Abstract
Plants are frequently under attack by multiple herbivores and can be infested at their shoots as well as their roots. As a consequence, plant metabolites are readily induced, mediated by phytohormones such as salicylic acid and jasmonic acid. Thereby, cross-talk between signal transduction pathways may occur if different herbivores attack the plant simultaneously. In turn, modifications in the plant metabolic pattern can affect herbivores infesting local and systemic tissue. Here, an integrative approach combining metabolomics and performance experiments was used to study the induction of plant metabolites in Arabidopsis thaliana by the specialist aphid Brevicoryne brassicae feeding on shoots and the generalist nematode Heterodera schachtii infesting root tissue. In contrast to most other studies, low infestation rates typical for the decisive early stages of infestation were used. Moreover, the consequences of induction responses on plant-mediated indirect interactions between these herbivores were investigated. In aphid-treated plants, several metabolites including glucosinolates, important defence compounds of Brassicaceae, were reduced in the shoot, but only minute changes took part in the systemic root tissue. Primary metabolites as well as phytohormones were not altered 3 days post infestation. In contrast, nematodes did not evoke significant metabolic alterations locally or systemically. In accordance, nematode presence did not affect aphid population growth, whereas aphids mediated a considerably reduced nematode infestation. These results demonstrate that plants respond in a very fine-tuned way to different challenges. Although they show only minute systemic responses to low herbivore stress, these changes can have pronounced effects on plant-mediated interactions between herbivores.
Key words: Aphids, glucosinolates, induction, metabolomics, nematodes, phytohormones
Introduction
Plants are frequently confronted with attacks by herbivores that can infest their shoots and roots simultaneously. The first plant reactions upon cell disruption by herbivores are changes in plasma membrane potential, calcium signalling, and production of reactive oxygen species, followed by phytohormone release and gene activation leading to changes in metabolite composition (Maffei et al., 2007). These responses to different herbivores are highly species specific and are regulated by a fine-tuned interplay between the central phytohormones jasmonic acid (JA), salicylic acid (SA), ethylene, and others (Pieterse et al., 2009), which occur in free and conjugated forms (Ogawa et al., 2010). JA is considered to be involved mainly in signalling responses to chewing herbivores, cell content feeders, and necrotrophic pathogens, whereas the SA pathway is induced in particular by biotrophic pathogens and herbivores with piercing-sucking mouthparts, such as aphids (Bostock, 2005; Glazebrook, 2005). For example, a series of SA-associated genes was activated in Arabidopsis thaliana (L.) Heynh. in response to infestation by the aphid Brevicoryne brassicae (L.) (Hemiptera: Aphididae) at early interaction time points, but JA was likewise found to be involved in defence responses to aphids (Mewis et al., 2005; Kuśnierczyk et al., 2007, 2008, 2011; Morkunas et al., 2011). In contrast to the rather well-studied plant signalling reactions to aboveground herbivores, far less is known about plant responses to belowground feeding organisms such as nematodes (Gutjahr and Paszkowski, 2009). Comparisons between resistant and susceptible plant cultivars indicated that the resistance against the root-knot nematode Meloidogyne javanica (Treub) and the cyst nematode Heterodera schachtii (Schmidt) (both Tylenchida: Heteroderidae), which feed on special feeding cells of the vascular system, depends at least partly on SA signalling (Branch et al., 2004; Wubben et al., 2008). Simultaneous infestation by different herbivores may further lead to a cross-talk between signalling pathways (Pieterse et al., 2009).
Initiation of the signalling cascades can trigger changes in primary and secondary metabolites not only locally but also in systemic tissue (Baldwin et al., 1994). Thus, spatially segregated above- and belowground feeding herbivores on a shared host plant can become indirectly connected and influence each other mediated by the induced systemic changes in plant quality (Bezemer and van Dam, 2005; Gonzáles-Megías and Müller, 2010). The effects of aboveground on belowground herbivores and vice versa have achieved increasing attention during recent years, whereas the former perspective was in focus more often (reviewed by Erb et al., 2008). Within such interactions, herbivores feeding at one compartment can have beneficial, negative, or neutral effects on the counterparts depending on many parameters such as the system under examination, experimental conditions, and the time sequence of infestation (van Dam et al., 2003; Soler et al., 2007; Kaplan et al., 2008; Vandegehuchte et al., 2010; Erb et al., 2011). Thereby, indirect interguild interactions remain surprisingly little studied (Soler et al., 2012).
Glucosinolates (GS) are a group of secondary metabolites that has been well studied with regard to induction by herbivores and plant-mediated interactions (van Dam et al., 2005; Gonzáles-Megías and Müller, 2010). GS are nitrogen- and sulphur-containing compounds, which are important feeding stimulants for specialist herbivores, whereas they act as a deterrent on generalists (Renwick, 2002; Halkier and Gershenzon, 2006). When plant tissue is injured, the GS are hydrolysed by separately stored thioglucosidases, the myrosinases, mainly to (iso-)thiocyanates and nitriles or other compounds, depending on various conditions (Bones and Rossiter, 1996, 2006). Especially the isothiocyanates act toxic to many organisms including nematodes (Zasada and Ferris, 2003; Halkier and Gershenzon 2006). GS are constitutively present in plant tissues of the order Capparales in different qualitative and quantitative composition (Brown et al., 2003) but their abundance can change following herbivore feeding in very species-specific ways and depending on the feeding pattern (Textor and Gershenzon, 2009). Among other herbivore taxa, several aphid species cause alterations in GS quantities in the shoot tissue of A. thaliana leading to reductions or increases of certain GS, depending on the infesting aphid species, the number and the developmental stage of aphids used for primary infestation (Mewis et al., 2005, 2006; Kim and Jander, 2007; Kuśnierczyk et al., 2008; Bidart-Bouzat and Kliebenstein, 2011). Previous studies were either designed to investigate progressed aphid–plant interactions and/or used considerable high infestation rates, whereas studies concentrating on early GS responses to herbivore attack rates are rare. Furthermore, the interaction of a plant with its antagonists starts with minimal infestation rates, which are thus more realistic. Moreover, the outcome of plant-mediated interactions between above- and belowground herbivores may be particularly relevant in stages when both species start to establish on the shared host plant, as early population growth should be highly dependent on plant quality.
A plethora of studies on the induction of plant responses to herbivory has focused on target metabolites such as GS (van Dam et al., 2005; Kim and Jander, 2007; Soler et al., 2007, 2012). Thereby many further metabolic plant responses may be missed (Macel et al., 2010; Sutter and Müller, 2011). Metabolomics approaches are thus increasingly used as important analytical tools to investigate treatment-dependent responses of organisms (Viant, 2008). Moreover, compared to the often-studied transcriptome or proteome, the metabolome is closer to the phenotype (Dettmer et al., 2007). Within metabolomics, metabolic fingerprinting aims at screening all metabolites in an untargeted, semi-quantitative approach. In contrast, metabolite profiling is used to analyse a smaller set of known metabolites with similar chemical properties (Hall, 2006). Both methods are useful complementary approaches to investigate the specificity of plant responses to challenges.
The aims of the present mechanistic study were to analyse the plant responses to singular and simultaneous herbivory by the aboveground specialist aphid B. brassicae and the belowground generalist H. schachtii at an early stage of infestation and to investigate the consequences on plant-mediated interactions between these herbivores in the model plant A. thaliana, which can serve as potential host for both herbivore species. To overcome the limitations of individual analytical platforms and to gain a comprehensive insight into plant metabolic changes caused by single and simultaneously applied biotic stress, metabolite profiling of primary metabolites and analyses of specific targets, namely the phytohormones JA, SA, and salicylic acid glucoside (SAG), and the GS as important target defence compounds of A. thaliana, were combined with metabolic fingerprinting.
Materials and methods
Plant growth and herbivore rearing
Two seeds of A. thaliana ecotype Columbia-0 were sown per pot (6cm diameter) in a sterilised mixture (1:1) of fine silica sand (particle size 0.1–0.5mm, Quarzwerke, Frechen, Germany) and washed coarse sand (particle size < 1mm). One week after germination, the weaker seedling per pot was removed. Plants were grown in a growth chamber (AR-41L2, Percival Scientific, Perry, USA) under a 8/16 light/dark cycle with 60% relative humidity and a light intensity of approximately 110 µE•m–2•s–1. Pots were watered three times per week as needed and fertilised with a slightly modified Hoagland solution, containing 3.5 mmol l–1 nitrate (Hoagland and Arnon, 1950). Aphids of the specialist B. brassicae were taken from a culture that originated from a single female and were reared on non-flowering plants of Brassica oleracea convar. capitata var. sabauda L. Vertus 2 (Kiepenkerl, Bruno Nebelung, Everswinkel, Germany). Four generations before the start of the experiment, aphids were transferred to A. thaliana plants for acclimatisation. Aphids were kept at room temperature under a 16/8 light/dark cycle.
