Abstract
Plasmodesmata mediate direct cell-to-cell communication in plants. One of their significant features is that primary plasmodesmata formed at the time of cytokinesis often undergo structural modifications, by the de novo addition of cytoplasmic strands across cell walls, to become complex secondary plasmodesmata during plant development. Whether such modifications allow plasmodesmata to gain special transport functions has been an outstanding issue in plant biology. Here we present data showing that the cucumber mosaic virus 3a movement protein (MP):green fluorescent protein (GFP) fusion was not targeted to primary plasmodesmata in the epidermis of young or mature leaves in transgenic tobacco (Nicotiana tabacum) plants constitutively expressing the 3a:GFP fusion gene. Furthermore, the cucumber mosaic virus 3a MP:GFP fusion protein produced in planta by biolistic bombardment of the 3a:GFP fusion gene did not traffic between cells interconnected by primary plasmodesmata in the epidermis of a young leaf. In contrast, the 3a MP:GFP was targeted to complex secondary plasmodesmata and trafficked from cell to cell when a leaf reached a certain developmental stage. These data provide the first experimental evidence, to our knowledge, that primary and complex secondary plasmodesmata have different protein-trafficking functions and suggest that complex secondary plasmodesmata may be formed to traffic specific macromolecules that are important for certain stages of leaf development.
Plasmodesmata are intercellular connections that allow direct cell-to-cell communication in plants. Traditionally, they were thought to be able to permit only passive diffusion of small molecules, but recent studies have demonstrated that plasmodesmata can also mediate cell-to-cell trafficking of macromolecules such as proteins and nucleic acids of viral origin (Fujiwara et al., 1993; Noueiry et al., 1994; Ding et al., 1995, 1997; Waigmann and Zambryski, 1995; Nguyen et al., 1996; Canto et al., 1997; Itaya et al., 1997; Rojas et al., 1997) and of plant origin (Bostwick et al., 1992; Fisher et al., 1992; Nakamura et al., 1993; Sakuth et al., 1993; Jackson et al., 1994; Ishiwatari et al., 1995; Lucas et al., 1995; Schobert et al., 1995; Perbal et al., 1996; Balachandran et al., 1997; Clark et al., 1997; Kühn et al., 1997; Murillo et al., 1997). These findings indicate that intercellular trafficking of macromolecules through plasmodesmata is potentially an important means of coordinating developmental and physiological processes at the transcellular level (Lucas et al., 1993; Lucas, 1995; Mezitt and Lucas, 1996; Ding, 1997, 1998; Jackson and Hake, 1997; Ding et al., 1998).
A prominent feature of plasmodesmata is their structural modifications associated with plant development. Previous work (Ding et al., 1992, 1993) showed that, at an early stage of tobacco (Nicotiana tabacum) leaf development, mesophyll cells are connected solely by simple primary plasmodesmata that are formed at the time of cytokinesis. During leaf maturation, however, the majority of these primary plasmodesmata undergo structural modification to become highly branched by laterally fusing with neighboring primary plasmodesmata and by the de novo addition of new cytoplasmic strands across the existing cell walls. The resulting branched plasmodesmata are known as secondary plasmodesmata (Jones, 1976; Ding et al., 1992, 1993; Ding and Lucas, 1996) or, more specifically, complex secondary plasmodesmata (Ding, 1998). Modification of primary plasmodesmata to form complex secondary plasmodesmata is likely a general phenomenon in plant development (Jones, 1976; Ding et al., 1993; Volk et al., 1996) and may be of functional significance.
Studies of the interactions between the 30-kD MP of TMV and plasmodesmata suggest that primary and complex secondary plasmodesmata differ in some functional aspects. In TMV MP-transgenic tobacco plants, MP is localized to complex secondary plasmodesmata in the mesophyll and bundle sheath of mature leaves (Ding et al., 1992; Moore et al., 1992) but not to any primary plasmodesmata in young and mature leaves (Ding et al., 1992). Furthermore, MP is able to modify the size-exclusion limit of complex secondary plasmodesmata in the mesophyll and bundle sheath from 1 kD to greater than 10 kD in mature leaves but not that of primary plasmodesmata in young leaves (even though MP is produced in such leaves) (Deom et al., 1990; Ding et al., 1992).
In contrast, Waigmann et al. (1994) showed that microinjected recombinant TMV MP produced from engineered Escherichia coli is able to increase the size-exclusion limit of plasmodesmata in the mesophyll of young leaves of nontransgenic tobacco plants, implying that TMV MP might interact with primary plasmodesmata in young leaves. Why the TMV MP has different effects on the plasmodesmata size-exclusion limit in young leaves of transgenic (Deom et al., 1990; Ding et al., 1992) and nontransgenic tobacco plants (Waigmann et al., 1994) is not well understood. Nevertheless, these observations raise a number of important issues concerning the functions of different plasmodesmata: Is localization to complex secondary but not primary plasmodesmata a peculiar feature of TMV MP, or does it have broader implications in protein-plasmodesmata interactions? And is such preferential localization indicative of the different abilities of these plasmodesmata to support intercellular trafficking of a particular protein? Resolution of these issues is necessary to advance our understanding of the functions of plasmodesmata in plant growth and development. To resolve these issues, further studies are needed to determine how other proteins interact with plasmodesmata. More important, such studies must include testing directly the ability of primary or complex secondary plasmodesmata to facilitate intercellular trafficking of a specific protein, in addition to examining how the protein is localized to plasmodesmata and how it affects the size-exclusion limit of plasmodesmata.
Intercellular protein trafficking has been mainly studied by microinjection techniques. In developing alternative methods, we recently utilized biolistic bombardment (Sanford, 1988) to deliver a DNA construct into epidermal cells of mature tobacco leaves to produce a CMV 3a MP:GFP fusion directly in planta. The fusion protein produced in this manner trafficked from cell to cell, as detected under a fluorescence microscope (Itaya et al., 1997). CMV 3a MP:GFP fusion produced during infection of an engineered CMV expressing the 3a:GFP gene also trafficked from cell to cell in tobacco leaf epidermis (Canto et al., 1997). These data are consistent with previous microinjection results showing cell-to-cell trafficking of a recombinant CMV 3a MP in mature tobacco leaf mesophyll (Ding et al., 1995).
We used an integrative approach that included structural analysis, immunolabeling, and biolistic bombardment to study the functions of primary and complex secondary plasmodesmata in trafficking CMV 3a MP:GFP in tobacco leaf epidermis. In this report we show that biolistically produced 3a MP:GFP fusion protein did not traffic between epidermal cells interconnected by primary plasmodesmata in young leaves. As these primary plasmodesmata became complex secondary plasmodesmata during further leaf development, 3a MP:GFP began to traffic between epidermal cells. Furthermore, in transgenic tobacco plants constitutively producing 3a MP:GFP, this fusion protein was localized to complex secondary but not to primary plasmodesmata as a function of leaf development. The implications of these findings in studying the functions of different plasmodesmata in plant growth and development are discussed.
MATERIALS AND METHODS
Plant Material
Tobacco (Nicotiana tabacum L. cv Samsun NN) plants were grown in a growth chamber under a day/night temperature regime of 24°C for 15 h/18°C for 9 h. During the day the light level was approximately 260 μmol m−2 s−1. Nine- to ten-week-old plants were used for the experiments.
Gene Constructs for Tobacco Transformation
Construction of expression vectors pRTL2-MP3a:GFP and pRTL2-GFP was described previously (Itaya et al., 1997). These vectors contain, respectively, the CMV 3a:GFP fusion gene and the GFP gene under the control of the CaMV 35S promoter. The 35S:3a:GFP fusion gene and the 35S:GFP gene inserted in pRTL2-MP3a:GFP and pRTL2-GFP (Itaya et al., 1997) were amplified by PCR using primer SacI-35S (CGAGCTCGCATGCCTGCAGGTCA) and primer term-HindIII (AAGCTTGCATGCCTGCAGGTCA). The PCR products were ligated into binary vector pBIN19 using a DNA-ligation kit (Takara Shuzo, Ltd., Otsu, Japan) following the manufacturer's instructions. Recombinant plasmids were used to transform Escherichia coli (DH5α) using the protocol of Pope and Kent (1996). Transformed E. coli were grown on Luria-Bertani (Km 50 μg mL−1) plates for selection. Purified plasmids from isolated colonies were digested by SacI and HindIII to check the size of inserts. The constructs were also sequenced to confirm correct DNA sequences. Positive plasmids were bombarded into tobacco leaves to check further the function of genes.
