 100 µM ABA for 3, 6, 12, and 24h, and low phosphate (10 µM phosphate for 72h, then recovered for 72h in MS medium with 1.25mM phosphate), respectively. (b) Expression of the BnCBL1 gene in roots of B. napus under salt stress, osmotic stress, and ABA treatment, and in leaves of B. napus under low phosphate stress. Total RNA was isolated from roots of 7-d-old seedlings treated with 150mM NaCl, 200mM mannitol, 100 µM ABA for 1,3, 6, and 12h and low phosphate (10 µM phosphate for 72h, then recovered for 72h in MS medium with normal phosphate content), respectively. (c) BnCIPK6 and (d) BnCBL1 expression in B. napus tissues. Total RNAs were isolated from roots (1), hypocotyls (2), cotyledons (3), stems (4), leaves (5), and flowers (6) of B. napus, respectively. Relative value of BnCIPK6 and BnCBL1 expression was shown as a percentage of BnACT2 expression activity; ck, untreated roots (control). Mean values and standard errors (bar) were shown from three independent experiments. Independent t tests for equality of means demonstrated that there was significant difference (*P value <0.05) or very significant difference (**P value <0.01) between untreated (control) and treated roots or leaves.
BnCIPK6 promoter is salt/osmotic stress- and ABA-inducible
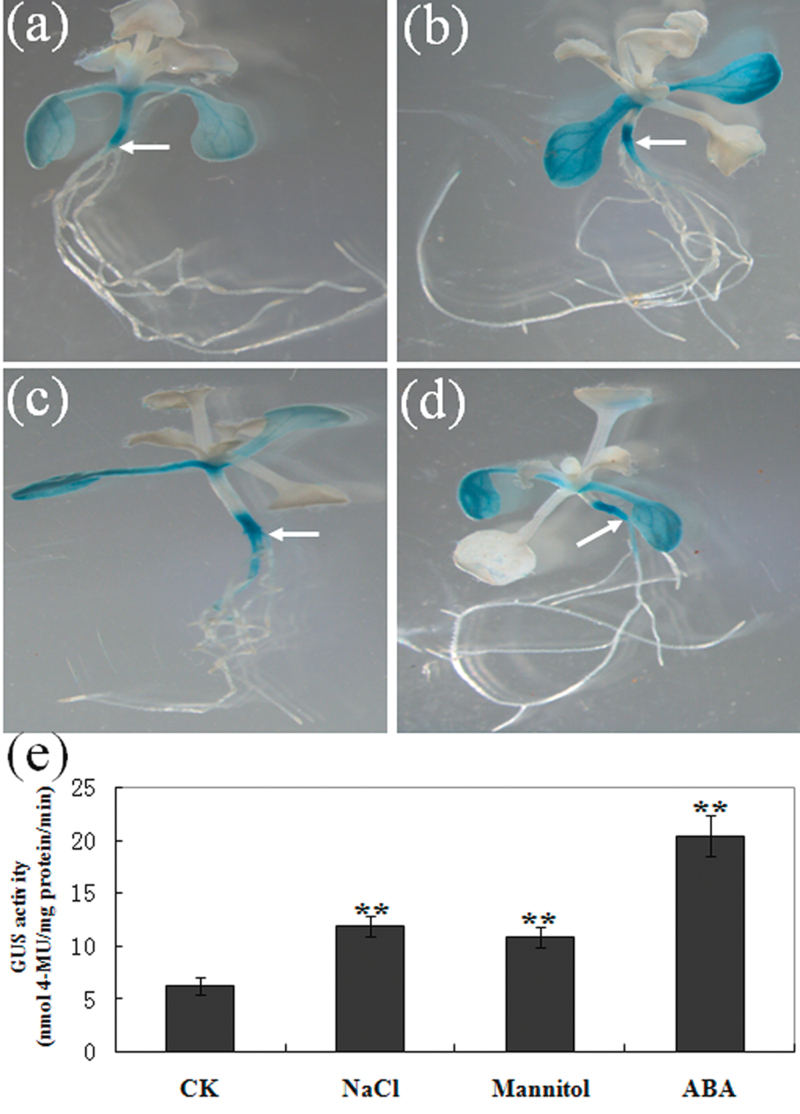
To investigate whether the BnCIPK6 promoter is induced by abiotic stresses and hormone treatments, a 1.2kb BnCIPK6 5’-flanking region before the translational initiation codon (ATG) was cloned upstream of the GUS reporter gene in the pBI101 vector, giving rise to the BnCIPK6p:GUS chimeric gene. The construct was introduced into Arabidopsis. A total of 30 transgenic Arabidopsis plants (lines) were obtained. Histochemical assay revealed that GUS staining was detected at relatively high levels in hypocotyls and cotyledons in 12-d-old seedlings, but weak or no GUS signals in other tissues (Fig. 3a). GUS activities were significantly increased in cotyledons and hypocotyls of the transgenic seedlings (12-d-old) for 6h after 150mM NaCl, 300mM mannitol, and 100 µM ABA treatments, compared with those of mock treatments (Fig. 3b–d). It should be noted that GUS activities were detected in the maturation zone of roots after these treatments, but not in those of control transgenic seedlings. Quantitative analysis further confirmed that GUS activity was higher in the salinity, osmotic, and ABA-treated transgenic plants than those of the mock treatment (Fig. 3e). These results suggested that the BnCIPK6 promoter is salt-/osmotic stress-/ABA-inducible.
Fig. 3.
