Background: GPCRs adopt multiple active states, resulting in pathway-biased signaling.
Results: A novel method for quantifying stimulus bias was applied to mutational analysis of the M2 muscarinic GPCR.
Conclusion: We established that aromatic residues in an allosteric site regulate the activation state of the receptor.
Significance: The combination of mutagenesis with statistical quantification of stimulus bias results in enriched structure-function relationships at GPCRs.
Keywords: Cell Signaling, Drug Discovery, G Protein-coupled Receptors (GPCR), Mutagenesis, Receptor Structure-Function, Biased Agonism, Functional Selectivity, Muscarinic Receptor
Abstract
A key characteristic of G protein-coupled receptors (GPCRs) is that they activate a plethora of signaling pathways. It is now clear that a GPCR coupling to these pathways can be regulated selectively by ligands that differentially drive signaling down one pathway in preference to another. This concept, termed stimulus bias, is revolutionizing receptor biology and drug discovery by providing a means of selectively targeting receptor signaling pathways that have therapeutic impact. Herein, we utilized a novel quantitative method that determines stimulus bias of synthetic GPCR ligands in a manner that nullifies the impact of both the cellular background and the “natural bias” of the endogenous ligand. By applying this method to the M2 muscarinic acetylcholine receptor, a prototypical GPCR, we found that mutation of key residues (Tyr-802.61 and Trp-993.28) in an allosteric binding pocket introduces stimulus bias in response to the atypical ligands AC-42 (4-n-butyl-1-(4-(2-methylphenyl)-4-oxo-1-butyl)piperidine HCl) and 77-LH-28-1 (1-(3-(4-butyl-1-piperidinyl)propyl)- 3,3-dihydro-2(1H)-quinolinone). By comparing stimulus bias factors among receptor internalization, G protein activation, extracellular-regulated protein kinase 1/2 (ERK1/2) signaling, and receptor phosphorylation, we provide evidence that Tyr-802.61 and Trp-993.28 act either as molecular switches or as gatekeeper residues that introduce constraints limiting the active conformation of the M2 muscarinic acetylcholine receptor and thereby regulate stimulus bias. Furthermore, we provide evidence that downstream signaling pathways previously considered to be related to each other (i.e. receptor phosphorylation, internalization, and activation of ERK1/2) can act independently.
Introduction
The GPCR5 superfamily is the largest family of cell surface receptors in the human genome (1). Because of their ubiquitous cell surface expression, interaction with multiple intracellular proteins, and contribution to a vast array of physiological and pathophysiological processes, GPCRs are commonly pursued in drug discovery programs and represent the target of ∼30% of all medicines (2). It has been suggested that much of GPCR signaling is mediated essentially by two processes. The first is classical coupling of the receptor to heterotrimeric G proteins, resulting in the activation of signaling cascades such as phospholipase C/calcium and adenylyl cyclase (3). In addition, GPCRs are considered to signal in a G protein-independent fashion to pathways such as mitogen-activated protein kinases, ERK1/2, and Jun kinase via receptor phosphorylation and interaction with arrestin adaptor proteins (4). Traditionally, a single active conformation of the receptor was thought to engage with each modality of this bimodal signaling mechanism (5). However, recent studies have indicated that this is not the case and that ligands can preferentially drive signaling down one arm of the bimodal pathway in preference to the other through the generation of multiple, distinct, active states (5–7). This concept has been variously termed biased agonism, functional selectivity, ligand-directed trafficking of receptor stimulus, or stimulus bias and has been used to account for the physiological/clinical properties of a number of GPCR ligands (8–11).
The fact that the physiological impact of numerous GPCR ligands has been attributed to stimulus bias reveals the potential of this concept in the development of ligands that can preferentially drive signaling to pathways that have therapeutic benefit. The importance of stimulus bias can extend further and impact on regulatory processes such as receptor internalization, down-regulation, and desensitization. There are reports, for instance, of ligands that are neutral antagonists or inverse agonists for acute signal transduction but are positive agonists for receptor regulation processes (12–16). Conversely, there is also evidence of positive agonists (with respect to acute signaling) that are incapable of inducing receptor GPCR internalization (17). Thus, it is evident that acute signaling efficacy (neutral, positive, or inverse) does not necessarily correlate with efficacy for GPCR regulation processes, and it is now acknowledged that an ability to quantify such differences in GPCR ligand functional selectivity can have a profound impact on drug discovery (18).