Plasmids containing the correct genes were then transferred via electroporation into Agrobacterium tumefaciens strain LBA 4404, which carries the helper plasmid pAL4404. Competent A. tumefaciens cells were first prepared by growth at 29°C in YM medium (0.04% [w/v] yeast extract, 1% [w/v] mannitol, 0.01% [w/v] NaCl, 0.02% [w/v] MgSO4·7H2O, 0.05% [w/v] K2HPO4·3H2O, pH 7.0, and 500 μg mL−1 streptomycin) for approximately 2 d until the A600 reached 0.7. The culture was chilled on ice for 15 min and then centrifuged at 5000 rpm for 15 min at 4°C. The pellet was washed with 7 mL of sterile Hepes, pH 7.0 (0.2-μm filter sterilization), and resuspended in 200 μL of sterile Hepes containing 10% (v/v) glycerol. Cells were split into 45-μL aliquots, frozen in liquid nitrogen, and stored at −20°C.
For electroporation, competent cells were thawed on ice and mixed with approximately 0.3 μg of plasmid and then incubated on ice for 2 min. The mixture was transferred to a prechilled electroporation cuvette (0.2-cm gap) and electroporated at a field strength of 2.5 kV cm−1 with a Gene Pulser (Bio-Rad). One milliliter of prechilled YM broth was added to the cuvette immediately after electroporation. The mixture was incubated with gentle shaking for 1 h at 29°C. One hundred microliters of the culture was spread onto selective YM plates (kanamycin 50 μg mL−1 and streptomycin 500 μg mL−1) and incubated at 29°C for 2 to 3 d. Colony PCR was performed to check for the presence of the correct insert in A. tumefaciens.
Tobacco Transformation
A standard leaf-disc transformation method (Horsch et al., 1985) was used to generate transformants of tobacco expressing 3a:GFP and GFP. Transformants initially growing in the selection medium were further selected by fluorescence microscopic examination for expression of 3a MP:GFP and GFP. Positive transformants were then transferred to soil in pots and were allowed to grow to maturity in a growth chamber.
Among the 16 primary transformants generated, three were found to show 3a MP:GFP production in all epidermal cells during initial screening by fluorescence microscopy. These transgenic lines were designated AI3a:GFP1, AI3a:GFP2, and AI3a:GFP3. These plants were self-fertilized and seeds were collected. The seeds were planted in soil directly, and progeny plants expressing the 3a:GFP or GFP gene were selected by fluorescence microscopic examination. Immunoblot analysis was performed to confirm the presence of 3a MP:GFP and GFP in these plants, as described below.
Immunoblot Analysis of CMV 3a MP:GFP- and GFP-Transgenic Tobacco Plants
Crude protein extracts were prepared from the transgenic and nontransgenic control plants following the protocol of Vaquero et al. (1994). Fresh leaves (0.1 g) were ground in liquid nitrogen and homogenized in 1 mL of grinding buffer (10 mm KCL, 5 mm MgCl2, 0.4 m Suc, 10% [v/v] glycerol, and 10 mm 2-mercaptoethanol in Tris-HCl buffer, pH 7.5). The mixture was centrifuged at 1000 rpm for 10 min at 4°C. The supernatant was collected and used for dot blotting.
For immunoblot analysis, 10 μL of the crude protein extract was blotted onto nitrocellulose membranes. The membranes were incubated in a blocking buffer consisting of 5% (w/v) nonfat dry milk in TBST (25 mm Tris-HCl, pH 7.4, 140 mm NaCl, 2.7 mm KCl, and 0.1% Tween 20) for 1 h and then incubated with a rabbit-derived polyclonal antibody against GFP (Clontech, Palo Alto, CA) overnight. After several buffer washes, the membranes were treated for 1 h with a goat-derived anti-rabbit IgG antibody conjugated to alkaline phosphatase. After the membranes were washed again with buffer, color reaction was carried out using a detection kit (Boehringer Mannheim) following the manufacturer's instructions.
CMV 3a MP:GFP and Callose Colocalization
Leaf blades from 3a MP:GFP-transgenic tobacco plants were submerged in a fixative (3.7% paraformaldehyde, 0.2% picric acid, 50 mm potassium phosphate, and 5 mm EGTA, pH 6.8) and cut into 2- × 3-mm segments. The leaf segments were then fixed in the same fixative for 2 h at room temperature.
After buffer (50 mm potassium phosphate and 5 mm EGTA, pH 6.8) washes, the segments were gradually infiltrated with a 2:1 mixture of 20% (w/v) Suc:Tissue Tek optimal cutting temperature compound (Ted Pella, Inc., Redding, CA). The infiltration was completed in three steps: 30% (v/v) of the mixture for 30 min, 60% for 30 min, and 100% for 1 h.
Two to three infiltrated leaf segments were stacked in one drop of absolute Tissue Tek on a piece of laboratory film (Parafilm, American National Can, Greenwich, CT) at room temperature and were then frozen in a CTD cryostat (International Equipment Co., Needham Heights, MA). Ten-micrometer cryosections were obtained and attached to a microscope slide coated with a mixture of 1% (w/v) gelatin and 0.1% (w/v) chrome alum. The slides with tissue sections were incubated on a warming plate at 40°C for at least 2 h before further processing.
The slides were incubated in PBS buffer (140 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4, and 1.8 mm KH2PO4, pH 7.4) containing 3% (w/v) BSA (fraction V, fatty acid free, Boehringer Mannheim) and 1% (v/v) Nonidet P-40 (Sigma) for 30 min at room temperature to wash away the optimal cutting temperature compound, block nonspecific antibody binding, and extract autofluorescent compounds from the sectioned tissues. Afterward, the sections were incubated in a monoclonal antibody against callose (Biosupplies Australia, Parkville, Australia) diluted 1:50 in PBS containing 1% BSA and 0.05% (v/v) Triton X-100 for 1.5 to 2 h at 37°C. Following PBS washes for 10 to 15 min, the sections were incubated in a goat-derived and Texas Red-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) at 1:50 dilution in PBS containing 1% BSA and 0.05% Triton X-100 for 1 to 1.5 h at 37°C. The sections were washed with PBS, embedded in a mounting medium, and covered with a coverslip. The slides were placed in the dark for at least 1 h prior to microscopic examination.
The labeled sections were examined with either an epifluorescence microscope (Optiphot-2, Nikon) or a confocal microscope (model MRC-1024, Bio-Rad). Two sets of filter combinations were used with the epifluorescence microscope. GFP was detected with a filter set consisting of a blue excitation filter (420–490 nm), a dichroic mirror (510 nm), and a green barrier filter (520–560 nm). Texas Red fluorescence was detected with a filter set consisting of a green excitation filter (546/10 nm), a 580-nm dichroic mirror, and an orange-red barrier filter (590 nm). Fluorescent images were acquired with a CCD (charge-coupled device) camera (model C2400, Hamamatsu Photonic Systems, Bridgewater, NJ) and processed using an image-processing system (Argus 20, Hamamatsu).
Other sections were examined using a confocal microscope at the imaging center at the Department of Genetics and Cell Biology, University of Minnesota, St. Paul. The confocal microscope was attached to an inverted microscope (Diaphot, Nikon) equipped with a 15-mW krypton/argon laser. For 3a MP:GFP and Texas Red visualization, the 488-/568-nm excitation lines were used with a 585-nm double-dichroic mirror and a 585-nm ELP20 barrier filter. Digital images were collected on a personal computer (model 300 ProSignia, Compaq Computer Corporation, Houston, TX) using LaserSharp software (version 2.1, Bio-Rad). Images were further analyzed using imaging software (version 1.62 NIH Image, National Institutes of Health, Bethesda, MD).
Tissue Processing for Electron Microscopy
For structural analysis of plasmodesmata during leaf development, tobacco leaves were initially fixed in 4% (v/v) glutaraldehyde/0.1% (v/v) tannic acid and then fixed in 2% (w/v) osmium tetroxide, as described by Ding et al. (1992). The fixed samples were then embedded in Spurr's medium (Spurr, 1996). Thin sections of 60 to 70 nm were stained with 2% (w/v) uranyl acetate in 70% (v/v) methanol and then lead citrate for transmission electron microscopic examination (Ding et al., 1992).
For immunolabeling, leaf tissues from 3a MP:GFP-transgenic and nontransgenic control tobacco plants were fixed in 2% (w/v) paraformaldehyde/0.5% (v/v) glutaraldehyde and embedded in London White resin for obtaining thin sections (Ding et al., 1992).