Analysis of BnCIPK6 promoter activity in Arabidopsis plants under NaCl, mannitol, and abscisic acid (ABA) treatments. 12-d-old transgenic Arabidopsis seedlings containing the BnCIPK6 promoter fused to the GUS reporter gene were treated without (control) or with 150mM NaCl, 300mM mannitol, and 100 µM ABA for 6h. (a) A control seedling; (b) a seedling with NaCl treatment; (c) a seedling with mannitol treatment; (d) a seedling with ABA treatment. (e) Measurement and quantitative analysis of GUS activity in BnCIPK6p:GUS transgenic Arabidopsis plants under NaCl, mannitol or ABA treatments. Mean values and standard errors (bar) were shown from three independent experiments. Independent t tests for equality of means demonstrated that there was very significant difference between CK and NaCl-, mannitol-, or ABA-treated transgenic plants (**P value <0.01).
Assay of BnCIPK6 kinase activity in vitro
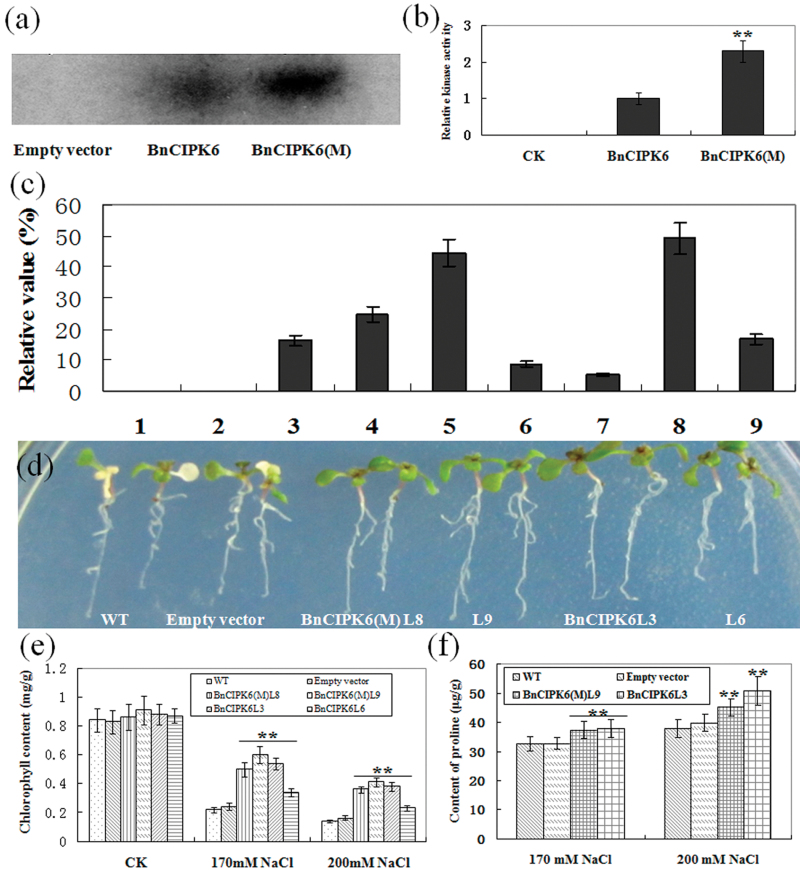
To characterize the kinase activity of BnCIPK6 biochemically, MBP-BnCIPK6 and MBP-BnCIPK6M (T182D, i.e. Thr182 was substituted by Asp in BnCIPK6, hereafter referred to as BnCIPK6M) proteins, as well as empty MBP protein from Escherichia coli, were expressed. As shown in Fig. 4a and 4b, BnCIPK6M mutant protein exhibited 2.3-fold higher autophosphorylation activity than that of BnCIPK6 protein, suggesting that the 182nd threonine residue in BnCIPK6 may be a critical target site for the protein activation by upstream kinase(s).
Fig. 4.
Over-expression of BnCIPK6 and BnCIPK6M in Arabidopsis enhances plant tolerance to salt stress. (a) In vitro phosphorylation assay of recombinant MBP-BnCIPK6 and MBP-BnCIPK6M fusion proteins. (b) Quantitative analysis of autophosphorylation activities of BnCIPK6 and BnCIPK6M. (c) Quantitative RT-PCR analysis of BnCIPK6 and BnCIPK6M expression in transgenic Arabidopsis. (1) WT; (2) PMD vector; (3–6) BnCIPK6M transgenic lines L5, L8, L9, and L10; (7–9) BnCIPK6 transgenic lines L1, L3, and L6. (d) One-week-old seedlings were transferred and grew for 5 d on MS medium supplemented with 170mM NaCl. (e) Statistical analysis of chlorophyll content of leaves of seedlings grown on MS medium containing 170mM and 200mM NaCl for 7 d. (f) Measurement of proline content in seedlings of wild-type and transgenic lines treated with 170mM and 200mM NaCl for 24h. Mean values and standard errors (bar) were shown from three independent experiments (n >50 seedlings per each line). Independent t tests for equality of means demonstrated that there was very significant difference between wild type and transgenic plants (**P value <0.01). (This figure is available in colour at JXB online.)
Over-expression of BnCIPK6 and BnCBL1 in Arabidopsis enhances plant tolerance to salt stress
The coding sequences of BnCIPK6 and BnCIPK6M fused to the CaMV 35S promoter were introduced into Arabidopsis, and over 30 homozygous transgenic lines (T2 and T3 generations) were obtained. Expression levels of BnCIPK6 and BnCIPK6M in the transgenic plants were examined by RT-PCR analysis (Fig. 4c). Two transgenic lines (L8 and L9) with higher BnCIPK6M expression and two lines (L3 and L6) with higher BnCIPK6 expression were selected for analysng their phenotypes under various treatment conditions. When 1-week-old seedlings grew on MS medium under normal conditions, there was no difference between wild type and transgenic plants. When 1-week-old seedlings were transferred and vertically cultured on MS medium containing a range of NaCl concentrations, both BnCIPK6 and BnCIPK6M transgenic lines displayed better salt tolerance than that of wild-type plants (Fig. 4d; see Supplementary Table S3 at JXB online). Statistical analysis indicated that there were significant differences in chlorophyll content, and proline content between BnCIPK6/BnCIPK6M transgenic lines and control plants under salt stress (Fig. 4e, 4f).