Cysts of the generalist nematode H. schachtii were obtained from a loess substrate (kindly provided by Dr. Björn Niere from Julius Kühn Institute, Münster, Germany) using a 200 µm sieve and incubated with 3mM ZnCl2 using the Baermann funnel technique (Baermann, 1917). For root inoculation, freshly hatched second-instar nematodes were counted under a light microscope and their concentration adjusted to a density of 250 larvae ml–1.
Plant treatments
After 8 weeks of growth, plants of comparable habitus were divided in four groups. One group of plants was left untreated as control (C), the second group was infested with aphids (A), the roots of the third plant group were inoculated with nematodes (N), and plants of the fourth group were treated with both aphids and nematodes (AN). For the aphid treatments, 10 adult apterous aphids were distributed over the rosette of each A and AN plant using a moistened fine brush. C and N plants were slightly streaked with a clean brush likewise to expose them to the same mechanical treatment. Four holes (diameter ca. 0.5cm) with a distance of 1cm from the plant centre were pierced into the sand of each pot and 1ml of the nematode suspension was filled into each hole around the N and AN plants (resulting in 1000 nematodes per pot), whereas 1ml of water was added to each whole around the C and A plants, respectively. Plants of the four treatment groups were treated in alternate order. Both, deposition of herbivores at the beginning and harvest of plant tissue at the end of the experiment were started around noon.
For chemical analyses, 10 replicates per group were established. For the assessment of nematode infestation rates, eight additional plants had to be prepared of the N and AN treatments, respectively, as the nematode counting is an invasive technique (see below). The experimental set-up was repeated in total three times independently (primary metabolites were only measured in two experiments and phytohormones once).
Testing reciprocal plant-mediated effects between the herbivores and harvest
Three days after infestation (dpi) with aphids and nematodes, plants were harvested in the same sequence as during experiment preparation. To investigate whether the aphids and nematodes influenced each other indirectly via the host plant, the numbers of herbivores were assessed. Juvenile and adult aphids were counted separately. Roots of additional N and AN plants prepared for nematode counting were cleaned from the growth substrate, fixed in 4% formaldehyde (AppliChem, Darmstadt, Germany) and stained with acid fuchsin as described by Byrd et al. (1983) but without bleaching. Nematodes were counted in root segments under a light microscope (×50, Zeiss Axiolab, Göttingen, Germany).
To measure the biomass and for subsequent chemical analyses, plants of all treatments were harvested. Aphids were carefully removed from the leaves with water and a brush. Aphid-free plants (C and N) encountered the same washing procedure. Afterwards, plants were carefully removed from the pots and sand was washed from the roots under a moderate water flow. Plants were shortly and gently dried between paper tissues and total shoot and root biomasses were determined. Subsamples of approximately 100mg per shoot were deposited in 2ml Eppendorf tubes (for metabolic fingerprinting and GS analyses) and the remaining shoot tissue was placed in 15ml Falcon tubes (for metabolite profiling and phytohormone analyses). As roots were rather small (111±21mg, mean ± SD), root samples could only be taken for metabolic fingerprinting and GS analyses. All samples were immediately frozen in liquid nitrogen and stored at –80 °C until analyses.
Phytohormone analyses of shoot samples
The phytohormones JA and free SA as well as the conjugated form SAG were analysed according to the protocol by Forcat et al. (2008) with some modifications. In short, maximally 14mg freeze-dried powdered leaf material was used for a 2-fold extraction with 10% methanol containing 1% acetic acid in water. For each extraction, samples were vortexed in solvent for 2min, incubated on ice for 30min, and centrifuged. The combined supernatants of each sample were filtered through 0.2 µm PTFE membrane syringe filters (4mm, Phenomenex, Torrance, USA). Subsequently, samples were analysed by ultra-high performance liquid chromatography (UPLC) equipped with a Kinetex column (50×2.1mm, 1,7 µm, C18, Phenomenex) with a crude catcher coupled with a time-of-flight (TOF) mass spectrometer (1290 Infinity UPLC and 6210 TOF-MS, Agilent Technologies, Santa Clara, USA). Samples were separated at a 6min gradient from 95% ultrapure water (TKA, Thermo Electron LED, Niederelbert, Germany) to 95% acetonitrile (LC-MS grade, Fisher Scientific UK, Loughborough, UK) both containing 0.1% formic acid (~98%, Fluka, Seelze, Germany), followed by a cleaning and column equilibration step. The column temperature was 35 °C and a flow rate of 0.4ml min–1 was used. The MS was equipped with a dual ESI source and samples were measured in negative ionization mode. The source parameters were set to drying gas flow 11ml min–1, gas temperature 350 °C, and nebulizer pressure 276 kPa. The MS parameters were adjusted to fragmentor voltage –130V, capillary voltage 3500V, and skimmer voltage 60V. JA and SA were quantified by external calibration curves and SAG was quantified in relation to the SA calibration curve.
Metabolite profiling of shoot samples
For analysis of primary metabolites, about 4mg freeze-dried plant material were homogenised with a ball mill (Retsch MM 301, Retsch, Haan, Germany) for 30 s at 25 Hz and extracted in 1ml 80% methanol (LC-MS grade, Fisher Scientific UK) with ribitol (99%, Sigma-Aldrich Chemie, Steinheim, Germany) as internal standard. Aliquots of the supernatants were dried and chemically derivatised by sequential methoxylamination and trimethylsilylation at 37 °C for 90 and 30min, respectively. After centrifugation, supernatants were analysed by GC coupled with MS (Focus GC-DSQ II, Thermo Electron, Rodano, Italy) on a VF-5 MS column (30 m × 0.25mm i.d., 10 m guard column, Varian, Palo Alto, CA, USA) in electron impact positive ionization mode operating at 70eV. Injection occurred in a 250 °C hot liner with 1:20 split, the helium carrier gas flow was set to 1ml min–1 and the transfer line had a temperature of 250 °C. The GC oven temperature of 80 °C was held for 3min and a temperature ramp of 5 °C min–1 up to 350 °C was used. Blank samples containing no biological material were measured at the beginning and end of each sequence and n-alkanes (C8–C40, Sigma-Aldrich-Fluka, Steinheim, Germany) were analysed under the same conditions to determine Kovats indices (Kovats, 1958). Primary metabolites were identified by comparison of mass spectra and retention times to those of standards, to the online Golm database (http://gmd.mpimp-golm.mpg.de/analysisinput.aspx; Kopka et al., 2005; Hummel et al., 2010), and to Kovats indices. All reference substances were purchased from Sigma-Aldrich-Fluka, Merck (Darmstadt, Germany), or Roth (Karlsruhe, Germany), respectively. Metabolites were quantified in relation to the peak area of the internal standard.
Metabolic fingerprinting and glucosinolate analyses of shoot and root samples
For metabolic fingerprinting and GS analyses, frozen samples were homogenised with a pre-cooled ball mill at 25 Hz for 30 s, and extracted 3-fold in 0.6ml of 90% methanol at 4 °C with 1 µg ml–1 hydrocortisone (>98%, Sigma-Aldrich Chemie) as internal standard on ice. Supernatants were filtered through 0.2 µm PTFE membrane syringe filters (15mm; Phenomenex). Samples were kept at 4 °C and analysed with UPLC-ESI-TOF-MS equipped with a Grom-Sil 120 ODS-4-HE-column (150×2mm, 3 µm, Alltech Grom, Rottenburg-Hailfingen, Germany) and an equivalent guard column (10×2mm, 3 µm). Samples were separated at a 19min gradient from 98% ultrapure water to 98% acetonitrile both containing 0.1% formic acid, with a flow of 0.8ml min–1 followed by a cleaning and column equilibration step. Measurements were done in positive and negative mode from 100 to 1000 m/z with the instrument parameters as described above despite a fragmentor voltage of ± 140V, a nebulizer pressure of 379 kPa, and a gas flow of 12ml min–1. Blank samples containing no biological material were measured at the beginning, in between and at the end of the measurements, and a reference sample from a bulk of A. thaliana plants was run at the beginning and at the end to check for instrumental reproducibility.