Immunolabeling of CMV 3a MP:GFP
Immunolabeling of 3a MP:GFP was performed as described previously (Ding et al., 1992). Thin sections of tobacco leaf samples from 3a MP:GFP-transgenic tobacco plants and from nontransgenic control tobacco plants were first incubated for 1 h in TBST buffer, which consisted of 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 2% (w/v) BSA, and 0.1% (v/v) Tween 20 (Sigma). Afterward, the sections were incubated for 1 h in a rabbit-derived polyclonal antibody (IgG) against CMV 3a MP (C. Masuta, unpublished data) at 1:200 dilutions. Following TBST buffer washes, the sections were incubated for 1 h in a goat-derived and 10-nm gold-conjugated anti-rabbit IgG antibody (Sigma). After buffer and distilled-water washes, the sections were stained with 2% (w/v) uranyl acetate in methanol followed by lead citrate. All sections were then examined with an electron microscope (model JEM-100CX II, JEOL) operated at 80 kV.
Biolistic Bombardment and Fluorescence Microscopy
Biolistic bombardment and fluorescence microscopy were essentially as described previously (Itaya et al., 1997). A segment of detached tobacco leaf from the tip or the base was immediately placed in a Petri dish containing wet filter paper. The fresh cut end of the leaf segment was covered with a wet cotton ball. The gene constructs were delivered into the lower epidermis of the leaf segment using a biolistic PDS 1000/He system (Bio-Rad) at a pressure of 1300 p.s.i. After bombardment, the leaf samples were kept in Petri dishes at 24°C as described above. Images of GFP were detected and processed as described previously (Itaya et al., 1997).
RESULTS
Development of Complex Secondary Plasmodesmata between Epidermal Cells
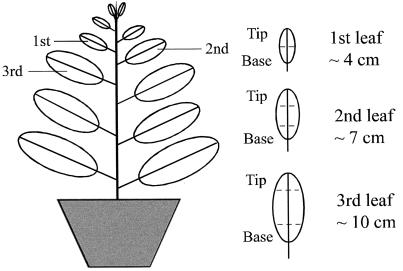
We focused our investigation on plasmodesmatal functions in tobacco leaf epidermis because this tissue is well suited for biolistic bombardment and fluorescence microscopic observation with little tissue manipulation (Itaya et al., 1997). The central issue under investigation was whether primary and complex secondary plasmodesmata function differently in interacting with and supporting intercellular trafficking of 3a MP:GFP. Therefore, it was necessary to obtain detailed information about plasmodesmal structure in the leaf epidermis at various developmental stages. Figure 1 gives a schematic view of an approximately 9-week-old tobacco plant with the leaves studied in detail highlighted. Preliminary studies showed that CMV 3a MP:GFP produced in the epidermal cells of a 4-cm or younger leaf did not traffic from cell to cell, in contrast to older leaves. Thus, we arbitrarily defined this young leaf as the first leaf for convenience of description. The second and third leaves were the next successively older leaves. The trafficking patterns of 3a MP:GFP in the epidermis of leaves at successive developmental stages are presented in a later section.
Figure 1.
Schematic view of a 10-week-old tobacco plant showing the leaves used in the present study. For the first, second, and third leaves, both the tip and the base regions (defined by the dashed lines) were investigated in terms of the structure and development of plasmodesmata and the interactions between CMV 3a MP:GFP and plasmodesmata.
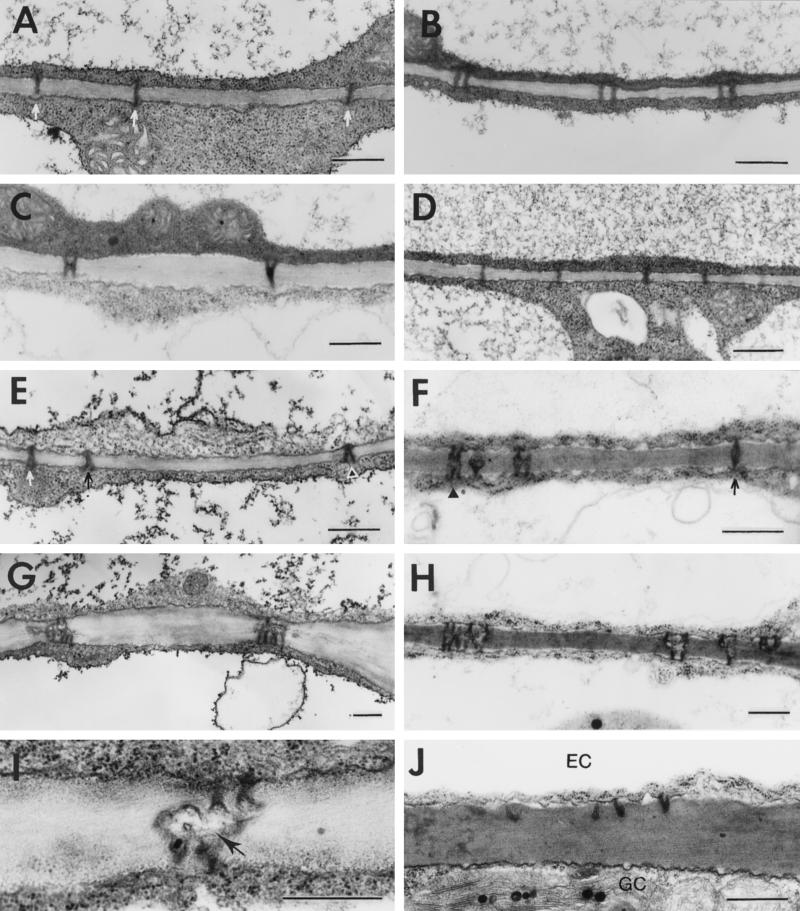
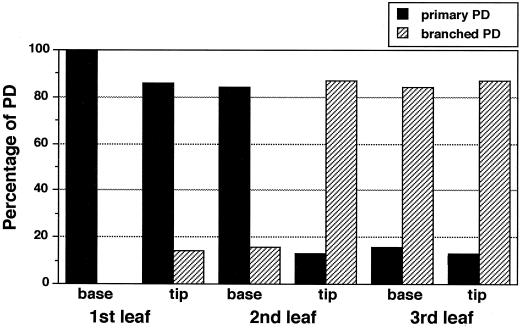
Because maturation of a tobacco leaf progresses from the tip to the base regions (Turgeon, 1989), the tip region in a leaf at an early stage of development will be more developed than the base region. Previous work with mesophyll tissue of tobacco showed that modification of primary plasmodesmata into complex secondary plasmodesmata also progresses from tip to base in a developing leaf (Ding et al., 1992). Therefore, we studied the structure of epidermal plasmodesmata in both the tip and the base regions of each experimental leaf. As shown in Figures 2A and 3, all plasmodesmata connecting epidermal cells in the base of the first leaf were simple primary plasmodesmata. In the tip of this leaf, although approximately 86% of the plasmodesmata were still primary, 14% had become branched (Figs. 2, B and C, and 3). These branched plasmodesmata were either H or Y shaped (Fig. 2C) and had no central cavity. The H-shaped plasmodesmata were apparently derived from the fusion of two neighboring primary plasmodesmata and were previously called “modified primary plasmodesmata” (Ding et al., 1993). The Y-shaped plasmodesmata most likely came from the de novo addition of a new cytoplasmic strand to a primary plasmodesmata across the existing cell walls.
Figure 2.
Structure and development of plasmodesmata between epidermal cells during tobacco leaf maturation. A, Primary plasmodesmata (arrows) in the base of the first leaf (see Fig. 1 for numbering of leaves). B, Primary plasmodesmata in the tip of the first leaf. C, H- and Y-shaped branched plasmodesmata in the tip of the first leaf. D, Primary plasmodesmata in the base of the second leaf. E, Primary (arrows) and branched (arrowhead) plasmodesmata in the base of the second leaf. F, Primary (arrow) and branched (arrowhead) plasmodesmata in the tip of the second leaf. G, Branched plasmodesmata in the tip of the second leaf. H, Branched plasmodesmata in the base of the third leaf. I, Branched plasmodesmata in the tip of the third leaf. Note the presence of a central cavity in the plasmodesmata (arrow). J, Truncated plasmodesmata between an epidermal cell (EC) and a mature guard cell (GC). Scale bars = 0.5 μm.
Figure 3.
Distribution of primary versus branched plasmodesmata (PD) between epidermal cells during tobacco leaf development. Total numbers of plasmodesmata examined for the different leaf regions were 315 for the base of first leaf, 474 for the tip of first leaf, 547 for the base of second leaf, 559 for the tip of second leaf, 380 for the base of third leaf, and 480 for the tip of third leaf. The plasmodesmata were counted from 200 to 300 cellular interfaces derived from two different tobacco plants.