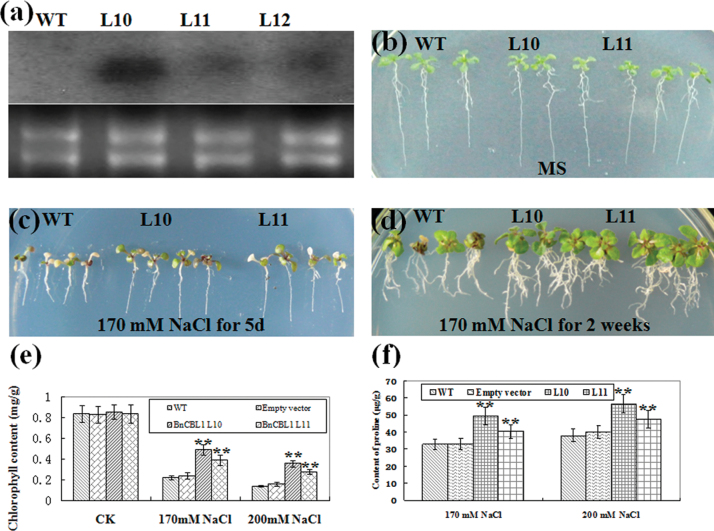
Similarly, in order to determine whether BnCBL1 is also involved in salt signalling, the BnCBL1 gene was introduced into Arabidopsis plants. Over 20 homozygous transgenic lines (T2 and T3 generations) were obtained, of which the lines with high BnCBL1 expression were selected for more detailed analysis (Fig. 5a; see Supplementary Fig. S4 at JXB online). The results also revealed that BnCBL1 transgenic seedlings showed increased NaCl-tolerance, compared with the wild type (Fig. 5; see Supplementary Table S4 at JXB online). These data indicated that over-expression of BnCIPK6, BnCIPK6M, and BnCBL1 significantly enhanced plant tolerance to salt stress.
Fig. 5.
Over-expression of BnCBL1 in Arabidopsis enhances plant tolerance to salt stress. (a) Northern blotting analysis of BnCBL1 expression in transgenic Arabidopsis. (b) One-week-old seedlings were transferred and grown for 5 d on MS medium as controls. (c) One-week-old seedlings were transferred and grown for 5 d on MS medium supplemented with 170mM NaCl. (d) One-week-old seedlings were transferred and grown for 2 weeks on MS medium supplemented with 170mM NaCl. (e) Statistical analysis of chlorophyll content of leaves. The seedlings were grown on MS medium containing 170mM and 200mM NaCl for 5 d. (f) Measurement of proline content. The seedlings of wild-type and transgenic lines were treated with 170mM and 200mM NaCl for 24h. Mean values and standard errors (bar) were shown from three independent experiments (n >50 seedlings per each line). Independent t tests for equality of means demonstrated that there was very significant difference between wild-type and transgenic plants (**P value <0.01). WT, wild type; L10, L11, and L12, BnCBL1 transgenic lines 10, 11, and 12. (This figure is available in colour at JXB online.)
Over-expression of BnCIPK6 and BnCBL1 in Arabidopsis enhances plant tolerance to low phosphate stress
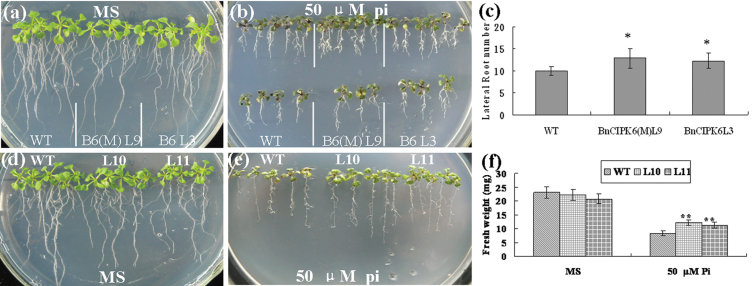
Six-day-old BnCBL1, BnCIPK6, and BnCIPK6M transgenic seedlings were transferred and vertically cultured at 50 µM low phosphate medium for 9 d. It was found that BnCIPK6 and BnCIPK6M transgenic seedlings grew better than wild-type plants under low phosphate conditions, whereas there was no significant difference between the transgenic plants and the wild type under phosphate-sufficient conditions (Fig. 6a, 6b). In addition, BnCIPK6 and BnCIPK6M transgenic seedlings have more and longer lateral roots than those of the wild type (Fig. 6c; see Supplementary Fig. S5 at JXB online). The fresh weight of BnCIPK6 and BnCIPK6M transgenic plants was also larger than that of the wild type. Likewise, BnCBL1 transgenic seedlings displayed higher low-phosphate tolerance than that of the wild type at the concentrations tested (Fig. 6d, 6e, 6f; see Supplementary Fig. S6 at JXB online). Collectively, these results suggested that BnCBL1 and BnCIPK6 may be involved in plant response and tolerance to low-phosphate.
Fig. 6.
Over-expression of BnCIPK6, BnCIPK6M, and BnCBL1 in Arabidopsis enhances plant tolerance to phosphorous starvation. (a–c) Transgenic Arabidopsis plants over-expressing BnCIPK6M and BnCIPK6 were transferred and grown for 9 d on MS medium and low phosphate medium. (a) Six-day-old seedlings were transferred and grown on MS medium. (b) Six-day-old seedlings were transferred and grown on MS medium with 50 µM phosphate (low phosphate, LP). (c) Statistical analysis of the lateral root number. (d–f) Transgenic Arabidopsis plants over-expressing BnCBL1 transferred and grown for 9 d on MS medium and low-phosphate medium. (d) One-week-old seedlings were transferred and grown on MS medium as controls. (e) One-week-old seedlings were transferred and grown on low- phosphate medium (50 µM Pi). (f) Statistical analysis of plant fresh weight. Mean values and standard errors (bar) were shown from three independent experiments (n >50 seedlings per each line). Independent t-tests for equality of means demonstrated that there was significant difference (*P value <0.05) or very significant difference (**P value <0.01) between wild-type and transgenic plants. (This figure is available in colour at JXB online.)