We have recently found that stimulus bias can be demonstrated at the M2 muscarinic acetylcholine receptor (mAChR), a prototypical family A GPCR, with respect to ligand-specific activation of acute signaling pathways such as [35S]GTPγS binding, intracellular calcium mobilization, and ERK1/2 phosphorylation (19). Importantly, we hypothesized that potential mechanisms underlying this bias were the differential engagement of specific constraints in both orthosteric and allosteric binding pockets on this GPCR. The purpose of the current study was thus to investigate whether such stimulus bias extends to two processes involved in the regulation of ligand activity at the M2 mAChR, namely receptor internalization and phosphorylation, and whether we could identify residues on the receptor that contribute to ligand-specific effects on these processes. We also exploited a novel approach for quantifying stimulus bias between pathways and mutant receptors in a manner that can be evaluated statistically (20–22). Collectively, our findings provide evidence for the existence of ligand-specific GPCR conformations both at the level of acute signaling and at longer term receptor regulation events, providing a framework by which the phenomenon can be quantified to assist in the interpretation of structure-function studies.
EXPERIMENTAL PROCEDURES
Materials
Mouse anti-V5 antibody was from Invitrogen, rabbit anti-M2 was from Alomone Labs (Jerusalem), and anti-rabbit HRP-conjugated and anti-mouse HRP-conjugated antibodies were sourced from Chemicon (Melbourne, Australia). Immobilon ECL detection reagents were from Millipore. Protein A-Sepharose was purchased from GE Healthcare. [3H]-N-Methylscopolamine (NMS), [3H]quinuclidinyl benzilate (QNB), and [32P]orthophosphate (10 mCi/ml) were obtained from PerkinElmer Life Sciences. AC-42 and 77-LH-28-1 were a generous gift from GlaxoSmithKline (Harlow, UK). N-Desmethylclozapine (NDMC) was sourced from Tocris Biosciences (Bristol, UK). All other reagents were purchased from Sigma-Aldrich and were of an analytical grade.
Receptor Mutagenesis and Generation of Stable Cell Lines
Mutations were introduced into the wild type human M2 mAChR in pENTR/D-TOPO using site-directed mutagenesis, prior to transfer to pEFS/FRT/V5-DEST as described previously (23, 24), which resulted in the insertion of the V5 epitope tag at the C terminus of the receptor. This receptor sequence, referred to as “wild type” throughout this study, had previously been shown to possess equivalent pharmacological properties to the non-tagged human M2 mAChRs (25). The primers for mutant receptor constructs have been reported previously (19, 26). Wild type and mutant receptor constructs were isogenically integrated into CHO-FlpIn cells (Invitrogen) as described previously (23, 24). Stably transfected cell lines were subsequently maintained in complete DMEM supplemented with 5% FBS, 16 mm HEPES, and 200 μg/ml hygromycin B at 37 °C in a humidified incubator containing 5% CO2, 95% O2. Non-transfected CHO-FlpIn cells (CHO-FlpIn-nt) were maintained in similar media except that zeocin (50 μg/ml) was used instead of hygromycin B as the selection agent.