In the base of the second leaf, approximately 84% of the plasmodesmata were primary and 16% were either H or Y shaped (Figs. 2, D and E, and 3). This pattern was similar to that found in the tip of the first leaf. In the tip of the second leaf, primary plasmodesmata were only about 13% of the total, and 87% of the plasmodesmata had become branched. In contrast to the H- or Y-shaped branched plasmodesmata found in the tip of the first leaf and at the base of the second leaf, a prominent feature of the branched plasmodesmata in the tip of the second leaf was that multiple branches (more than three in many cases) were confluent at a central cavity in the middle lamellar region of the cell walls (Fig. 2, F and G). This structural feature and distribution pattern of the branched plasmodesmata were maintained throughout leaf development. As shown in Figures 2, H and I, and 3, structures and percentages of branched plasmodesmata in the base and tip of the third leaf were very similar to those found at the tip of the second leaf. Thus, modification of primary plasmodesmata into highly branched plasmodesmata occurred most dramatically and appeared to reach a maximal level in the tip of the second leaf.
Based on the data presented above, the highly branched plasmodesmata between tobacco epidermal cells were apparently formed utilizing similar mechanisms, as found for the formation of complex secondary plasmodesmata between mesophyll cells (Ding et al., 1992, 1993). Following the terminology used for tobacco mesophyll (Ding et al., 1992, 1993; Ding and Lucas, 1996; Ding, 1998), we will refer to the highly branched plasmodesmata found in the tip of the second leaf and older leaves as “complex secondary plasmodesmata.” The conditions and limitations of the usage of this term have been discussed previously (Ding et al., 1992; Ding, 1998). The full development of complex secondary plasmodesmata from the tip of the second leaf onward was directly correlated with the ability of these leaf tissues to allow plasmodesmal localization and cell-to-cell trafficking of 3a MP:GFP, as discussed below.
Truncated plasmodesmata were observed on an epidermal cell side that abutted a mature guard cell (Fig. 2J). Whether these plasmodesmata had been formed previously or they were formed after the plasmodesmata on the guard cell side were completely sealed during leaf maturation was not investigated. Nevertheless, our observation that truncated plasmodesmata exist between epidermal and mature guard cells is consistent with earlier findings by Willmer and Sexton (1979) and Willie and Lucas (1984) in tobacco and several other species. 3a MP:GFP was found to be targeted to these plasmodesmata in a transgenic tobacco plant (see below).
CMV 3a MP:GFP Fusion Protein Was Localized to Complex Secondary Plasmodesmata, and Not Primary Plasmodesmata, during Leaf Development in Transgenic Tobacco
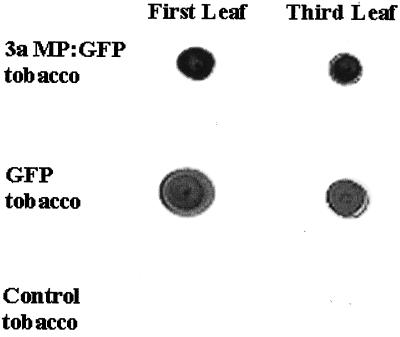
To determine how 3a MP:GFP would interact with plasmodesmata during leaf development, we generated transgenic tobacco plants expressing the CMV 3a:GFP fusion gene under the control of the constitutive CaMV 35S promoter (see Methods for details). Transgenic line AI3a:GFP1 showed a higher level of 3a MP:GFP fluorescence than other lines and was therefore chosen for detailed study. The presence of the fusion protein in leaves of different developmental stages was also confirmed by immunoblot analysis, as shown in Figure 4.
Figure 4.
Immunoblot analysis showing the presence of 3a MP:GFP and GFP in transgenic tobacco plants expressing these proteins. The control is a nontransgenic tobacco plant. The fusion protein was detected with a rabbit-derived polyclonal antibody against GFP and then with a goat-derived anti-rabbit IgG antibody conjugated to alkaline phosphatase.
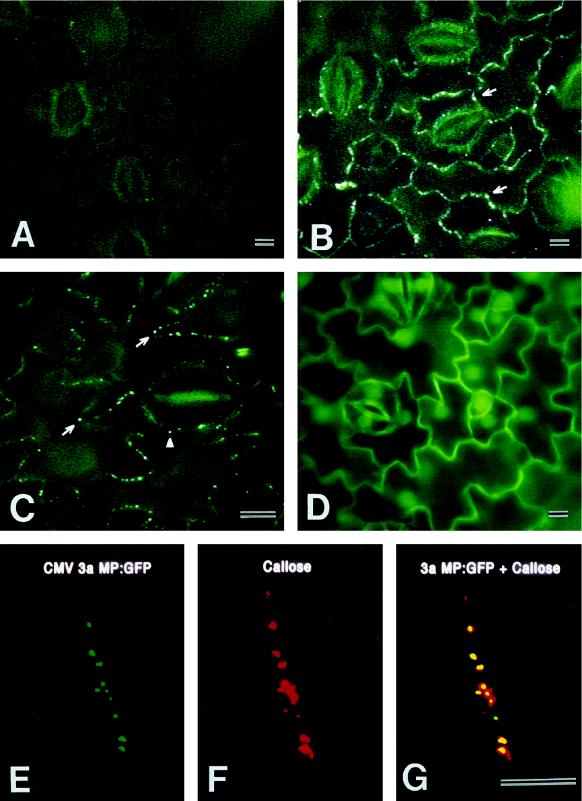
Detailed analysis of AI3a:GFP1 plants revealed the appearance of distinct green fluorescent dots in the cell walls of epidermis and mesophyll as a function of leaf development. In the base of the second leaf (Fig. 5A) and the entire first leaf (data not shown), there were no green fluorescent dots in the cell walls of epidermal cells overlaying the mesophyll. In the tip of the second leaf, green fluorescent dots started to appear in the walls of epidermal cells (Fig. 5B). In the third (Fig. 5C) and older leaves, green fluorescent dots were detected in the epidermal cell walls from the tip to the base region of every leaf. Some fluorescent dots also appeared to be present in the cell walls adjoining epidermal and guard cells (Fig. 5C, arrowhead). Transgenic tobacco plants producing GFP alone did not exhibit any fluorescent dots in the cell walls of any leaves (Fig. 5D). GFP accumulated in the nucleus and cytoplasm of every cell.
Figure 5.
Localization of CMV 3a MP:GFP to plasmodesmata during leaf development in a transgenic tobacco plant (AI3a:GFP1). A, Base of second leaf. Green fluorescence is visible in the cytoplasm of all epidermal and guard cells. There are no fluorescent dots in the cell walls. The first leaf showed the same image. B, Tip of the same second leaf as in A. Green fluorescent dots (arrows) are present in the cell walls between epidermal cells. C, Tip of third leaf. Green fluorescent dots (arrows) are prominent between all epidermal cells. In addition, such dots are also visible in the part of epidermal cell wall abutting a mature guard cell (arrowhead). D, Tip of third leaf from a transgenic tobacco plant expressing the GFP gene only. GFP fluorescence is prominent in all epidermal and guard cells and accumulates in the nucleus of every cell. Note the absence of green fluorescent dots in the walls between the cells. E, Confocal image of 3a MP:GFP fluorescent dots in walls between mesophyll cells in the tip of a third leaf. This image was obtained from a cryosection of leaf tissue. F, Confocal image of callose in the same cryosection, detected with a monoclonal anti-callose antibody and a Texas Red-conjugated secondary antibody. G, Superimposed confocal images of E and F demonstrating colocalization of 3a MP:GFP and callose. Scale bars = 40 μm.
We performed immunolabeling studies with transgenic tobacco plants to determine whether the green fluorescent dots of 3a MP:GFP in the cell walls represented localization of the fusion protein in plasmodesmata. Since callose is typically deposited in the cell wall area immediately surrounding the orifice of a plasmodesmata, especially when a tissue is wounded or during aldehyde fixation (Hughes and Gunning, 1980; Northcote et al., 1989; Vaughn et al., 1996), a colocalization of callose and green fluorescent dots would indicate that the latter represent 3a MP:GFP in plasmodesmata. This strategy has been used to localize TMV MP:GFP to plasmodesmata in tobacco infected with an engineered TMV (Oparka et al., 1997) and 3a MP:GFP to plasmodesmata in tobacco infected with potato virus X expressing a 3a:GFP fusion gene (Blackman et al., 1998).
Using a monoclonal antibody against callose (1,3-β-glucan) and a Texas Red-conjugated secondary antibody to label cryosections, we localized callose to the cell walls as punctate dots in the epidermis and mesophyll of the 3a MP:GFP-transgenic tobacco plants. Confocal microscopic examination of the distribution patterns of 3a MP:GFP fluorescent dots and callose staining on any given section indicated that 3a MP:GFP and callose were colocalized in most cases (Fig. 5, E–G).