Activation of BnCIPK6 confers Arabidopsis plants hypersensitive to abscisic acid (ABA)
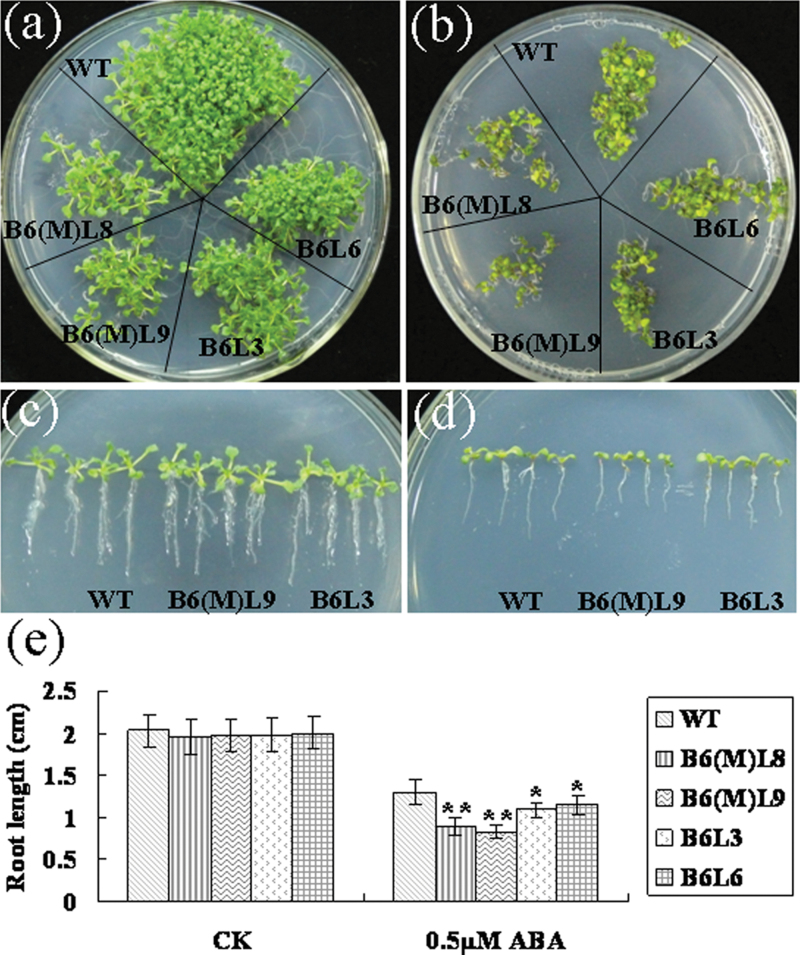
To determine whether BnCIPK6 protein is involved in the ABA signalling pathway, the transgenic lines were tested under exogenous ABA treatment. When germinated and grown on MS medium, BnCIPK6 and BnCIPK6M transgenic plants grew almost the same as the wild type (Fig. 7a, 7c). When cultured on MS medium with 0.25–1 µM ABA for several days, however, both seed germination and primary root growth of BnCIPK6M and BnCIPK6 transgenic plants were severely inhibited, compared with wild-type plants (Fig. 7b; see Supplementary Table S5 at JXB online). When seedlings were grown on MS medium containing 0.5 µM ABA, the root length of the BnCIPK6M transgenic lines was shorter than that of the wild type (Fig. 7d). Measurement and statistical analysis indicated that the root length of two BnCIPK6M transgenic lines was significantly less than 70% of the wild type, whereas the root length of the BnCIPK6 lines was over 80% of the wild type on MS medium with 0.5 µM ABA (Fig. 7e). These results demonstrated that both germination and post-germination growth of BnCIPK6M transgenic plants were more ABA-sensitive than those of BnCIPK6 transgenic plants, suggesting that activation of BnCIPK6 may be important for its participation in ABA signalling transduction.
Fig. 7.
Over-expression of BnCIPK6M in Arabidopsis results in plants that are hypersensitive to abscisic acid (ABA). (a) Seeds of wild-type and transgenic lines germinated and were grown on MS medium without ABA for 2 weeks. (b) Seeds of wild-type and transgenic lines germinated and were grown on MS medium with 0.5 µM ABA for 2 weeks. (c) Root length of wild-type and transgenic seedlings grown on MS medium for 2 weeks. (d) Root length of wild-type and transgenic seedlings grown on MS medium containing 0.5 µM ABA for 2 weeks. (e) Statistical analysis of the root length of transgenic Arabidopsis plants over-expressing BnCIPK6 and BnCIPK6M grown on MS medium without (CK) or with 0.5 µM ABA for 2 weeks. Mean values and standard errors (bar) were shown from three independent experiments (n >50 seedlings per each line). Independent t tests for equality of means demonstrated that there was significant difference (*P value <0.05) or very significant difference (**P value <0.01) between wild-type and transgenic plants. WT, wild type; B6(M)L8 and L9, BnCIPK6M transgenic line 8 and 9; B6L3 and L6, BnCIPK6 transgenic line 3 and 6. (This figure is available in colour at JXB online.)