Based on these LC-MS data, 19 GS could be extracted from negative ionization data. GS were identified by their exact masses with a maximal mass error of 2 ppm, by their UV spectra and by comparison to standards, where available. They were quantified based on the relation of their peak areas to the peak area of the internal standard hydrocortisone, incorporating response factors that had been calculated by co-injection of the hydrocortisone and 2-propenyl GS, p-hydroxybenzyl GS, and indol-3-ylmethyl GS (Phytoplan, Heidelberg, Germany and Glucosinolates.com, Copenhagen, Denmark) as representatives for aliphatic, aromatic, and indole GS, respectively, in various concentrations.
Data analyses and statistics
Peak picking and data alignment of GC-MS data was done in XCalibur software (version 2.0.5, Thermo Electron) using the specific mass fragments of the compounds. These data were normalised to the sample dry mass and the internal standard intensity.
LC-MS chromatograms in centroid mode were exported in mzdata.xml format from Agilent MassHunter Qualitative Analysis software (version B.01.03, Agilent Technologies). Isotopes were eliminated during the procedure to reduce redundant data originating from one chemical substance. Peak picking, grouping of associated peaks, and retention time alignment was performed using the xcms software package (version 2.14.1, Smith et al., 2006; Tautenhahn et al., 2008) in the statistical software R (version 2.12.2), R Development Core Team 2010. The parameter settings were peak finding: method = ‘centWave’, ppm = 25, peakwidth = c(5,20), snthresh = 10, prefilter = c(3,500), fitgauss=T; peak grouping: bw = 10/5, minfrac = 0.7, mzwid = 0.025; retention time correction algorithm: ‘obiwarp’. The resulting data matrices of positive and negative ionization mode measurements were pooled for further analyses. Data were normalised to sample freshweight and related to the intensity of the internal standard. For noise subtraction and removal of signals originating from the solvents, only those features were used for further analyses, whose mean intensities over all samples were ≥100 times higher than the mean blank intensity or that occurred in biological samples only (blank feature subtraction). Note that the number of features (peaks) is higher than the number of metabolites, as fragments and adducts may occur.
Statistical analyses were performed with the software R except otherwise stated. Physiological plant parameters (root and shoot biomasses and root/shoot ratio) as well as primary metabolites and phytohormone concentrations of plants of different treatment groups were compared with Kruskal–Wallis tests followed by multiple comparison tests using the package nparcomp (type = Tukey), as data were not normally distributed. Data were corrected for multiple testing by Bonferroni, where applicable. The numbers of herbivores of one guild on plants infested either with a single or two herbivore species were compared with a Mann–Whitney U test (aphids) and a t-test (nematodes), as the latter data were normally distributed.
GS concentrations as well as the ratio of aliphatic to indole GS were analysed for correlations with physiological parameters using Spearman’s correlation tests. Those parameters that were correlated with GS data were included in further analyses as covariates. The differences of total, aliphatic, and indole GS concentrations as well as the ratio of aliphatic to indole GS in shoots and roots between the control and the three different treatments were tested using generalized linear models (GLM), with treatment as fixed effect and shoot/root ratio or root biomass as covariables, as significant correlations were found. The analyses were initiated with saturated models and were simplified following the Akaike information criterion leading to the minimal adequate models. GLM were fitted with Gaussian distribution and tested for normal distribution of the residuals (using QQ plots and Shapiro–Wilk tests).
LC-MS metabolic fingerprinting data consist of hundreds of features and thus are very complex. To reduce this complexity, multivariate analysis techniques were used both unsupervised with principle component analyses (PCA) and in a supervised way using group (treatment) membership as the categorical Y-variable in orthogonal partial least-squares discriminant analyses (OPLS-DA). Data were centred and scaled to unit variance prior to analyses. PCA of metabolic fingerprints of shoots and roots of the four treatment groups were used to get an overview over the general pattern in the datasets. The resulting scores of the samples (PC1, PC2, PC3, and additionally PC1–PC3 for shoot data, as in Taylor et al., 2009), were tested for significant differences with Kruskal–Wallis tests followed by multiple comparison tests as described above. To gain a deeper insight into the data structure, OPLS-DA were performed with SIMCA software (version 12.01, Umetrics, Umeå, Sweden) for each pair of treatments. The optimal number of latent variables for each model was evaluated using cross validation with the autofit routine. The quality of the resulting models was evaluated with the routine 1/7th out cross validation based on the residuals with R2X(cum) (cumulative modelled variation in X), R2Y(cum) (cumulative modelled variation in Y) and Q2cum (model predictive ability parameter according to cross validation). The models were tested for overfitting with ANOVA based on the cross-validated predictive residuals (Eriksson et al., 2008). Loadings and variable importance plots (VIPs) were used to delineate the metabolites that were responsible for class separation between the two plant groups of each analysis. Only metabolites with a VIP value of 1 or higher were rated as important (Eriksson et al., 2006).
Results
Physiological plant parameters
Feeding by the herbivores (aphids, nematodes, and combined treatment) had no effect on the biomass of the herbivore-treated A. thaliana plants at 3 dpi. The shoot and root biomasses of the three treated plant groups as well as the root/shoot ratios were similar as the values of the control plant group at this time point (Table 1).
Table 1.
Shoot and root fresh biomass, root/shoot ratio, and shoot phytohormone concentrations in control, aphid-, nematode-, and aphid + nematode-treated plants at 3 days after infestation
| Parameter | Treatment | χ2 | P | |||
|---|---|---|---|---|---|---|
| Control | Aphid | Nematode | Aphid + nematode | |||
| Shoot biomass (mg freshweight) | 373.1±72.9 | 383.9±40.7 | 361.9±49.6 | 365.6±53.0 | 0.91 | 0.82 |
| Root biomass (mg freshweight) | 118.2±26.5 | 107.7±12.6 | 112.2±23.2 | 107.9±18.9 | 0.75 | 0.86 |
| Root/shoot ratio | 0.32±0.08 | 0.28±0.05 | 0.31±0.07 | 0.30±0.05 | 2.42 | 0.49 |
| JA (ng (mg dryweight)–1) | 0.55±0.16 | 0.73±0.23 | 0.61±0.30 | 0.65±0.19 | 4.58 | 0.21 |
| SA (ng (mg dryweight)–1) | 2.76±0.91 | 2.42±0.24 | 2.64±1.05 | 2.66±0.72 | 0.99 | 0.80 |
| SAG (ng (mg dryweight)–1) | 3.53±2.24 | 1.56±0.55 | 3.26±3.99 | 2.53±3.00 | 4.59 | 0.21 |
Values are means ± SD. Statistical analyses were done with Kruskal–Wallis tests (df = 3, χ2 and P values are shown). There were 10 replicates per treatment (data are shown from one representative experiment of three independent experiments for morphological data). JA = jasmonic acid; SA = salicylic acid; SAG = salicylic acid glucoside
Phytohormones
Feeding by aphids and nematodes separately and in combination did not result in significantly changed shoot JA, SA, or SAG concentrations (Table 1).
Primary metabolites
In total, 54 peaks were found by metabolite profiling via GC-MS analyses in shoots, whereof 33 compounds could be identified at least putatively (Supplementary Table S1, available at JXB online). The primary metabolism was rather stable, as it was not influenced by any type of herbivory. Neither severe changes in the abundances of individual metabolites nor in total abundances of groups of metabolites, such as amino acids and sugars, were found between the four plant treatments. Solely glyceric acid, ethanolamine, and an unidentified compound were significantly affected by plant treatment (Kruskal–Wallis test, df = 3, P < 0.05, but not significant after Bonferroni correction).