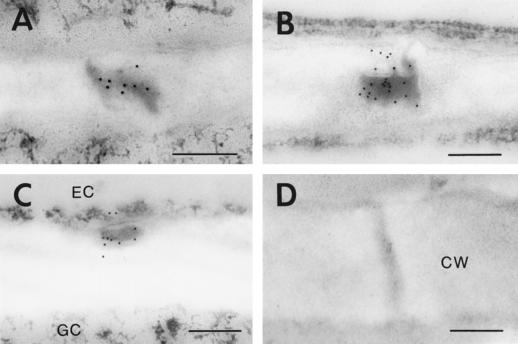
Our data therefore demonstrated that the green fluorescent dots in the cell walls were 3a MP:GFP localized to plasmodesmata. Furthermore, 3a MP:GFP localization to plasmodesmata was a function of leaf development in transgenic tobacco plants. Specifically, this pattern suggests that 3a MP:GFP might be localized to complex secondary plasmodesmata, as TMV MP does in the mesophyll of transgenic tobacco plants (Ding et al., 1992). To test this possibility, we performed further immunolabeling experiments at the electron microscope level using a rabbit-derived polyclonal antibody (IgG) against CMV 3a MP and a goat-derived, 10-nm gold-conjugated secondary antibody against rabbit IgG. By this method, we localized 3a MP:GFP to complex secondary plasmodesmata in the mesophyll and epidermal cells of transgenic leaves (Fig. 6, A and B). In addition, 3a MP:GFP was localized to the truncated plasmodesmata between guard cells and epidermal cells (Fig. 6C). 3a MP:GFP was absent from primary plasmodesmata in the third (Fig. 6D) and younger (data not shown) leaves. Therefore, we concluded that the green fluorescent dots observed at the fluorescence microscope level represented localization of the 3a MP:GFP specifically to complex secondary plasmodesmata.
Figure 6.
Immunolabeling of CMV 3a MP:GFP fusion protein to complex secondary plasmodesmata in transgenic tobacco plants. The fusion protein was detected with a polyclonal anti-CMV 3a MP antibody and a 10-nm gold-conjugated secondary antibody. A, Complex secondary plasmodesmal between two mesophyll cells showing gold labeling. B, Complex secondary plasmodesmal between two epidermal cells showing gold labeling. C, Truncated plasmodesmal between an epidermal cell (EC) and a guard cell (GC) showing gold labeling. D, Primary plasmodesmata from a third leaf showing no gold labeling. CW, Cell wall. Scale bars = 0.25 μm.
Cell-to-Cell Trafficking of CMV 3a MP:GFP Fusion Protein in Tobacco Leaf Epidermis Was Correlated with the Development of Complex Secondary Plasmodesmata during Leaf Maturation
Having established that 3a MP:GFP was localized to complex secondary plasmodesmata as a function of leaf development in transgenic tobacco plants, we then asked whether this localization pattern was indicative of the capabilities of primary and/or complex secondary plasmodesmata to facilitate 3a MP:GFP cell-to-cell trafficking in tobacco leaves. To answer this question, we used a biolistic bombardment method to deliver plasmid pRTL2–3a:GFP, which carries the 3a:GFP fusion gene under the control of the CaMV 35S promoter (Itaya et al., 1997) into the epidermis of nontransgenic tobacco leaves at successive developmental stages to produce 3a MP:GFP fusion protein and then monitored its trafficking or nontrafficking function.
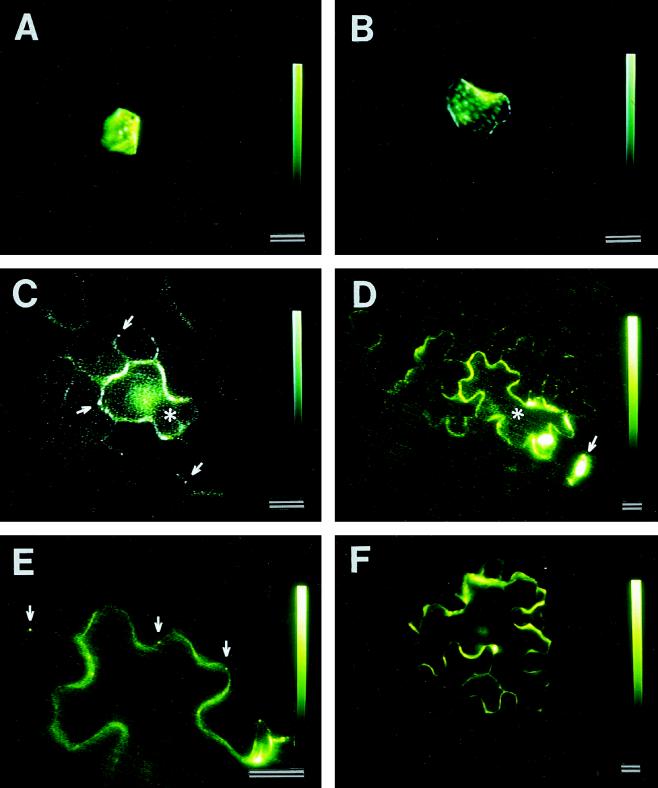
When the plasmid was bombarded into either the base or the tip of the first leaf, the 3a MP:GFP fusion protein was produced in epidermal cells. The green fluorescence was confined to the cytoplasm. There were no fluorescent dots in the cell walls, indicating that the fusion protein was not targeted to plasmodesmata. The fusion protein also did not move into neighboring cells (Fig. 7A; Table I).
Figure 7.
Cell-to-cell trafficking of CMV 3a MP:GFP in tobacco leaf epidermis as a function of leaf development. The fusion protein was produced upon biolistic bombardment of the 3a:GFP fusion gene. A, 3a MP:GFP produced in an epidermal cell in the tip of the first leaf, which does not traffic into neighboring cells. B, 3a MP:GFP produced in an epidermal cell in the base of the second leaf, which also remains in a single cell. C, 3a MP:GFP produced in an epidermal cell (asterisk) in the tip of the second leaf, which trafficks into neighboring cells. Note the presence of green fluorescent dots in the walls of the cell producing the fusion protein and in neighboring cells (arrows). D, 3a MP:GFP produced in the base of the third leaf, which trafficks from cell to cell. The asterisk denotes the cell producing the protein. The arrow points to a guard cell that produces 3a MP:GFP but does not permit cell-to-cell trafficking of the fusion protein. E, High-magnification view of D showing the presence of fluorescent dots in the cell walls. F, 3a MP:GFP produced in an epidermal cell in the base of the third leaf. The fusion protein was not targeted to complex secondary plasmodesmata, as determined by the lack of green fluorescent dots in the cell walls. The protein does not traffic into neighboring cells. Scale bars = 20 μm.
Table I.
Cell-to-cell trafficking of CMV 3a MP:GFP fusion protein in the epidermis of tobacco
| Leaf No. | Leaf Section | 3a MP:GFP | 3a MP (M5):GFP | GFP |
|---|---|---|---|---|
| Onea | 2 /174 | 0 /40 | 0 /200 | |
| Two | Base | 0 /34 | –b | – |
| Tip | 9 /20 | – | – | |
| Three | Base | 14 /25 | 0 /53 | 0 /75 |
| Tip | 7 /13 | – | – |
The proteins (fusion or nonfusion) were produced in planta after plasmids containing the respective genes were delivered into the tobacco epidermis by a particle gun. Three to four leaves from different plants were used in each experiment, except for the first leaf, for which 10 samples were used. The data are the number of cells permitting cell-to-cell trafficking of a protein over the total cells producing the protein.
For the first leaf, identical results were obtained when the genes were bombarded into either the tip or the base regions, so the data were pooled.
–, Not done.
In the base of the second leaf the 3a MP:GFP produced in a cell was not targeted to plasmodesmata and did not traffic out of the cell (Fig. 7B; Table I). In contrast, the fusion protein produced in the tip of the same leaf was targeted to plasmodesmata, as manifested by the presence of fluorescent dots in the cell walls, and trafficked from cell to cell (Fig. 7C; Table I). In the third leaf, 3a MP:GFP was targeted to plasmodesmata and was able to traffic from cell to cell in both the base (Fig. 7, D and E) and the tip regions (Table I).