Receptor Internalization Assays
CHO-FlpIn cells stably expressing the human M2 mAChR or various mutants thereof were seeded into 48-well plates at a density of 100,000 cells/well and allowed to adhere overnight. Growth medium was aspirated, and cells were washed twice with phosphate-buffered saline (PBS) and serum-starved overnight in DMEM supplemented with 16 mm HEPES. For time course experiments, cells were stimulated with a concentration of agonist that was ∼10× its previously determined Ki (19). Following agonist stimulation, ligand-containing DMEM was aspirated, and cells were placed immediately on ice and washed three times with ice-cold PBS. Cells were then incubated with 0.5–2 nm [3H]NMS for 4 h at 4 °C in CHO-HEPES buffer (110 mm NaCl, 5.4 mm KCl, 1.8 mm CaCl2, 1 mm MgSO4, 25 mm d-glucose, 20 mm HEPES, and 58.4 mm sucrose, pH 7.4), with the exception of the M2 Y4036.51A and Y1043.33A mutants, where 1–3 nm [3H]QNB was used, as these constructs showed markedly reduced [3H]NMS affinity (19). Nonspecific binding was determined with 10 μm atropine, with the exception of Y1043.33A and Y4036.51A, where 1 mm atropine was used. After 4 h, cells were washed three times with ice-cold PBS and then lysed with 0.2 m NaOH. Lysates were transferred to scintillation vials, and 4 ml of Ultima GoldTM scintillation mixture was added to each. Radioactivity was counted using a Packard 1600 TR liquid scintillation counter.
Receptor Phosphorylation Assays
CHO-FlpIn cells stably expressing the M2 mAChR or various mutants thereof were seeded into 12- well plates and allowed to adhere and grow such that they were an 80–90% confluent monolayer on the day of assay. Growth medium was aspirated, and cells were washed twice with phosphate free-Krebs buffer (10 mm HEPES, 118 mm NaCl, 4.3 mm KCl, 1.17 MgSO4, 1.3 mm CaCl2, 25 mm NaHCO3, and 11.7 mm glucose, pH 7.4) and labeled with 50 mCi of [32P]orthophosphate for 1 h at 37 °C. Cells were stimulated with agonists (at a ∼10× Ki concentration) for 5 min and then lysed on ice with radioimmunoprecipitation assay buffer (10 mm Tris, 10 mm EDTA, 500 mm NaCl, 1% v/v Nonidet P-40, and 0.5% w/v sodium deoxycholate, pH 7.4). M2 mAChR constructs were immunoprecipitated using anti-V5 antibody and protein A-Sepharose. The immunoprecipitated receptor was resolved on 10% SDS-PAGE, transferred to nitrocellulose membrane, and visualized with autoradiography. To ensure equal protein loading, immunoblotting was performed on nitrocellulose membranes, which were probed overnight at 4 °C with anti-M2 antibody diluted 1/5000 in TBST (20 mm Tris base, 140 mm NaCl, and 0.1% v/v Tween-20) with 5% skim milk powder. Membranes were washed three times for 15 min with TBST and then probed with secondary antibody for 1 h at room temperature (anti-rabbit HRP-conjugated sheep-raised immunoglobulin, 1/5000 in TBST with 5% skim milk powder). Bands were visualized using enhanced chemiluminescence and quantified using densitometry (ChemidocTM, Bio-Rad).
Data Analysis
Agonist concentration-response curves were fitted empirically to a three-parameter logistic equation using Prism 5.01 (GraphPad, San Diego),
 |
where bottom and top are the lower and upper plateaus, respectively, of the concentration-response curve, [A] is the molar concentration of agonist, and EC50 is the molar concentration of agonist required to generate a response halfway between the top and the bottom. To compare agonist profiles and quantify stimulus bias (functional selectivity) between the M2 wild type and mutant receptors, agonist concentration-response curves were fitted to the following form of the operational model of agonism (20–22, 27),
 |
where Em is the maximal possible response of the system; basal is the basal level of response; KA denotes the equilibrium dissociation constant of the agonist (A); τ is an index of the signaling efficacy of the agonist and is defined as RT/KE, where RT is the total number of receptors and KE is the coupling efficiency of each agonist-occupied receptor; and n is the slope of the transducer function that links occupancy to response. The analysis assumes that the maximal system responsiveness (Em) and the transduction machinery utilized for a given cellular pathway are the same for all agonists, such that the Em and transducer slope (n) are shared between agonists. The ratio, τ/KA (determined as a logarithm i.e. log(τ/KA)) is referred to herein as the “transduction coefficient” (21), as this composite parameter is sufficient to describe agonism and bias for a given pathway, i.e. stimulus-biased agonism can result from either a selective affinity (KA−1) of an agonist for a given receptor state(s) and/or a differential coupling efficacy (τ) toward certain pathways. To cancel the impact of cell-dependent effects on the observed agonism at each pathway, the log(τ/KA) values were then normalized to that determined for the endogenous agonist, ACh, at each pathway to yield a “normalized transduction coefficient,” Δlog(τ/KA), i.e. Δlog(τ/KA) = log(τ/KA)test − log(τ/KA)ACh. Finally, to determine the actual bias of each agonist for different signaling pathways, the Δlog(τ/KA) values were evaluated statistically between the pathways. The ligand bias of an agonist for one pathway, j1, over another, j2, is given as
where
 |
A lack of functional selectivity will thus result in bias values not substantially different from 1 between pathways and, hence, log(bias) values not significantly different from zero. To account for the propagation of error associated with the determination of composite parameters, the following equation was used.