Glucosinolates
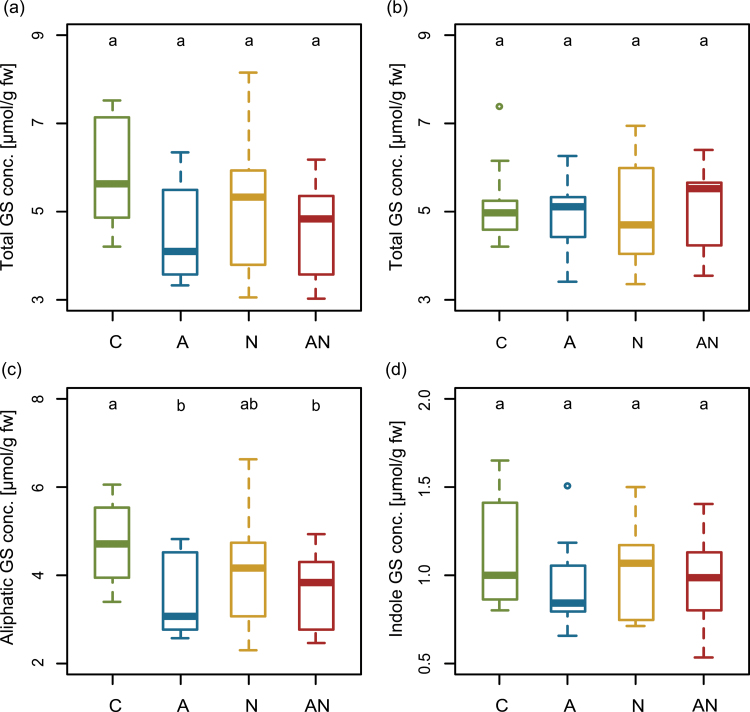
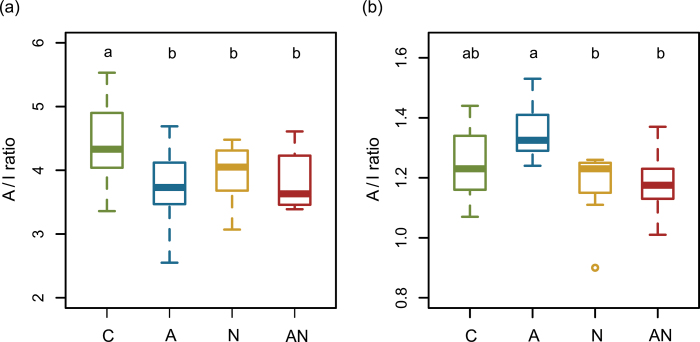
The total shoot GS concentration was not affected by the different treatments (GLM, Fig. 1a). However, aliphatic GS were significantly reduced by aphids and also by the combined herbivore treatment (GLM, A vs. C P = 0.022, AN vs. C P = 0.042; Fig. 1c). Nematode infestation had no effect on aliphatic GS (N vs. C P = 0.163; Fig. 1c). In contrast to aliphatic GS, total indole GS concentrations were not altered by any treatment (Fig. 1d). The ratio of aliphatic to indole GS was significantly reduced by all three treatments compared to the control plants (A vs. C P = 0.001, N vs. C P = 0.0499, AN vs. C P = 0.007; Fig. 2a).
Fig. 1.
Total glucosinolate (GS) concentrations (conc.) in (a) shoots and (b) roots and concentrations of (c) aliphatic and (d) indole GS in the shoots of control (C)-, aphid (A)-, nematode (N)-, and aphid + nematode (AN)- treated Arabidopsis thaliana plants at 3 days after infestation. The horizontal line in the boxes indicates the median, the boxes indicate the 25% and 75% percentiles, the whiskers extend to the lowest and highest value within the 1.5 × interquartile range, respectively, and values outside that range are depicted as dots. Different lowercase letters indicate significant differences at P < 0.05 (analysed with GLM, n = 10 per treatment; data are shown from one representative experiment of three independent experiments) (this figure is available in colour at JXB online).
Fig. 2.
Ratios of aliphatic and indole (A/I ratio) glucosinolates (GS) in (a) shoots and (b) roots of control (C)-, aphid (A)-, nematode (N)- and aphid + nematode (AN)- treated Arabidopsis thaliana plants 3 days after infestation. The horizontal line in the boxes indicates the median, the boxes indicate the 25% and 75% percentiles, the whiskers extend to the lowest and highest value within the 1.5 × interquartile range, respectively, and values outside that range are depicted as dots. Different lower case letters indicate significant differences at P < 0.05 (analysed with GLM, n = 10 per treatment; data are shown from one representative experiment of three independent experiments) (this figure is available in colour at JXB online).
In the roots, no significant differences were found for total GS nor for aliphatic or indole GS between plants of different herbivore treatments and the control (Fig. 1b). However, individual GS differed significantly between the treatments (Supplementary Table S2); three short-chain aliphatic GS (3-methylsulphinylpropyl GS, 4-methylsulphinylbutyl GS, and 5-methylsulphinylpentyl GS) were significantly reduced in A compared to C plants (P = 0.024, 0.017, and 0.007, respectively). In contrast, 6-methylsulphinylhexyl GS was significantly reduced by nematodes (P = 0.024). The ratio of aliphatic to indole GS in the roots was shifted in response to the different herbivore treatments; it was significantly higher in A compared to N (P = 0.001) and AN plants (P < 0.001), respectively, and marginal higher compared to C plants (P = 0.077; Fig. 2b).
Metabolic fingerprints
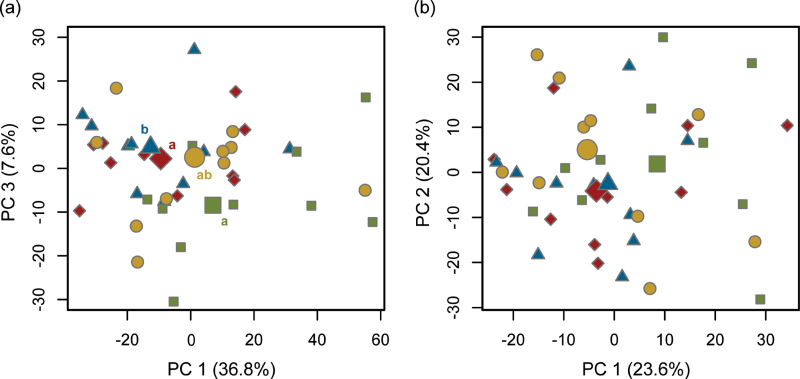
In the shoot material, 1673 features were found (after blank feature subtraction) over all plants by LC-TOF-MS measurements. The fingerprints of the different plant treatment groups could be separated along the PC1-PC3 axis (Kruskal–Wallis test, df = 3, χ2 = 10.31, P = 0.016; Fig. 3a). Thereby, scores of A plants differed mostly from C plants (Tukey post-hoc test, P = 0.039), whereas AN plants differed from C plants by tendency only (P = 0.066). Nematodes did not lead to systemic changes in the shoot metabolite pattern compared to C plants (P = 0.437). Principle component 2 (describing 10.9% of the variance) did not contribute to class separation (χ2 = 1.31, P = 0.727) indicating that it bore mostly non-induced biological variation as well as instrumental variation. For the roots, metabolic fingerprints (1061 features after blank feature subtraction) were not separated between treatments in a PCA (Kruskal–Wallis tests for scores of PC1 χ2 = 3.30, P = 0.347; for PC 2 χ2 = 1.70, P = 0.637; for PC 3 χ2 = 3.48, P = 0.324; Fig. 3b).
Fig. 3.
PCA scores plot of metabolic fingerprints of Arabidopsis thaliana from (a) shoots and (b) roots of control plants (green boxes) and plants under different herbivore impact (aphid, blue triangles; nematode, yellow circles; aphid + nematode, red diamonds; n = 10 per treatment; data are shown from one representative experiment of three independent experiments). Large symbols represent the score median of each treatment group. The percentage of total variance explained by the given principal component is declared in brackets. Different lowercase letters in (a) indicate significant differences at P < 0.05 between the scores of the orthogonal PC1–PC3 axes (analysed with Kruskal–Wallis tests followed by nonparametric Tukey post-hoc tests). No significant differences were found for root fingerprints.