The cell-to-cell trafficking pattern of 3a MP:GFP in nontransgenic tobacco plants was positively correlated with the localization pattern of 3a MP:GFP in transgenic plants. This fusion protein was not localized to and did not traffic through primary plasmodesmata in young leaves. At a leaf developmental stage at which complex secondary plasmodesmata became predominant (the tip of the second leaf in the present study), the fusion protein was localized to complex secondary plasmodesmata and trafficked from cell to cell. Therefore, the formation of complex secondary plasmodesmata appears to be necessary for 3a MP:GFP cell-to-cell trafficking.
In control experiments GFP alone or GFP fused to mutant M5 of 3a MP, which was shown previously to be incapable of trafficking from cell to cell (Itaya et al., 1997), did not traffic from cell to cell in either first or third leaves (Table I). These results confirmed that cell-to-cell trafficking of 3a MP:GFP during tobacco leaf development was a specific function mediated by 3a MP.
Targeting to Complex Secondary Plasmodesmata Was Required for CMV 3a MP:GFP Trafficking
When 3a MP:GFP trafficked from cell to cell in the tip of the second leaf and the entire third leaf, green fluorescent dots were observed in the walls of cells producing this fusion protein and the surrounding cells—an indication that the fusion protein was targeted to complex secondary plasmodesmata. The fusion protein never trafficked out of a cell that did not exhibit green fluorescent dots in the cell walls of these leaves (Fig. 7F). Because our extensive electron microscopic studies revealed that complex secondary plasmodesmata predominantly connected all epidermal cells in these leaves, the lack of fluorescent dots in a cell such as that shown in Figure 7F indicates that factors yet to be identified contributed to the lack of targeting of 3a MP:GFP to complex secondary plasmodesmata. Therefore, targeting to complex secondary plasmodesmata is apparently required for 3a MP:GFP intercellular trafficking.
DISCUSSION
We have demonstrated that the ability of CMV 3a MP:GFP to interact with plasmodesmata and to traffic between tobacco leaf epidermal cells is a function of leaf development. Specifically, our data indicated that 3a MP:GFP does not interact with and traffic cell to cell through primary plasmodesmata in young tobacco leaves, and yet the same fusion protein is able to interact with and traffic through complex secondary plasmodesmata formed during further leaf development. Primary and complex secondary plasmodesmata therefore have different protein-trafficking functions. It is unlikely that such differences are significant for only the intercellular trafficking of a viral protein.
We suggest that primary and complex secondary plasmodesmata can differentially traffic endogenous macromolecules that are critical for specific stages of plant development. This is consistent with the view that complex secondary plasmodesmata are probably important for later stages of leaf development, based on the observation that arrested formation of complex secondary plasmodesmata in the mesophyll of a transgenic tobacco plant expressing an acid invertase is correlated with accelerated leaf senescence (Ding et al., 1993). Because modification of primary plasmodesmata into complex secondary plasmodesmata appears to be a general phenomenon associated with plant development (Jones, 1976; Ding et al., 1992, 1993; Ding and Lucas, 1996; Volk et al., 1996), elucidating the specific functions of such modifications should enhance our understanding of cell-to-cell communication in plant development.
Vaquero et al. (1996) previously showed immunolocalization of CMV 3a MP in the cell walls, and presumably to plasmodesmata, in CMV-infected as well as in CMV 3a MP-transgenic tobacco plants. Blackman et al. (1998) showed clear and conclusive evidence for plasmodesmatal localization of 3a MP during CMV infection and of 3a MP:GFP expressed from a potato virus X vector. Those studies, however, did not distinguish between primary and complex secondary plasmodesmata and did not examine the localization pattern of 3a MP or 3a MP:GFP as a function of leaf development. Our data extended those observations by showing that 3a MP:GFP is localized exclusively to complex secondary plasmodesmata, not to primary plasmodesmata of young or mature leaves of transgenic tobacco plants. This pattern of localization is similar to that of TMV MP in transgenic tobacco (Ding et al., 1992). Thus, preferential interaction with complex secondary plasmodesmata is not a peculiar feature of a particular protein (TMV MP or CMV 3a MP); rather, it is likely a function shared by many proteins. These pooled data further support the view that primary and complex secondary plasmodesmata have unique trafficking functions that are of broad biological significance.
The molecular basis underlying the different abilities of primary and complex secondary plasmodesmata to allow 3a MP:GFP to traffic from cell to cell remains to be elucidated. There are a number of possibilities. First, during leaf development, modification of primary plasmodesmata into complex secondary plasmodesmata is accompanied by changes in the biochemical composition of these plasmodesmata, so complex secondary plasmodesmata may possess the factor (receptor?) that is necessary for 3a MP:GFP trafficking. Second, a cytosolic factor that recognizes and shuttles 3a MP:GFP to plasmodesmata is expressed only at certain stages of leaf development. It may be this factor, and not plasmodesmata structure or biochemical components, that dictates developmental regulation of 3a MP:GFP trafficking. Third, specific interactions among 3a MP:GFP, a cytosolic factor, and a plasmodesmal component are necessary for trafficking, and both the necessary cytosolic factor and the plasmodesmal component are expressed only at a certain leaf developmental stage. Irrespective of the specific mechanisms involved, our data suggest that tobacco leaf development may provide a useful system for biochemical and molecular characterization of cellular factors that participate in intercellular protein trafficking through plasmodesmata.
In search of cellular factors essential for plasmodesma-mediated protein trafficking, it should be noted that a putative protein kinase that can phosphorylate the TMV 30-kD MP has been detected in the cell walls of tobacco leaf mesophyll. In particular, the activity of this kinase is also a function of tobacco leaf development, being absent from young leaves and present in more mature leaves (Citovsky et al., 1993). Thus, there is a possibility that this kinase is a component of complex secondary plasmodesmata in tobacco leaves (Citovsky et al., 1993). Citovsky et al. (1993) speculated that phosphorylation of TMV MP may lead to its inactivation and then accumulation in complex secondary plasmodesmata in transgenic tobacco plants, thereby sequestering this protein to this location (possibly as a plant defense mechanism).
The data in the present study suggest the possibility that, contrary to the speculation of Citovsky et al. (1993), the phosphorylation of an MP such as CMV 3a MP, and perhaps even TMV MP, is required for intercellular trafficking. Accumulation of 3a MP:GFP and TMV MP (Ding et al., 1992) in complex secondary plasmodesmata might therefore be due to a different type of protein modification. Alternatively, phosphorylation of an MP may lead to its accumulation in a complex secondary plasmodesmata, as suggested by Citovsky et al. (1993), and a different mechanism is responsible for the trafficking of MP through the plasmodesmata. Further experimental studies in this direction should provide important insights into the mechanisms that regulate intercellular protein trafficking through plasmodesmata.
Targeting of 3a MP:GFP to truncated plasmodesmata between the epidermal and mature guard cells was unexpected, because these plasmodesmata apparently do not have intercellular transport functions (Erwee et al., 1985; Palevitz and Hepler, 1985; Ding et al., 1997). Nevertheless, this finding implies that protein targeting to plasmodesmata is an autonomous function of a cell and is not necessarily coupled to protein translocation through plasmodesmata or protein release from plasmodesmata for import into a neighboring cell. Thus, targeting to and release from plasmodesmata may be separate steps in intercellular protein trafficking that require specific interactions between the trafficking protein and dedicated cellular factors (Carrington et al., 1996; Ding, 1998).
Although our investigation was not specifically aimed at providing a mechanistic explanation of how CMV moves from cell to cell, the finding that 3a MP:GFP does not traffic from cell to cell in a young leaf raises the question of how a virus such as CMV, which can infect young leaves (Cooper and Dodds, 1995; Ding et al., 1995), moves intercellularly. Based on the observation that CMV virions are absent from plasmodesmata in infected tobacco cells and that 3a MP can traffic CMV RNA from cell to cell, Ding et al. (1995) suggested that CMV moves from cell to cell as a ribonucleoprotein complex. CMV replicase (Gal-On et al., 1994) and coat protein (Suzuki et al., 1991; Boccard and Baulcombe, 1993; Canto et al., 1997) also appear to be required for CMV cell-to-cell movement. Blackman et al. (1998) recently proposed that a CMV ribonucleoprotein complex that moves from cell to cell consists of CMV RNA, 3a MP, and coat protein. Thus, it is possible that CMV 3a MP will traffic from cell to cell through primary plasmodesmata in young leaves in the presence of other viral factors under infection conditions and that such trafficking is necessary for viral genome trafficking. Alternatively, cell-to-cell trafficking of 3a MP can enhance viral genome trafficking but is not absolutely required. These possibilities need to be tested in future studies.