 |
Internalization time courses were fitted to the following equation for monoexponential decay,
where Bt is equal to the specific binding of radioligand at time t, B0 is the specific binding of radioligand in the absence of agonist pretreatment (t = 0), and K is the rate constant, such that t½ = 0.69/K.
All affinity, potency, and transduction ratio parameters were estimated as logarithms (28). All results are expressed as the mean ± S.E. Statistical analyses were performed where appropriate using one-way ANOVA with the Dunnett's or Neuman-Keuls post test or Student's t test, and statistical significance was taken as p < 0.05.
RESULTS
C-terminal Epitope Tagging Does Not Affect M2 mAChR Internalization
To facilitate subsequent immunoprecipitation experiments, a V5 epitope tag was incorporated into the C termini of all M2 mAChR constructs used in this study. To ensure that the V5 tag did not unduly influence receptor regulation, internalization experiments were performed on CHO-FlpIn cells stably expressing an untagged human M2 mAChR for comparison. At both the (V5-tagged) wild type and untagged (data not shown) M2 mAChRs, the prototypical orthosteric agonists, ACh and CCh, caused rapid internalization of 80–90% of cell surface receptors with half-lives of 2–3 min (Fig. 1A and Tables 1 and 2). Following treatment with the orthosteric agonist arecoline, ∼40% of receptors remained at the surface after 30 min, and the rate of internalization was slower with a t½ of 5–8 min. The orthosteric partial agonist pilocarpine, as well AC-42, 77-LH-28-1, and McN-A-343, did not mediate internalization over the 30-min time course and was thus not pursued further for internalization studies of the wild type M2 mAChR (Fig. 1B). McN-A-343 has been shown previously to interact with the M2 mAChR as a bitopic ligand, i.e. concomitantly bridging both orthosteric and allosteric sites (26). It is possible that AC-42 and 77-LH-28-1 share this mechanism (19, 29), and thus they are referred to as “atypical” agonists herein. NDMC appeared to induce some internalization (Fig. 1B).
FIGURE 1.
Agonist-mediated internalization of wild type M2 mAChRs. CHO-FlpIn cells stably expressing the V5-tagged wild type M2 mAChR (A and B) were exposed to 100 μm ACh (■), 100 μm CCh (●), 100 μm arecoline (▴),1 μm pilocarpine (⧫), 30 μm 77-LH-28-1 (□), 30 μm AC-42 (△), 30 μm NDMC (◊), or 300 μm McN-A-343 (○) for up to 30 min prior to quantification of cell surface receptors with [3H]NMS. Concentration-response curves were constructed for the indicated agonists at the V5-tagged wild type M2 mAChR (C) following 30 min of agonist stimulation. Data represent the mean ± S.E. of n = 3–5 experiments performed in triplicate; error bars not shown lie within the dimensions of the symbol.
TABLE 1.