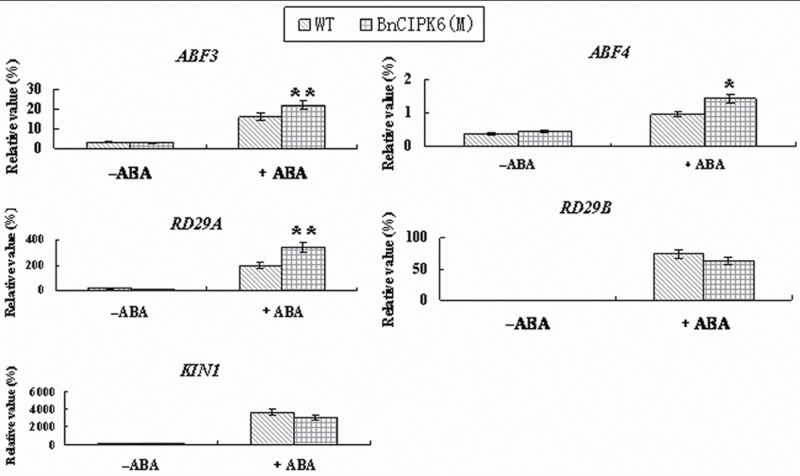
To investigate further the mechanism of the constitutively activated kinase, BnCIPK6M, involved in the ABA signalling pathway, it was examined whether expression of ABA-responsive genes (such as RD29A, RD29B, KIN1, ABF3, and ABF4) in BnCIPK6M transgenic lines, served as markers for monitoring ABA and stress response pathways. As shown in Fig. 8, expression levels of ABF3, ABF4, and RD29A in the transgenic plants were much higher than those in the wild type under ABA treatment although there was no significant difference in expression levels of those genes between the transgenic plants and the wild type in the absence of ABA. However, there was only slight or no significant decrease in mRNA levels of RD29B and KIN between wild-type and transgenic plants with or without ABA treatment. These results further suggest that the constitutively activated BnCIPK6, which displayed higher kinase activity, may be involved in the ABA signalling pathway, acting upstream of these marker genes. On the other hand, it was found that BnCBL1 over-expression transgenic plants did not show detectable phenotypic change under ABA treatments (data not shown) although BnCBL1 can interact with BnCIPK6, suggesting that other CBLs that interacted with BnCIPK6 protein may be involved in the response to ABA.
Fig. 8.
Quantitative RT-PCR analysis of expression of stress- and ABA-responsive genes in transgenic Arabidopsis plants over-expressing BnCIPK6M. Total RNA was isolated from 2-week-old seedlings without (–ABA) and with ABA treatment (+ABA) for 6h, respectively. Transcript levels of RD29A, RD29B, KIN1, ABF3, and ABF4 were determined by quantitative RT-PCR, using ACTIN2 as a quantification control. Independent t tests for equality of means demonstrated that there was (very) significant difference between wild-type and transgenic plants (*P value <0.05; **P value <0.01).
BnCIPK6 functionally complemented the defects of the atcipk6 mutant
The homozygous lines of the Arabidopsis cipk6 mutant (SM_3_39539) obtained from ABRC were identified by PCR using gene-specific and T-DNA-specific primers (3’ dSpm). To confirm that cipk6 is a transcript-null mutant, RT-PCR analysis was performed. As shown in Supplementary Fig. S7 at JXB online, no CIPK6 transcripts were detected in three lines of cipk6ko seedlings. Phenotypic analysis revealed that cipk6ko seedlings were more sensitive to salt and low phosphate stress, and confers the plant ABA-insensitive phenotype (our unpublished data).
To investigate whether BnCIPK6 performs a similar function as AtCIPK6, functional complementation analysis was performed. BnCIPK6, under the CaMV 35S promoter, was expressed in Arabidopsis cipk6 knockout mutants (cipk6/35S:BnCIPK6). RT-PCR analysis showed that strong BnCIPK6 expression was only detected in the complemented transgenic lines. Under normal growth conditions, cipk6/BnCIPK6 transgenic plants did not show noticeable phenotypes compared with wild-type and cipk6 mutant plants. By contrast, the cipk6/BnCIPK6 transgenic lines completely rescued the low-phosphate-sensitive and ABA-insensitive phenotypes of the cipk6 mutants under low phosphate stress and ABA treatment (see Supplementary Fig. S8 at JXB online), suggesting that both BnCIPK6 and AtCIPK6 proteins may perform similar function in plants.
Discussion
Previous studies revealed that the constitutively activated forms of CIPK activity might be generated through the substitution of one of the three conserved residues (serine, threonine, and tyrosine) to aspartate within the activation loop (Guo et al., 2001; Gong et al., 2002b ). Similarly, our results showed that substitution of Thr182 by Asp resulted in a higher autophosphorylation activity of BnCIPK6. It remains unclear so far whether these three residues are also the targets of phosphorylation in regulating the activity of CIPKs in vivo. However, the mechanism of phosphorylation-dependent activation of CIPKs suggests that CIPKs may be activated by other CIPK-phosphorylating kinases, such as CDPKs, MAPKs or other protein kinases, and are involved in the signalling cross-talk with other signalling pathways such as CDPKs and MAPKs (Kolukisaoglu et al., 2004). In our study, it was found that three protein kinases, including ITPK4, SNF1 kinase homologue 10, and phosphoribulokinase, were the interactors of BnCIPK6. These data may lay the foundation of explaining the activation mechanism of CIPKs in vivo in the future.