Canto et al. (1997) observed cell-to-cell spreading of biolistically produced GFP in both N. tabacum and Nicotiana benthamiana leaf epidermis. We have been unable to confirm that finding with our extensive experiments with tobacco. In the study of Canto et al. (1997), the spreading of free GFP from cell to cell was observed 5 d after bombardment. In our experiments we could detect GFP fluorescence approximately 9 to 10 h after plasmid bombardment. GFP fluorescence remained in single cells for 3 to 7 d, depending on the cells, and then disappeared completely. Our preliminary studies in Arabidopsis and cucumber indicate that biolistically produced GFP can indeed spread from cell to cell in some tissues (A. Itaya, Y.-M. Woo, and B. Ding, unpublished results); therefore, cell-to-cell spreading of free GFP may be associated with a particular plant species, tissue, growth condition, or development stage.
Our transgenic tobacco plants expressing 3a MP:GFP may have other applications. For instance, such plants may be used to map development of complex secondary plasmodesmata at the light microscope level by detecting the presence of 3a MP:GFP fluorescence dots in the cell walls. This should make it possible to examine the effects of various environmental and physiological factors on complex secondary plasmodesma formation in tobacco leaves. Since these plants now contain an endogenous and visible marker for complex secondary plasmodesmata, they may also be used to test whether a putative plasmodesmal protein is localized to plasmodesmata in immunolabeling studies at the light microscope level. This might be especially useful when the immunolabeling intensity is limited at the electron microscope level.
ACKNOWLEDGMENTS
We thank Peter Palukaitis for providing plasmids pET-MP3a and pET-MPm5 and James Carrington for plasmid pRTL2. We thank John Cushman, Yinghua Huang, Yi Li, and Zhenbiao Yang for technical advice concerning the generation of transgenic plants. David Meinke is acknowledged with special thanks for making his equipment available to us. We thank John Cushman, SueAnn Hudiberg, and Janet Rogers at the Oklahoma State University Recombinant DNA/Protein Resources Facility for the synthesis and purification of oligonucleotides, DNA sequencing, and use of equipment. We are grateful to Christine Fry of Nikon Corporation for loaning us oil lenses and a mercury lamphouse, and to Phoebe Doss at the Oklahoma State University Electron Microscopy Laboratory for the use of the electron microscope and for technical assistance. Finally, we thank Mark Sanders of the Imaging Center, Department of Genetics and Cell Biology, University of Minnesota at St. Paul for the use of the confocal microscope.
Abbreviations:
- CaMV
cauliflower mosaic virus
- CMV
cucumber mosaic virus
- GFP
green fluorescent protein
- MP
movement protein
- TMV
tobacco mosaic virus
Footnotes
This study was supported by a grant from The Samuel Roberts Noble Foundation (to B.D. and R.S.N.), by a grant from the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (no. 97-35303-4519 to B.D.), and by a Dean's Incentive Grant from the College of Arts and Sciences, Oklahoma State University (to B.D.).
LITERATURE CITED
- Balachandran S, Xiang Y, Schobert C, Thompson GA, Lucas WJ. Phloem sap proteins from Cucurbita maxima and Ricinus communis have the capacity to traffic cell to cell through plasmodesmata. Proc Natl Acad Sci USA. 1997;94:14150–14155. doi: 10.1073/pnas.94.25.14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman LM, Boevink P, Santa Cruz S, Palukaitis P, Oparka KJ. The movement protein of cucumber mosaic virus traffics into sieve elements in minor veins of Nicotiana clevelandii. Plant Cell. 1998;10:525–537. doi: 10.1105/tpc.10.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccard F, Baulcombe D. Mutational analysis of cis-acting sequences and gene function in RNA3 of cucumber mosaic virus. Virology. 1993;193:563–578. doi: 10.1006/viro.1993.1165. [DOI] [PubMed] [Google Scholar]
- Bostwick DE, Dannenhoffer JM, Skaggs MI, Lister RM, Larkins BA, Thompson GA. Pumpkin phloem lectin genes are specifically expressed in companion cells. Plant Cell. 1992;4:1539–1548. doi: 10.1105/tpc.4.12.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto T, Prior DAM, Hellward KH, Oparka KJ, Palukaitis P. Characterization of cucumber mosaic virus. 4. Movement protein and coat proteins are both essential for cell-to-cell movement of cucumber mosaic virus. Virology. 1997;237:237–248. doi: 10.1006/viro.1997.8804. [DOI] [PubMed] [Google Scholar]
- Carrington JC, Kasschau KD, Mahajan SK, Schaad MC. Cell-to-cell and long-distance transport of viruses in plants. Plant Cell. 1996;8:1669–1681. doi: 10.1105/tpc.8.10.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V, McLean BG, Zupan JR, Zambryski P. Phosphorylation of tobacco mosaic virus cell-to-cell movement protein by a developmentally regulated plant cell wall-associated protein kinase. Genes Dev. 1993;7:904–910. doi: 10.1101/gad.7.5.904. [DOI] [PubMed] [Google Scholar]
- Clark AM, Jacobsen KR, Bostwick DE, Dannenhoffer JM, Skaggs MI, Thompson GA. Molecular characterization of a phloem-specific gene encoding the filament protein, phloem protein 1 (PP1), from Cucurbita maxima. Plant J. 1997;12:49–61. doi: 10.1046/j.1365-313x.1997.12010049.x. [DOI] [PubMed] [Google Scholar]
- Cooper B, Dodds JA. Differences in the subcellular localization of tobacco mosaic virus and cucumber mosaic virus movement proteins in infected and transgenic plants. J Gen Virol. 1995;76:3217–3221. doi: 10.1099/0022-1317-76-12-3217. [DOI] [PubMed] [Google Scholar]
- Deom CM, Schubert K, Wolf S, Holt C, Lucas WJ, Beachy RN. Molecular characterization and biological function of the movement protein of tobacco mosaic virus in transgenic plants. Proc Natl Acad Sci USA. 1990;87:3284–3288. doi: 10.1073/pnas.87.9.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B. Cell-to-cell transport of macromolecules through plasmodesmata: a novel signalling pathway in plants. Trends Cell Biol. 1997;7:5–9. doi: 10.1016/S0962-8924(97)20041-3. [DOI] [PubMed] [Google Scholar]
- Ding B. Intercellular protein trafficking through plasmodesmata. Plant Mol Biol. 1998;38:279–310. [PubMed] [Google Scholar]
- Ding B, Haudenshield JS, Hull RJ, Wolf S, Beachy RN, Lucas WJ. Secondary plasmodesmata are specific sites of localization of the tobacco mosaic virus movement protein in transgenic tobacco plants. Plant Cell. 1992;4:915–928. doi: 10.1105/tpc.4.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Haudenshield JS, Willmitzer L, Lucas WJ. Correlation between arrested secondary plasmodesmal development and onset of accelerated leaf senescence in yeast acid invertase transgenic tobacco plants. Plant J. 1993;4:179–189. doi: 10.1046/j.1365-313x.1993.04010179.x. [DOI] [PubMed] [Google Scholar]
- Ding B, Itaya A, Woo Y-M (1999) Plasmodesmata and cell-to-cell communication in plants. Int Rev Cytol (in press)
- Ding B, Kwon M-O, Hammond R, Owens R. Cell-to-cell movement of potato spindle tuber viroid. Plant J. 1997;12:931–936. doi: 10.1046/j.1365-313x.1997.12040931.x. [DOI] [PubMed] [Google Scholar]
- Ding B, Li Q-b, Nguyen L, Palukaitis P, Lucas WJ. Cucumber mosaic virus 3a protein potentiates cell-to-cell trafficking of CMV-vRNA in tobacco plants. Virology. 1995;207:345–353. doi: 10.1006/viro.1995.1093. [DOI] [PubMed] [Google Scholar]
- Ding B, Lucas WJ (1996) Secondary plasmodesmata: biogenesis, special functions, and evolution. In M Smallwood, P Knox, D Bowles, eds, Membranes: Specialized Functions in Plants. BIOS Scientific Publishers, Inc., Oxford, UK, pp 489–506
- Erwee MG, Goodwin PB, Van Bel AJE. Cell-cell communication in the leaves of Commelina cyanea and other plants. Plant Cell Environ. 1985;8:173–178. [Google Scholar]