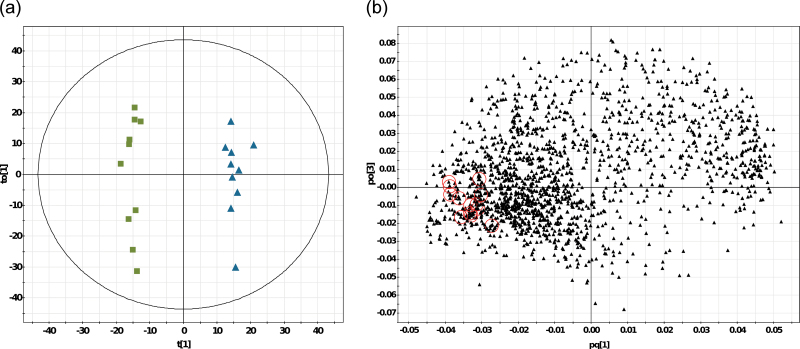
The computation of OPLS-DA confirmed that aphids had the strongest impact on A. thaliana leading to significant changes of the metabolic fingerprints in the aboveground plant tissue (Fig. 4a). The model for comparison of fingerprints of C and A plants consisted of one latent variable related to the Y-variable (explaining 14.9% of the variance) and three orthogonal latent variables not related to the differences between these plants (49.9% of variation). The scores plot significantly separated C and A plants with a relative high quality of the model (Table 2), whereas within the control group subgrouping along the y-axis was found. The analysis of the VIP revealed numerous features that were significantly different between C and A plants, with a high proportion of those features being downregulated in the plants stressed with aphids (Fig. 4b). Interestingly, most aliphatic GS were found in the loadings plot at the left outside margin, indicating downregulation in the A plants, and were thus among the metabolites that are most responsible for class separation (VIP > 1; Fig. 4b). Apart from GS, numerous other metabolites were either up- or downregulated by aphid feeding in the shoots of A. thaliana. For the comparison of fingerprints between AN and C plants, the model derived by OPLS-DA was not significant (Table 2), thus, less pronounced differences were evoked by the combination of both herbivore species compared to the effect of aphids alone. For the other treatment combinations R2X(cum), R2Y(cum) and Q2(cum) were either also low with P > 0.05 (CV-ANOVA) or no models could be fitted (Table 2).
Fig. 4.
OPLS-DA of metabolic fingerprints of Arabidopsis thaliana. (a) Scores plot of shoots of control plants (green boxes) and aphid-treated plants (blue triangles) (n = 10 per treatment; data are shown from one representative experiment of three independent experiments). The tolerance ellipse corresponds to 95% of the Hotelling’s T2 multivariate distribution. (b) Loadings plot. Red circles = identified glucosinolates with VIP > 1. The features at the left side were more abundant in the control plants compared to the aphid-treated plants, and vice versa (this figure is available in colour at JXB online).
Table 2.
Summary of pairwise OPLS-DA computation for shoot and root metabolic fingerprints acquired with UPLC-TOF-MS of control, aphid-, nematode-, and aphid + nematode-treated plants at 3 days after infestation
| Comparison | MD | R2X(cum) | R2Y(cum) | Q2(cum) | F | P |
|---|---|---|---|---|---|---|
| Shoot | ||||||
| C vs. A | 1+3 | 0.612 | 0.985 | 0.646 | 3.16 | 0.038 |
| C vs. N | nm | |||||
| C vs. AN | 1+0 | 0.384 | 0.399 | 0.235 | 2.61 | 0.102 |
| A vs. N | nm | |||||
| A vs. AN | nm | |||||
| N vs. AN | nm | |||||
| Root | ||||||
| C vs. A | 1+1 | 0.218 | 0.465 | 0.157 | 1.58 | 0.234 |
| C vs. N | nm | |||||
| C vs. AN | nm | |||||
| A vs. N | 1+4 | 0.630 | 0.990 | 0.577 | 1.92 | 0.157 |
| A vs. AN | 1+6 | 0.757 | 0.939 | 0.501 | 0.90 | 0.563 |
| N vs. AN | nm | |||||
There were 10 replicates per treatment (data are shown from one representative experiment of three independent experiments). The quality of the resulting models was evaluated with the routine 1/7th out cross validation based on the residuals with R2X(cum) (cumulative modelled variation in X), R2Y(cum) (cumulative modelled variation in Y), and Q2cum (model predictive ability parameter according to cross validation). The result from CV-ANOVA based on the cross-validated predictive residuals is listed for each model and significant results (indicating a valid model) are marked by bold numbers. MD = model dimensions (predictive + orthogonal components); nm = no model could be generated; C = control; A = aphid treatment; N = nematode treatment; AN = aphid + nematode treatment.
For the roots, none of the herbivore treatments revealed valuable OPLS-DA models, demonstrating that the fingerprints were not significantly different between treatments. Only for the comparison between root metabolic fingerprints of A and N plants a model with more or less satisfactory model quality criteria could be derived. However, the model was clearly overfitted (CV-ANOVA, F 8,11 = 1.92, P = 0.157).
Similar morphological and chemical pattern were also observed in two further independent experiments carried out under the same conditions (data not shown; primary metabolites were only measured twice and phytohormones once).
Reciprocal plant-mediated effects between the herbivores
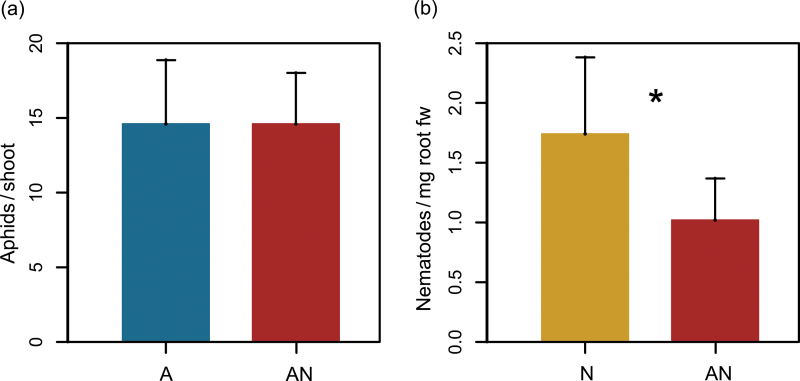
The total aphid number at time of harvest was unaffected by nematode presence, resulting in similar numbers of aphids on A- and AN-infested plants at 3 dpi (Mann–Whitney U test, U = 45.5, P = 0.752; Fig. 5a). Likewise, the abundances of adult aphids (A 9.1±1.1, AN 8.9±0.7, mean ± SD, n = 10 for A and AN plants) and juveniles (A 5.5±3.8, AN 5.7±3.3) did not differ significantly between A and AN plants (Mann–Whitney U tests, adults U = 48.5, P = 0.829, juveniles U = 44.5, P = 0.692). The data given here and shown in Fig. 5a are aphid counts from AN plants used for chemical analysis. In the additional plants taken for the invasive nematode counting, aphid numbers were comparable (15.4±2.6 total aphids, thereof 9.1±1.0 adult and 6.3±1.8 juvenile aphids, n = 8).
Fig. 5.
Abundance of (a) aphids of Brevicoryne brassicae on aphid- (A)- and aphid + nematode (AN)-treated shoots (n = 10) and (b) nematodes of Heterodera schachtii on nematode (N)- and AN-treated roots of Arabidopsis thaliana (n = 7–8) at 3 days after infestation. Values are means + SD; data are shown from one representative experiment of three independent experiments. Statistical analyses were performed with a Mann–Whitney U test (a) and a t-test (b): * = P < 0.05). As nematode counting is an invasive technique, separate plant sets had to be prepared for nematode counting and chemical analyses. The results for aphid abundances shown here (a) were taken from the plants used for chemical analyses; however, aphid numbers on AN plants for nematode counting were similar (see results) (this figure is available in colour at JXB online).
Total nematode numbers and nematode numbers per mg root freshweight were significantly affected by aphid presence, as nematode abundance was almost less than half in AN compared to N plants (t-test, t = 3.55, P = 0.01 for total nematode numbers and t = 2.65, P = 0.02 for numbers per mg root freshweight, respectively; Fig. 5b). A similar outcome was also observed in two further independent experiments under the same conditions (data not shown).
Discussion
Involvement of phytohormones in plant responses
Changes in plant chemistry in response to herbivory are generally mediated by phytohormone signalling (Pieterse et al., 2009). Whereas a plethora of studies demonstrated changes in JA- and SA-related gene expression levels due to induction events (Moran and Thompson, 2001; de Vos et al., 2005; Kuśnierczyk et al., 2007, 2008, 2011), only few studies actually measured the phytohormone concentrations in response to aphid or nematode feeding (Heidel and Baldwin, 2004; de Vos et al., 2005; Wubben et al., 2008), finding only in some cases an increased phytohormone level (Heidel and Baldwin 2004). In the present study’s treatment plants, neither JA nor free or conjugated SA were significantly affected (Table 1). These findings are in agreement with previous investigations, in which no SA and JA changes were found after 3 dpi in A. thaliana shoots infested with the generalist aphid Myzus persicae (de Vos et al., 2005). The JA and SA concentrations may have already levelled off after 3 dpi of feeding initiation. Alternatively, other signalling pathways may play a more important role in these interactions, which should be considered in future studies (de Vos et al., 2005). Nematodes also did not affect shoot phytohormone concentrations in the present study. Although the SA pathway is involved in resistance against nematodes (Branch et al., 2004; Wubben et al., 2008), likewise no significant SA increases in roots or shoots occurred in an earlier study with H. schachtii and A. thaliana (Wubben et al., 2008). Furthermore, also no increase in phytohormone concentrations caused by the synchronous infestation with aphids and nematodes was found. Thus, multiple stressors do not necessarily elevate phytohormone-signalling intensities in a cumulative way.