Yeast two-hybrid analysis revealed that BnCIPK6 is able to interact with Arabidopsis CBL1, CBL2, CBL3, and CBL9, indicating that the structure of CIPKs is conserved and CBL-CIPK binding specificity can cross the species barrier. To identify more interaction partners of BnCIPK6 in Brassica napus, BnCIPK6 protein was used as bait to screen the Brassica napus cDNA two-hybrid library, and 27 unique proteins were identified as positive clones, including two calcineurin B-like proteins, BnCBL1 and BnCBL3. A previous study indicated that four Arabidopsis CBL proteins, including CBL1, CBL4, CBL5, and CBL9, contain a myristoylation site at their N-terminus that plays an important role for protein-membrane attachment (Batistic and Kudla, 2004). Studies on protein localization displayed membrane targeting of AtCBL1 and AtCBL9 (Albrecht et al., 2003; Cheong et al., 2003; Pandey et al., 2004). In contrast to CBLs, CIPKs do not have any recognizable localization signal or the myristoylation site (Kolukisaoglu et al., 2004). Different localization of CIPKs may be dependent on their specific interaction partners, which could determine and regulate its localization (Batistic and Kudla, 2004). A previous localization analysis showed that AtCIPK1:GFP fusion proteins were observed at the plasma membrane, and to some extent also in the cytosol and nucleus. AtCIPK1 was recruited to the plasma membrane by interaction with AtCBL1 and AtCBL9, which localize to the plasma membrane (D’Angelo et al., 2006). A similar observation was also made in a study of AtCIPK23 subcellular localization (Xu et al., 2006; Cheong et al., 2007). In our study, BnCIPK6 was mainly localized at the plasma membrane and nucleus, whereas its interaction partner BnCBL1 was localized to the plasma membrane. These results suggest that the BnCBL1/BnCIPK6 complex may function in vivo by interacting with some membrane-localized proteins as their targets.
Progress has been made in understanding the salt-stress signalling pathway of Arabidopsis in recent years. The Arabidopsis SOS pathway includes three components, SOS1, SOS2, and SOS3, which collectively contribute to salt stress (Shi et al., 2000; Qiu et al., 2002). It was reported that SOS pathway also exists in rice and has a high degree of functional similarity to its Arabidopsis counterpart (Martínez-Atienza et al., 2007). Similarly, our data demonstrated that over-expression of BnCIPK6 and BnCIPK6M significantly enhanced plant tolerance to salt stress. Recently, a study indicated that CaCIPK6 is up-regulated by abiotic stresses (such as salinity and dehydration) and hormones (such as ABA and IAA). Over-expression of a constitutively activated mutant of CaCIPK6 promoted salt tolerance in transgenic tobacco, whereas the Arabidopsis cipk6 knockdown mutant was more sensitive to salt stress (Tripathi et al., 2009). These results together suggest that CIPK6 plays positive roles in conferring plant salt-tolerance. In this study, two calcineurin B-like proteins, BnCBL1 and BnCBL3, were identified as BnCIPK6 interacting proteins. Further study revealed that BnCBL1 was also involved in the plant response to salt stress. BnCBL1 transgenic seedlings displayed more salt-tolerance than that of the wild type. The data presented here implied that BnCBL1 and BnCIPK6 may function in the same salt signalling pathway.
Reverse genetics analyses have uncovered crucial functions of CBLs and CIPKs in the plant response to ABA. Previous studies reported that cipk3 and cbl9 loss-of-function mutants are hypersensitive to ABA (Kim et al., 2003; Pandey et al., 2004, 2008). Furthermore, the pks3 (cipk15) mutant shows ABA hypersensitive, revealing that PKS3/CIPK15 is a negative regulator of ABA signalling (Guo et al., 2002). Over-expression of CIPK20/PKS18 (T169D) rendered the transgenic plants hypersensitive to ABA, whereas RNAi plants showed insensitivity to ABA (Gong et al., 2002c ). Similarly, it was shown that both germination and post-germination growth of BnCIPK6M over-expression transgenic Arabidopsis were hypersensitive to ABA, whereas silencing of its homologous gene AtCIPK6 confers plant ABA-insensitive growth phenotypes. Furthermore, expression levels of ABF3, ABF4, and RD29A genes were much increased in the transgenic plants compared with the wild type after ABA treatment. Previous studies revealed that over-expression of ABF3 or ABF4 in Arabidopsis resulted in ABA hypersensitivity and other common ABA-associated phenotypes (Kang et al., 2002). The RD29A (responsive to desiccation) gene has been shown to be responsive to ABA, drought, cold, and salinity (Yamaguchi-Shinozaki and Shinozaki, 1994). Both CPK4 and CPK11 kinases phosphorylated two ABA-responsive transcription factors, ABF1 and ABF4, in vitro, suggesting that the two kinases may regulate ABA signalling through these transcription factors (Zhu et al., 2007). Based on our results, as well as those of published data, it is therefore speculated that BnCIPK6M kinase, a constitutively activated form of BnCIPK6, may regulate these transcription factors in the ABA signalling pathway.
Phosphate (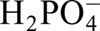 ) is an essential nutrient required for various basic biological functions in the plant life cycle (Raghothama, 1999), and is the major form that is most readily taken up and transported in plant cells (Tu et al., 1990). It was known that the phosphate concentration in soil, typically 10 µM or less, results in phosphorous starvation for plant growth and survival, which is one of the major limiting factors for crop production in cultivated soils (Chen et al., 2009). It is shown here that CIPK6 is involved in the response to phosphorous starvation. BnCIPK6 expression was strongly induced by low-phosphate stress in both roots and leaves of B. napus. The transgenic seedlings over-expressing BnCIPK6 and BnCIPK6M were obviously growing better than the wild type under low-phosphate conditions. BnCIPK6 and BnCIPK6M transgenic seedlings had more and longer lateral roots than that of wild-type plants under low-phosphate conditions. It should be mentioned that significant lateral roots differences were not observed between the wild type and cipk6 mutants in normal growth medium. This is inconsistent with a previous report that the lateral roots of cipk6 mutants are thinner and shorter than wild-type plants (Tripathi et al., 2009). Furthermore, over-expression of BnCBL1 in Arabidopsis enhances plant tolerance to low-phosphate stress. These results suggest that BnCBL1-BnCIPK6 may functionally interact with each other to be involved in response to low-phosphate stress. Further identification of CIPK6 substrates will be crucial toward a better understanding of its role in phosphorous starvation signalling.