- Fisher D, Wu Y, Ku MSB. Turnover of soluble proteins in the wheat sieve tube. Plant Physiol. 1992;100:1433–1441. doi: 10.1104/pp.100.3.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Giesmann-Cookmeyer D, Ding B, Lommel SA, Lucas WJ. Cell-to-cell trafficking of macromolecules through plasmodesmata potentiated by the red clover necrotic mosaic virus movement protein. Plant Cell. 1993;5:1783–1794. doi: 10.1105/tpc.5.12.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal-On A, Kaplan I, Roossinck MJ, Palukaitis P. The kinetics of infection of zucchini squash by cucumber mosaic virus indicates a function for RNA 1 in virus movement. Virology. 1994;205:280–289. doi: 10.1006/viro.1994.1644. [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry J, Hoffmann NL, Wallroth M, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Hughes JE, Gunning BES. Glutaraldehyde-induced deposition of callose. Can J Bot. 1980;58:250–258. [Google Scholar]
- Ishiwatari Y, Honda C, Kawashima I, Nakamura S-i, Hirano H, Mori S, Fujiwara T, Hayashi H, Chino M. Thioredoxin h is one of the major proteins in rice phloem sap. Planta. 1995;195:456–463. doi: 10.1007/BF00202605. [DOI] [PubMed] [Google Scholar]
- Itaya A, Hickman H, Bao Y, Nelson R, Ding B. Cell-to-cell trafficking of cucumber mosaic virus movement protein: green fluorescent protein fusion produced by biolistic gene bombardment in tobacco. Plant J. 1997;12:1223–1230. [Google Scholar]
- Jackson D, Hake S. Morphogenesis on the move: cell-to-cell trafficking of plant regulatory proteins. Curr Opin Genet Dev. 1997;7:495–500. doi: 10.1016/s0959-437x(97)80076-7. [DOI] [PubMed] [Google Scholar]
- Jackson D, Veit B, Hake S. Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development. 1994;120:405–413. [Google Scholar]
- Jones MGK. The origin and development of plasmodesmata. In: Gunning BES, Robards RW, editors. Intercellular Communication in Plants: Studies on Plasmodesmata. Berlin: Springer-Verlag; 1976. pp. 81–105. [Google Scholar]
- Kühn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB. Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science. 1997;275:1298–1300. doi: 10.1126/science.275.5304.1298. [DOI] [PubMed] [Google Scholar]
- Lucas WJ. Plasmodesmata: intercellular channels for macromolecular transport in plants. Curr Opin Cell Biol. 1995;7:673–680. doi: 10.1016/0955-0674(95)80109-x. [DOI] [PubMed] [Google Scholar]
- Lucas WJ, Bouche-Pillon S, Jackson DP, Nguyen L, Baker L, Ding B, Hake S. Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science. 1995;270:1980–1983. doi: 10.1126/science.270.5244.1980. [DOI] [PubMed] [Google Scholar]
- Lucas WJ, Ding B, Van der Schoot C. Plasmodesmata and the supracellular nature of plants. New Phytol. 1993;125:435–476. doi: 10.1111/j.1469-8137.1993.tb03897.x. [DOI] [PubMed] [Google Scholar]
- Mezitt LA, Lucas WJ. Plasmodesmal cell-to-cell transport of proteins and nucleic acids. Plant Mol Biol. 1996;32:251–273. doi: 10.1007/BF00039385. [DOI] [PubMed] [Google Scholar]
- Moore PJ, Fenczik CA, Deom CM, Beach RN. Developmental changes in plasmodesmata in transgenic tobacco expressing the movement protein of tobacco mosaic virus. Protoplasma. 1992;170:115–127. [Google Scholar]
- Murillo I, Cavallarin L, Segundo BS. The maize pathogenesis-related PRms protein localizes to plasmodesmata in maize radicles. Plant Cell. 1997;9:145–156. doi: 10.1105/tpc.9.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Hayashi H, Mori S, Chino M. Protein phosphorylation in the sieve tubes of rice plants. Plant Cell Physiol. 1993;34:927–933. [Google Scholar]
- Nguyen L, Lucas WJ, Ding B, Zaitlin M. Viral RNA trafficking is inhibited in replicase-mediated resistant transgenic tobacco plants. Proc Natl Acad Sci USA. 1996;93:12643–12647. doi: 10.1073/pnas.93.22.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcote DH, Davey R, Lay J. Use of antisera to localize callose, xylan and arabinogalactan in the cell plate, primary and secondary walls of plants cells. Planta. 1989;178:353–366. doi: 10.1007/BF00391863. [DOI] [PubMed] [Google Scholar]
- Noueiry AO, Lucas WJ, Gilbertson RL. Two proteins of a plant DNA virus coordinate nuclear and plasmodesmal transport. Cell. 1994;76:925–932. doi: 10.1016/0092-8674(94)90366-2. [DOI] [PubMed] [Google Scholar]
- Oparka KJ, Prior DAM, Santa Cruz S, Padgett HS, Beachy RN. Gating of epidermal plasmodesmata is restricted to the leading edge of expanding infection sites of tobacco mosaic virus (TMV) Plant J. 1997;12:781–789. doi: 10.1046/j.1365-313x.1997.12040781.x. [DOI] [PubMed] [Google Scholar]
- Palevitz BA, Hepler PK. Changes in dye coupling of stomatal cells of Allium and Commelina demonstrated by microinjection of Lucifer yellow. Planta. 1985;164:473–479. doi: 10.1007/BF00395962. [DOI] [PubMed] [Google Scholar]
- Perbal M-C, Haughn G, Saedler H, Schwarz-Sommer Z. Non-autonomous function of the Antirrhinum floral homeotic proteins DEFICIENS and GLOBOSA is exerted by their polar cell-to-cell trafficking. Development. 1996;122:3433–3441. doi: 10.1242/dev.122.11.3433. [DOI] [PubMed] [Google Scholar]
- Pope B, Kent HM. High efficiency 5 min transformation of Escherichia coli. Nucleic Acids Res. 1996;24:536–537. doi: 10.1093/nar/24.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas MR, Zerbini FM, Allison RF, Gilbertson RL, Lucas WJ. Capsid protein and helper component-proteinase function as potyvirus cell-to-cell movement proteins. Virology. 1997;237:283–295. doi: 10.1006/viro.1997.8777. [DOI] [PubMed] [Google Scholar]
- Sakuth T, Schobert C, Pecsvaradi A, Eichholz A, Komor E, Orlich G. Specific proteins in the sieve-tube exudate of Ricinus communis L. seedlings: separation, characterization and in vivo labelling. Planta. 1993;191:207–213. [Google Scholar]
- Sanford JC. The biolistic process. Trends Biotechnol. 1988;6:299–302. [Google Scholar]
- Schobert C, Grossmann P, Gottschalk M, Komor E, Pecsvaradi A, Nieden UZ. Sieve-tube exudate from Ricinus communis L. seedlings contains ubiquitin and chaperones. Planta. 1995;196:205–210. [Google Scholar]
- Spurr AR. A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kuwata S, Kataoka J, Masuta C, Nitta N, Takanami Y. Functional analysis of deletion mutants of cucumber mosaic virus RNA3 using an in vitro transcription system. Virology. 1991;183:106–113. doi: 10.1016/0042-6822(91)90123-s. [DOI] [PubMed] [Google Scholar]
- Turgeon R. Sink-source transition in leaves. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:119–138. [Google Scholar]
- Vaquero C, Sanz AI, Serra MT, García-Luque I. Accumulation kinetics of CMV RNA 3-encoded proteins and subcellular localization of the 3a protein in infected and transgenic tobacco plants. Arch Virol. 1996;141:987–999. doi: 10.1007/BF01718603. [DOI] [PubMed] [Google Scholar]
- Vaquero C, Turner AP, Demangeat G, Sanz A, Serra MT, Roberts K, García-Luque I. The 3a protein from cucumber mosaic virus increases the gating capacity of plasmodesmata in transgenic tobacco plants. J Gen Virol. 1994;75:3193–3197. doi: 10.1099/0022-1317-75-11-3193. [DOI] [PubMed] [Google Scholar]
- Vaughn KC, Hoffman JC, Hahn MG, Staehelin LA. The herbicide dichlobenil disrupts cell plate formation: immunogold characterization. Protoplasma. 1996;194:117–132. [Google Scholar]
- Volk GM, Turgeon R, Beebe DU. Secondary plasmodesmata formation in the minor-vein phloem of Cucumis melo L. and Cucurbita pepo L. Planta. 1996;199:425–432. [Google Scholar]
- Waigmann E, Lucas WJ, Citovsky V, Zambryski P. Direct functional assay for tobacco mosaic virus cell-to-cell movement protein and identification of a domain involved in increasing plasmodesmal permeability. Proc Natl Acad Sci USA. 1994;91:1433–1437. doi: 10.1073/pnas.91.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie AC, Lucas WJ. Ultrastructural and histochemical studies on guard cells. Planta. 1984;160:129–142. doi: 10.1007/BF00392861. [DOI] [PubMed] [Google Scholar]
- Willmer CM, Sexton R. Stomata and plasmodesmata. Protoplasma. 1979;100:113–124. [Google Scholar]