Effects on primary metabolism
In accordance with the only minute responses of JA and free or conjugated SA to herbivory, herbivore feeding by one species alone or by the combination of two species belonging to different guilds feeding in different compartments did also barely alter shoot primary metabolite composition in A. thaliana in the present study. Aphids solely depend on nutrients acquired from phloem sap and can thus act as strong sinks in order to receive sufficient supply with amino acids (Giordanengo et al., 2010). Sucking by aphids can indeed induce changes of amino acid composition in the host plants; however, this was only demonstrated at a very severe population size and when aphids caused macroscopic plant changes (Sandström et al., 2000; Girousse et al., 2005). Hofmann et al. (2010) detected a systemic induction of several primary metabolites in A. thaliana shoots following H. schachtii infestation but significant changes occurred only after later time points post infestation (15 dpi). Pronounced changes in primary metabolites may take place primarily locally in the phloem or near the nematode feeding sites, the syncytia, in the roots, and only after a longer time period of infestation also systemically.
Overall, a more severe infestation by herbivores might be necessary to evoke considerable plant responses. Likewise, when herbivore numbers increase during the cause of the infestation process, as can happen rapidly in aphids, a certain threshold may be reached at which the plant starts to invest strongly in chemical modifications and defence.
Changes in secondary metabolites and metabolic fingerprints
With regard to target defence metabolites, aliphatic GS concentrations in A. thaliana shoots were reduced by aphid infestation, whereas indole GS showed no reduction (Fig. 1). The reduction of aliphatic GS is in accordance with other studies on different A. thaliana ecotypes and aphid species although induction of certain indole GS in whole shoots or locally at feeding sites has been reported in addition (Kim and Jander, 2007; Kuśnierczyk et al., 2008). In contrast, Mewis et al. (2005, 2006) found an overall increase of aliphatic GS concentrations by B. brassicae at a progressed time point. All other mentioned studies (Mewis et al. 2005, 2006; Kim and Jander, 2007; Kuśnierczyk et al., 2008) applied much higher aphid infestation numbers of partly different species and instars than used in the present study, which may account for the different induction pattern. In turn, aliphatic GS in different A. thaliana ecotypes were positively correlated with B. brassicae performance (Kos et al., 2012). Thus, the reduction of aliphatic GS in the plant in response to aphid feeding may be highly adaptive to suppress further aphid population growth. Aphids also reduced several aliphatic GS in the roots and led to a shift in the aliphatic/indole GS ratio in the systemic tissue (Fig. 2b), indicating that the plants alter the allocation of defence compounds in a highly fine-tuned way. As far as is known, systemic effects of aphid feeding on root metabolites have never been investigated before.
In contrast to the effects of aphids, nematodes had neither an impact on the total GS concentrations nor on the groups of aliphatic and indole GS in systemic shoot tissue. Nevertheless, nematode feeding led to a slight shift in the ratio of aliphatic and indole GS (Fig. 2), indicating that these root feeders can also cause at least subtle changes in the major defence compounds of the Brassicaceae. In the roots, nematodes had only a minor effect on GS with a significant reduction of one aliphatic GS. Cyst nematodes have a destructive intracellular immigration behaviour (Wyss and Grundler, 1992) but are able to manipulate their host (Davis and Mitchum, 2005). Such manipulation may explain the relatively low plant reactions to nematode infestation in the present study and the reduction of aphid effects when they co-occurred with nematodes.
With regard to the metabolic fingerprints, treatment-specific changes could be clearly disentangled between the different treatment groups. Aphid feeding induced a significant shift in the metabolite pattern of the plant shoots (Table 2 and Figs. 3a and 4a), which was not only caused by changes in aliphatic GS (Supplementary Table S2) but also other plant metabolites contributed to these differences (Fig. 4b). A large proportion of metabolic signals, including the GS, was rather down- than upregulated in response to aphid feeding. In the metabolic fingerprints of Plantago lanceolata L. (Plantaginaceae) similarly mainly downregulation of few metabolites was found after attack by a specialised aphid at 3 dpi (Sutter and Müller, 2011). It needs to be determined whether this is a general feature for plant responses to low early infestation rates of phloem-sucking herbivores. As found for the GS pattern, nematodes had no effect on the overall shoot and root metabolic fingerprints and interestingly also diminished the aphid influence in the combined treatment on the shoots (Table 2 and Fig. 3a), suggesting some cross-talk in the plant responses. Overall, the data indicate that changes in GS clearly dominated the shifts in metabolic fingerprints of the experimental plants.
Plant-mediated asymmetric effects between aphids and nematodes
The above- and belowground feeding herbivores B. brevicoryne and H. schachtii had asymmetric effects on each other on the shared host plant A. thaliana (Fig. 5). Whereas nematode infestation did not influence the development of the aphids, aphid presence led to significantly reduced nematode infestation rates. Studies on indirect interactions between above- and belowground plant feeders provided contradictory results ranging from neutral effects through negative influences on both sides to contradirectional as well as asymmetrical interactions (Vandegehuchte et al., 2010). The various outcomes can most probably be attributed to different experimental designs or systems of investigation and mechanisms behind the interactions. Thereby, the sequence and the infestation rates with the different organisms are crucial for the outcome of the interaction (Erb et al., 2011; Soler et al., 2012).
Negative impacts of shoot on root herbivores, as found in this study (Fig. 5b) and also in a few other studies (Gange and Brown, 1989; Soler et al., 2007; Kaplan et al., 2009; Tiwari et al., 2009), may be mediated by reduced root biomass or lowered root quality as a consequence of shoot herbivore presence (Masters et al., 1993). However, changes in root biomass should only become apparent after more severe shoot herbivory and longer feeding periods. Indeed, low aphid densities usually do not affect the biomass of the host plant (Wurst and van der Putten, 2007) and in accordance, the low herbivore abundances did neither affect the biomass of the shoot and root nor the root/shoot ratio in the present study (Table 1). Next to changes in biomass and allocation pattern, sink competition was discussed to lead to interference between aphids and nematodes, but secondary metabolites were proposed to be more relevant (Kaplan et al., 2011). Such changes in plant secondary chemistry can occur rapidly, leading to plant-mediated indirect interactions between herbivores feeding at different tissues (Bezemer and van Dam, 2005; Maffei et al., 2007; Gonzáles-Megías and Müller, 2010). In the present experiment, aboveground aphid infestation led to a significant reduction of a few GS, indicating some changes of systemic plant quality, which may impair the development of H. schachtii. It is well known that herbivores can respond rather sensitively to changes in individual GS even if the overall GS concentrations do not change, as also their hydrolysis products differ in toxicity (Halkier and Gershenzon, 2006; Textor and Gershenzon, 2009). Alternatively (or additionally) to the potential effects of changed plant chemistry, aboveground feeding may alter root respiration as well as root exudation with consequences for the microfauna (Holland et al., 1996; Bardgett and Wardle, 2003; Vestergard et al., 2004). Although in natural situations root feeders might be able to infest a plant prior to aboveground feeders, nematodes are often patchily distributed and produce several generations per year. Therefore, aphid presence may have indeed a relevant impact on the belowground community. Further experiments are needed to investigate whether the sequence of infestation is of importance for the outcome of the reciprocal herbivore interactions in this system.
On the contrary, root nematodes had no effect on aphid performance in the present study (Fig. 5a). This is well mirrored in the fact that nematodes did not cause significant changes in the metabolic composition of the shoots at least within 3 days of infestation. Definitely, more studies are needed for a conclusive assessment of aphid–plant–nematode interactions (Kaplan et al., 2011), especially at early time points, when the organisms start to establish on their host plants.