) is an essential nutrient required for various basic biological functions in the plant life cycle (Raghothama, 1999), and is the major form that is most readily taken up and transported in plant cells (Tu et al., 1990). It was known that the phosphate concentration in soil, typically 10 µM or less, results in phosphorous starvation for plant growth and survival, which is one of the major limiting factors for crop production in cultivated soils (Chen et al., 2009). It is shown here that CIPK6 is involved in the response to phosphorous starvation. BnCIPK6 expression was strongly induced by low-phosphate stress in both roots and leaves of B. napus. The transgenic seedlings over-expressing BnCIPK6 and BnCIPK6M were obviously growing better than the wild type under low-phosphate conditions. BnCIPK6 and BnCIPK6M transgenic seedlings had more and longer lateral roots than that of wild-type plants under low-phosphate conditions. It should be mentioned that significant lateral roots differences were not observed between the wild type and cipk6 mutants in normal growth medium. This is inconsistent with a previous report that the lateral roots of cipk6 mutants are thinner and shorter than wild-type plants (Tripathi et al., 2009). Furthermore, over-expression of BnCBL1 in Arabidopsis enhances plant tolerance to low-phosphate stress. These results suggest that BnCBL1-BnCIPK6 may functionally interact with each other to be involved in response to low-phosphate stress. Further identification of CIPK6 substrates will be crucial toward a better understanding of its role in phosphorous starvation signalling.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Table S1. Primer sequences used in this study.
Supplementary Table S2. cDNA clones identified from the BnCIPK6 yeast two-hybrid screen.
Supplementary Table S3. Statistical analysis of relative green leaves and relative fresh weight of BnCIPK6 and BnCIPK6M transgenic Arabidopsis under a range of NaCl concentrations.
Supplementary Table S4. Statistical analysis of relative green leaves and relative fresh weight of BnCBL1 transgenic Arabidopsis under a range of NaCl concentrations.
Supplementary Table S5. Statistical analysis of the primary root length of BnCIPK6 and BnCIPK6M transgenic Arabidopsis under a range of ABA concentrations.
Supplementary Fig. S1. A schematic diagram of the domain structure of BnCIPK6.
Supplementary Fig. S2. BiFC assays of BnCIPK6 interaction with BnCBL1 in onion cells.
Supplementary Fig. S3. Yeast two-hybrid analysis for interactions between BnCIPK6 and ten AtCBL proteins.
Supplementary Fig. S4. Quantitative RT-PCR analysis of BnCBL1 expression in transgenic Arabidopsis.
Supplementary Fig. S5. Assay of lateral roots elongation of BnCIPK6 and BnCIPK6M transgenic plants growing on low phosphate medium.
Supplementary Fig. S6. Statistical analysis of plant dry weight of BnCBL1 transgenic plants under phosphorous starvation.
Supplementary Fig. S7. Identification of cipk6 loss-of-function mutants.
Supplementary Fig. S8. Characterization of Arabidopsis cipk6 knockout mutant expressing BnCIPK6.
Acknowledgements
This work was supported by the project from the Ministry of Agriculture of China for transgenic research (Grant No. 2011ZX08009-003), and the Chenguang Project of Wuhan Municipality (Grant No. 200950431185).
References
- Albrecht V, Ritz O, Linder S, Harter K, Kudla J. 2001. The NAF domain defines a novel protein–protein interaction module conserved in Ca2+-regulated kinases The EMBO Journal 20 1051–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht V, Weinl S, Blazevic D, D’Angelo C, Batistic O, Kolukisaoglu U, Bock R, Schulz B, Harter K, Kudla J. 2003. The calcium sensor CBL1 integrates plant responses to abiotic stresses The Plant Journal 36 457–470 [DOI] [PubMed] [Google Scholar]
- Batistic O, Kudla J. 2004. Integration and channeling of calcium signaling through the CBL calcium sensor/CIPK protein kinase network Planta 219 915–924 [DOI] [PubMed] [Google Scholar]
- Chen L, Ren F, Zhong H, Jiang WM, Li XB. 2010. Identification and expression analysis of genes induced by high-salinity and drought stresses in Brassica napus Acta Biochimica et Biophysica Sinica 42 154–164 [DOI] [PubMed] [Google Scholar]
- Chen YF, Li LQ, Xu Q, Kong YH, Wang H, Wu WH. 2009. The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis The Plant Cell 21 3554–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong YH, Kim KN, Pandey GK, Gupta R, Grant J, Luan S. 2003. CBL1, a calcium sensor that differentially regulates salt, drought, and cold responses in Arabidopsis The Plant Cell 15 1833–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong YH, Pandey GK, Grant J, Batistic O, Li L, Lee SC, Kim BG, Kudla J, Luan S. 2007. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis The Plant Journal 52 223–239 [DOI] [PubMed] [Google Scholar]
- D’Angelo C, Weinl S, Batistic O, et al. 2006. Alternative complex formation of the Ca2+-regulated protein kinase CIPK1 controls abscisic acid dependent and independent stress responses in Arabidopsis The Plant Journal 48 857–872 [DOI] [PubMed] [Google Scholar]
- Gietz D, StJean A, Woods RA, Schiestl RH. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Research. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Gong Z, Guo Y, Zhu JK. 2002. b Expression, activation, and biochemical properties of a novel Arabidopsis protein kinase Plant Physiology 129 225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Guo Y, Jagendorf AT, Zhu JK. 2002. a Biochemical characterization of the Arabidopsis protein kinase SOS2 that functions in salt tolerance Plant Physiology 130 256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Guo Y, Schumaker K, Zhu JK. 2004. The SOS3 family of calcium sensors and SOS2 family of protein kinases in Arabidopsis Plant Physiology 134 919–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Zhang C, Chen X, Gong Z, Zhu JK. 2002. c Constitutive activation and transgenic evaluation of the function of an Arabidopsis PKS protein kinase Journal of Biological Chemistry 277 42088–42096 [DOI] [PubMed] [Google Scholar]