In summary, only moderate plant metabolic responses were detected to realistic low levels of initial herbivore infestation. It may be adaptive for a plant not to overreact to a minute stress level, as in a natural environment plants can be attacked by various antagonists. In such situations, a well-balanced response to the different stressors is mandatory due to limited resources that have to be partitioned between growth, reproduction and defence. Nevertheless, nematodes seem to react highly sensitive to even low changes induced by herbivores feeding at the other compartment. As a consequence, minor impacts of shoot herbivores may have strong influences on the root herbivore community, which are mediated by the common host plant.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Table S1. Relative abundances (mean ± SD) of primary metabolites identified by GC-MS in Arabidopsis thaliana shoot tissue of control, aphid-, nematode-, and aphid + nematode-treated plants at 3 dpi.
Supplementary Table S2. Shoot and root glucosinolate and glucosinolate group concentrations (mean ± SD) in control, aphid-, nematode-, and aphid + nematode-treated plants at 3 dpi.
Supplementary Material
Acknowledgements
The authors thank Linda Ehlert for help with plant breeding, Jana Niekamp for assistance with nematode counting, Björn Niere for an initial nematode culture, Karsten Niehaus for providing standards of various primary metabolites, and Rabea Sutter for helpful editing of this manuscript. This work was funded by the grant MU 1829/9-1 of the Deutsche Forschungsgemeinschaft.
References
- Baermann G. 1917. Eine einfache Methode zur Auffindung von Ankylostomum (Nematoden) Larven in Erdproben Geneeskunding Tijdschrift voor Nederlandsch-Indië 57 131–137 [Google Scholar]
- Baldwin IT, Schmelz EA, Ohnmeiss TE. 1994. Wound-induced changes in root and shoot jasmonic acid pools correlate with induced nicotine synthesis in Nicotiana sylvestris Spegazzini and Comes Journal of Chemical Ecology 20 2139–2157 [DOI] [PubMed] [Google Scholar]
- Bardgett RD, Wardle DA. 2003. Herbivore-mediated linkages between aboveground and belowground communities Ecology 84 2258–2268 [Google Scholar]
- Bezemer TM, van Dam NM. 2005. Linking aboveground and belowground interactions via induced plant defenses Trends in Ecology and Evolution 20 617–624 [DOI] [PubMed] [Google Scholar]
- Bidart-Bouzat MG, Kliebenstein D. 2011. An ecological genomic approach challenging the paradigm of differential plant responses to specialist versus generalist insect herbivores Oecologia 167 677–689 [DOI] [PubMed] [Google Scholar]
- Bones AM, Rossiter JT. 1996. The myrosinase-glucosinolate system, its organisation and biochemistry Physiologia Plantarum 97 194–208 [Google Scholar]
- Bones AM, Rossiter JT. 2006. The enzymic and chemically induced decomposition of glucosinolates Phytochemistry 67 1053–1067 [DOI] [PubMed] [Google Scholar]
- Bostock RM. 2005. Signal crosstalk and induced resistance: straddling the line between cost and benefit Annual Review of Phytopathology 43 545–580 [DOI] [PubMed] [Google Scholar]
- Branch C, Hwang CF, Navarre DA, Williamson VM. 2004. Salicylic acid is part of the Mi-1-mediated defense response to root-knot nematode in tomato Molecular Plant–Microbe Interactions 17 351–356 [DOI] [PubMed] [Google Scholar]
- Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J. 2003. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana Phytochemistry 62 471–481 [DOI] [PubMed] [Google Scholar]
- Byrd DW, Kirkpatrick T, Barker KR. 1983. An improved technique for clearing and staining plant-tissues for detection of nematodes Journal of Nematology 15 142–143 [PMC free article] [PubMed] [Google Scholar]
- Davis EL, Mitchum MG. 2005. Nematodes. Sophisticated parasites of legumes Plant Physiology 137 1182–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos M, van Oosten VR, van Poecke RMP, van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Metraux JP, van Loon LC, Dicke M, Pieterse CMJ. 2005. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack Molecular Plant–Microbe Interactions 18 923–937 [DOI] [PubMed] [Google Scholar]
- Dettmer K, Aronov PA, Hammock BD. 2007. Mass spectrometry-based metabolomics Mass Spectrometry Reviews 26 51–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M, Robert CAM, Hibbard BE, Turlings TCJ. 2011. Sequence of arrival determines plant-mediated interactions between herbivores Journal of Ecology 99 7–15 [Google Scholar]
- Erb M, Ton J, Degenhardt J, Turlings TCJ. 2008. Interactions between arthropod-induced aboveground and belowground defenses in plants Plant Physiology 146 867–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson L, Johansson E, Kettaneh-Wold N, Trygg J, Wikström C, Wold S. Multi- and megavariate data analysis. Part I: basic principles and applications. Umeå, Sweden: Umetrics Academy; 2006. [Google Scholar]
- Eriksson L, Trygg J, Wold S. 2008. CV-ANOVA for significance testing of PLS and OPLS (R) models Journal of Chemometrics 22 594–600 [Google Scholar]
- Forcat S, Bennett MH, Mansfield JW, Grant MR. 2008. A rapid and robust method for simultaneously measuring changes in the phytohormones ABA, JA and SA in plants following biotic and abiotic stress Plant Methods 4, 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gange AC, Brown VK. 1989. Effects of root herbivory by an insect on a foliar-feeding species, mediated through changes in the host plant Oecologia 81 38–42 [DOI] [PubMed] [Google Scholar]
- Giordanengo P, Brunissen L, Rusterucci C, Vincent C, van Bel A, Dinant S, Girousse C, Faucher M, Bonnemain JL. 2010. Compatible plant-aphid interactions: how aphids manipulate plant responses Comptes Rendus Biologies 333 516–523 [DOI] [PubMed] [Google Scholar]
- Girousse C, Moulia B, Silk W, Bonnemain JL. 2005. Aphid infestation causes different changes in carbon and nitrogen allocation in alfalfa stems as well as different inhibitions of longitudinal and radial expansion Plant Physiology 137 1474–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens Annual Review of Phytopathology 43 205–227 [DOI] [PubMed] [Google Scholar]
- Gonzáles-Megías AG, Müller C. 2010. Root herbivores and detritivores shape above-ground multitrophic assemblage through plant-mediated effects Journal of Animal Ecology 79 923–931 [DOI] [PubMed] [Google Scholar]
- Gutjahr C, Paszkowski U. 2009. Weights in the balance: jasmonic acid and salicylic acid signaling in root-biotroph interactions Molecular Plant–Microbe Interactions 22 763–772 [DOI] [PubMed] [Google Scholar]
- Halkier BA, Gershenzon J. 2006. Biology and biochemistry of glucosinolates Annual Review of Plant Biology 57 303–333 [DOI] [PubMed] [Google Scholar]
- Hall RD. 2006. Plant metabolomics: from holistic hope, to hype, to hot topic New Phytologist 169 453–468 [DOI] [PubMed] [Google Scholar]
- Heidel AJ, Baldwin IT. 2004. Microarray analysis of salicylic acid- and jasmonic acid-signalling in responses of Nicotiana attenuata to attack by insects from multiple feeding guilds Plant, Cell and Environment 27 1362–1373 [Google Scholar]
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil California Agricultural Experiment Station Circular 347 1–32 [Google Scholar]
- Hofmann J, El Ashry A, Anwar S, Erban A, Kopka J, Grundler F. 2010. Metabolic profiling reveals local and systemic responses of host plants to nematode parasitism The Plant Journal 62 1058–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland JN, Cheng WX, Crossley DA. 1996. Herbivore-induced changes in plant carbon allocation: assessment of below-ground C fluxes using carbon-14 Oecologia 107 87–94 [DOI] [PubMed] [Google Scholar]
- Hummel J, Strehmel N, Selbig J, Walther D, Kopka J. 2010. Decision tree supported substructure prediction of metabolites from GC-MS profiles Metabolomics 6 322–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan I, Halitschke R, Kessler A, Sardanelli S, Denno RF. 2008. Constitutive and induced defenses to herbivory in above- and belowground plant tissues Ecology 89 392–406 [DOI] [PubMed] [Google Scholar]