- Gong SY, Huang GQ, Sun X, Li P, Zhao LL, Zhang DJ, Li XB. 2012. GhAGP31, a cotton non-classical arabinogalactan protein, is involved in response to cold stress during early seedling developmentple-style-spanPlant Biology 14,447ple-style-span–457 [DOI] [PubMed] [Google Scholar]
- Guo Y, Halfter U, Ishitani M, Zhu JK. 2001. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance The Plant Cell 13 1383–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Xiong L, Song CP, Gong D, Halfter U, Zhu JK. 2002. A calcium sensor and its interacting protein kinase are globar regulators of abscisic acid signaling in Arabidopsis Developmental Cell 3 233–244 [DOI] [PubMed] [Google Scholar]
- Ho CH, Lin SH, Hu HC, Tsay YF. 2009. CHL1 functions as a nitrate sensor in plants Cell 138 1184–1194 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants The EMBO Journal 6 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JY, Choi HI, Im MY, Kim SY. 2002. Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling The Plant Cell 14 343–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KN, Cheong Y, Pandey G, Grant J, Luan S. 2003. CIPK3, a calcium sensor-associated protein kinase that regulates abscisic acid and cold signal transduction in Arabidopsis The Plant Cell 15 411–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolukisaoglu U, Weinl S, Blazevic D, Batistic O, Kudla J. 2004. Calcium sensors and their interacting protein kinases: genomics of the Arabidopsis and rice CBL-CIPK signaling networks Plant Physiology 134 43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan WZ, Kim BG, Li L, Cheong YH, Pandey GK, Lu G, Buchanan BB, Luan S. 2007. A protein phosphorylation/dephosphorylation network regulates a plant potassium channel Proceedings of the National Academy of Sciences, USA 104 15959–15964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Kim BG, Cheong YH, Pandey GK, Luan S. 2006. A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis Proceedings of the National Academy of Sciences USDA 103 12625–12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XB, Cai L, Cheng NH, Liu JW. 2002. Molecular characterization of the cotton GhTUB1 gene that is preferentially expressed in fiber Plant Physiology 130 666–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XB, Fan XP, Wang XL, Cai L, Yang WC. 2005. The cotton ACTIN1 gene is functionally expressed in fibers and participates in fiber elongation The Plant Cell 17 859–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S, Kudla J, Rodriguez-Concepcionc M, Yalovsky S, Gruissem W. 2002. Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants The Plant Cell 54 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Atienza J, Jiang X, Garciadeblas B, Mendoza I, Zhu JK, Pardo JM, Quintero FJ. 2007. Conservation of the Salt Overly Sensitive pathway in rice Plant Physiology 143 1001–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Guo Y, Halfter U, Zhu JK. 2003. A novel domain in the protein kinase SOS2 mediates interaction with the protein phosphatase 2C ABI2 Proceedings of the National Academy of Sciences, USA 100 11771–11776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey G, Cheong YH, Kim KN, Kudla J, Luan S. 2004. The calcium sensor calcineurin B-like 9 modulates abscisic acid sensitivity and biosynthesis in Arabidopsis The Plant Cell 16 1912–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey G, Grant J, Cheong YH, Kim BG, Li L, Luan S. 2008. Calcineurin B-like protein CBL9 interactes with target kinase CIPK3 in the regulation of ABA response in seed germination Molecular Plant 2 238–248 [DOI] [PubMed] [Google Scholar]
- Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK. 2002. Regulation of SOS1, a plasma membrane Nat/Ht exchanger in Arabidopsis thaliana, by SOS2 and SOS3 Proceedings of the National Academy of Sciences, USA 99 8436–8441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan R, Lin H, Mendoza I, Zhang Y, Cao W, Yang Y, Shang M, Chen S, Pardo JM, Guo Y. 2007. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress The Plant Cell 19 1415–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghothama KG. 1999. Phosphate acquisition Annual Review of Plant Physiology and Plant Molecular Biology 50 665–693 [DOI] [PubMed] [Google Scholar]
- Rodriguez P. 1998. Protein phosphatase 2C (PP2C) function in higher plants Plant Molecular Biology 38 919–927 [DOI] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper J. 2002. Calcium at the crossroads of signaling The Plant Cell 14 401–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Ishitani M, Kim CS, Zhu JK. 2000. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter Proceedings of the National Academy of Sciences, USA 97 6896–6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi V, Parasuraman B, Laxmi A, Chattopadhyay D. 2009. CIPK6, a CBL-interacting protein kinase is required for development and salt tolerance in plant The Plant Journal 58 778–790 [DOI] [PubMed] [Google Scholar]
- Tu SI, Cavanaugh JR, Boswell RT. 1990. Phosphate uptake by excised maize root tips studied by in vivo P nuclear magnetic resonance spectroscopy Plant Physiology 93 778–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH. 2006. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis Cell 125 1347–1360 [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. 1994. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress The Plant Cell 6 251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZT, Zhou Y, Li Y, Shao SQ, Li BY, Shi HY, Li XB. 2010. Interactome analysis of the six cotton 14-3-3s that are preferentially expressed in fibres and involved in cell elongation Journal of Experimental Botany 61 3331–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK, Liu J, Xiong L. 1998. Genetic analysis of salt tolerance in Arabidopsis: evidence for a critical role of potassium nutrition The Plant Cell 10 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SY, Yu XC, Wang XJ, et al. 2007. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis The Plant Cell 19 3019–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.