- Kaplan I, Sardanelli S, Denno RF. 2009. Field evidence for indirect interactions between foliar-feeding insect and root-feeding nematode communities on Nicotiana tabacum Ecological Entomology 34 262–270 [Google Scholar]
- Kaplan I, Sardanelli S, Rehill BJ, Denno RF. 2011. Toward a mechanistic understanding of competition in vascular-feeding herbivores: an empirical test of the sink competition hypothesis Oecologia 166 627–636 [DOI] [PubMed] [Google Scholar]
- Kim JH, Jander G. 2007. Myzus persicae (green peach aphid) feeding on Arabidopsis induces the formation of a deterrent indole glucosinolate The Plant Journal 49 1008–1019 [DOI] [PubMed] [Google Scholar]
- Kopka J, Schauer N, Krueger S, et al. 2005. GMD@CSB.DB: the Golm Metabolome Database Bioinformatics 21 1635–1638 [DOI] [PubMed] [Google Scholar]
- Kos M, Houshyani B, Achhami BB, Wietsma R, Gols R, Weldegergis BT, Kabouw P, Bouwmeester HJ, Vet LEM, Dicke M, van Loon JJA. 2012. Herbivore-mediated effects of glucosinolates on different natural enemies of a specialist aphid Journal of Chemical Ecology 38 100–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats E. 1958. Gas-chromatographische Charakterisierung organischer Verbindungen.1. Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone Helvetica Chimica Acta 41 1915–1932 [Google Scholar]
- Kuśnierczyk A, Tran DHT, Winge P, Jørstad TS, Reese JC, Troczyńska J, Bones AM. 2011. Testing the importance of jasmonate signalling in induction of plant defences upon cabbage aphid (Brevicoryne brassicae) attack BMC Genomics12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuśnierczyk A, Winge P, Jørstad TS, Troczyńska J, Rossiter JT, Bones AM. 2008. Towards global understanding of plant defence against aphids – timing and dynamics of early Arabidopsis defence responses to cabbage aphid (Brevicoryne brassicae) attack Plant, Cell and Environment 31 1097–1115 [DOI] [PubMed] [Google Scholar]
- Kuśnierczyk A, Winge P, Midelfart H, Armbruster WS, Rossiter JT, Bones AM. 2007. Transcriptional responses of Arabidopsis thaliana ecotypes with different glucosinolate profiles after attack by polyphagous Myzus persicae and oligophagous Brevicoryne brassicae Journal of Experimental Botany 58 2537–2552 [DOI] [PubMed] [Google Scholar]
- Macel M, van Dam NM, Keurentjes JJB. 2010. Metabolomics: the chemistry between ecology and genetics Molecular Ecology Resources 10 583–593 [DOI] [PubMed] [Google Scholar]
- Maffei ME, Mithofer A, Boland W. 2007. Before gene expression: early events in plant-insect interaction Trends in Plant Science 12 310–316 [DOI] [PubMed] [Google Scholar]
- Masters GJ, Brown VK, Gange AC. 1993. Plant mediated interactions between aboveground and belowground insect herbivores Oikos 66 148–151 [Google Scholar]
- Mewis I, Appel HM, Hom A, Raina R, Schultz JC. 2005. Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects Plant Physiology 138 1149–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewis I, Tokuhisa JG, Schultz JC, Appel HM, Ulrichs C, Gershenzon J. 2006. Gene expression and glucosinolate accumulation in Arabidopsis thaliana in response to generalist and specialist herbivores of different feeding guilds and the role of defense signaling pathways Phytochemistry 67 2450–2462 [DOI] [PubMed] [Google Scholar]
- Moran PJ, Thompson GA. 2001. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways Plant Physiology 125 1074–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkunas I, van Chung M, Gabrys B. 2011. Phytohormonal signaling in plant responses to aphid feeding Acta Physiologica Plantarum 33 2057–2073 [Google Scholar]
- Ogawa T, Ara T, Aoki K, Suzuki H, Shibata D. 2010. Transient increase in salicylic acid and its glucose conjugates after wounding in Arabidopsis leaves Plant Biotechnology 27 205–209 [Google Scholar]
- Pieterse CMJ, Leon-Reyes A, van der Ent S, van Wees SCM. 2009. Networking by small-molecule hormones in plant immunity Nature Chemical Biology 5 308–316 [DOI] [PubMed] [Google Scholar]
- R Developmental Core Team. 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. URL http://www.R-project.org. [Google Scholar]
- Renwick JAA. 2002. The chemical world of crucivores: lures, treats and traps Entomologia Experimentalis et Applicata 104 35–42 [Google Scholar]
- Sandström J, Telang A, Moran NA. 2000. Nutritional enhancement of host plants by aphids–a comparison of three aphid species on grasses Journal of Insect Physiology 46 33–40 [DOI] [PubMed] [Google Scholar]
- Smith CA, Want EJ, O’Maille G, Abagyan R, Siuzdak G. 2006. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification Analytical Chemistry 78 779–787 [DOI] [PubMed] [Google Scholar]
- Soler R, Badenes-Pérez FR, Broekgaarden C, Zheng SJ, David A, Boland W, Dicke M. 2012. Plant-mediated facilitation between a leaf-feeding and a phloem-feeding insect in a brassicaceous plant: from insect performance to gene transcription Functional Ecology 26 156–166 [Google Scholar]
- Soler R, Bezemer TM, Cortesero AM, van der Putten WH, Vet LEM, Harvey JA. 2007. Impact of foliar herbivory on the development of a root-feeding insect and its parasitoid Oecologia 152 257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter R, Müller C. 2011. Mining for treatment-specific and general changes in target compounds and metabolic fingerprints in response to herbivory and phytohormones in Plantago lanceolata New Phytologist 191 1069–1082 [DOI] [PubMed] [Google Scholar]
- Tautenhahn R, Böttcher C, Neumann S. 2008. Highly sensitive feature detection for high resolution LC/MS BMC Bioinformatics 9, 504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NS, Weber RJM, Southam AD, Payne TG, Hrydziuszko O, Arvanitis TN, Viant MR. 2009. A new approach to toxicity testing in Daphnia magna: application of high throughput FT-ICR mass spectrometry metabolomics Metabolomics 5 44–58 [Google Scholar]
- Textor S, Gershenzon J. 2009. Herbivore induction of the glucosinolate-myrosinase defense system: major trends, biochemical bases and ecological significance Phytochemistry Reviews 8 149–170 [Google Scholar]
- Tiwari S, Youngman RR, Lewis EE, Eisenback JD. 2009. European corn borer (Lepidoptera: Crambidae) stalk tunneling on root-knot nematode (Tylenchida: Heteroderidae) fitness on corn Journal of Economic Entomology 102 602–609 [DOI] [PubMed] [Google Scholar]
- van Dam NM, Harvey JA, Wäckers FL, Bezemer TM, van der Putten WH, Vet LEM. 2003. Interactions between aboveground and belowground induced responses against phytophages Basic and Applied Ecology 4 63–77 [Google Scholar]
- van Dam NM, Raaijmakers CE, van der Putten WH. 2005. Root herbivory reduces growth and survival of the shoot feeding specialist Pieris rapae on Brassica nigra Entomologia Experimentalis et Applicata 115 161–170 [Google Scholar]
- Vandegehuchte ML, de la Peña E, Bonte D. 2010. Interactions between root and shoot herbivores of Ammophila arenaria in the laboratory do not translate into correlated abundances in the field Oikos 119 1011–1019 [Google Scholar]
- Vestergard M, Bjørnlund L, Christensen S. 2004. Aphid effects on rhizosphere microorganisms and microfauna depend more on barley growth phase than on soil fertilization Oecologia 141 84–93 [DOI] [PubMed] [Google Scholar]
- Viant MR. 2008. Recent developments in environmental metabolomics Molecular Biosystems 4 980–986 [DOI] [PubMed] [Google Scholar]
- Wubben MJE, Jin J, Baum TJ. 2008. Cyst nematode parasitism of Arabidopsis thaliana is inhibited by salicylic acid (SA) and elicits uncoupled SA-independent pathogenesis-related gene expression in roots Molecular Plant–Microbe Interactions 21 424–432 [DOI] [PubMed] [Google Scholar]
- Wurst S, van der Putten WH. 2007. Root herbivore identity matters in plant-mediated interactions between root and shoot herbivores Basic and Applied Ecology 8 491–499 [Google Scholar]
- Wyss U, Grundler FMW. 1992. Heterodera schachtii and Arabidopsis thaliana, a model host-parasite interaction Nematologica 38 488–493 [Google Scholar]
- Zasada IA, Ferris H. 2003. Sensitivity of Meloidogyne javanica and Tylenchulus semipenetrans to isothiocyanates in laboratory assays Phytopathology 93 747–750 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.