Half-life (min−1) of agonist-mediated internalization of M2 mAChR constructs expressed in CHO-FlpIn cells
Data represent the mean ± S.E. for n = 3–6 experiments determined in triplicate. Internalization of M2 mAChRs was measured by quantifying the amount of cell surface receptors remaining with [3H]NMS following agonist treatment, except where indicated with an asterisk, where [3H]QNB was used to label receptors. ND, not determined, as the response was highly variable; NA, not applicable, as no apparent agonist-mediated internalization was observed in time course experiments.
| Receptor construct | ACh | CCh | Arecoline | AC-42 | 77-LH-28-1 | NDMC |
|---|---|---|---|---|---|---|
| Wild type M2 | 2.4 ± 0.1 | 2.7 ± 0.9 | 4.8 ± 0.9a | NA | NA | 4.3 ± 0.6 |
| Untagged M2 | 2.1 ± 0.1 | 2.6 ± 0.7 | 8.1 ± 3.1 | NA | NA | ND |
| Y802.61A | 3.8 ± 0.6 | 1.9 ± 0.8 | 5.9 ± 1.5 | 0.7 ± 0.2 | 0.8 ± 0.3 | NA |
| W993.28A | 5.8 ± 0.7b | 10.3 ± 4.0 | 17.0 ± 6.6a | 0.3 ± 0.0 | 0.4 ± 0.1 | ND |
| Y1043.33A* | NA | NA | NA | 0.7 ± 0.3 | NA | 0.3 ± 0.0 |
| Y177A | 4.0 ± 0.9 | 3.9 ± 1.5 | 8.4 ± 1.4a | ND | NA | NA |
| Y4036.51A* | NA | NA | NA | NA | NA | NA |
a Significantly different from ACh value at same receptor; p < 0.05, one-way ANOVA, Dunnett's post test.
b Significantly different from wild type value; p < 0.05, one-way ANOVA, Dunnett's post test.
TABLE 2.
Percentage of M2 mAChR remaining at the cell surface after 30-min exposure to agonists as determined from concentration-response curves
Data represent the mean ± S.E. for n = 3–4 experiments determined in triplicate. Internalization of M2 mAChRs was measured by quantifying the amount of cell surface receptors remaining with [3H]NMS following agonist treatment, except where indicated with an asterisk, where [3H]QNB was used to label receptors. ND, concentration-response relationship could not be defined, despite evidence of internalization in time course assays; NA, not applicable, as no apparent agonist-mediated internalization was observed in time course experiments.
| Receptor construct | ACh | CCh | Arecoline | AC-42 | 77-LH-28−1 | NDMC |
|---|---|---|---|---|---|---|
| Wild type M2 | 19.1 ± 3.1 | 16.9 ± 1.6 | 38.0 ± 1.1 | NA | NA | ND |
| Y802.61A | 46.4 ± 5.8a | 46.2 ± 6.7a | 56.4 ± 6.4a | 48.8 ± 4.4 | 50.4 ± 2.5 | ND |
| W993.28A | 55.1 ± 1.4a | 55.1 ± 2.1a | 62.0 ± 1.7a | 8.2 ± 2.0 | 7.3 ± 1.4 | ND |
| Y1043.33A* | NA | NA | NA | 33.1 ± 8.8 | NA | 19.3 ± 1.9 |
| Y177A | 39.3 ± 9.7a | 43.1 ± 11.4a | 57.2 ± 8.6 | ND | NA | NA |
| Y4036.51A* | NA | NA | NA | NA | NA | NA |
a Significantly different from wild type value; p < 0.05, one-way ANOVA, Dunnett's post test.
Determination of Agonist Transduction Coefficients Facilitates the Quantification of Stimulus Bias across Different Pathways
Concentration-response curves for M2 mAChR internalization were subsequently constructed following 30 min of stimulation with various agonists (Fig. 1C). At both the V5-tagged (Table 3) and untagged (not shown) wild type receptors, ACh was the most potent agonist, whereas CCh and arecoline were roughly equipotent. Unfortunately, the limited solubility of NDMC prevented complete definition of its concentration-response relationship.
TABLE 3.
Potencies (pEC50) for agonist-mediated internalization of M2 mAChR constructs expressed in CHO-FlpIn cells
pEC50 denotes the negative logarithm of the molar agonist concentration required to produce a half-maximal response. Data represent the mean ± S.E. for n = 3–4 experiments determined in triplicate. Internalization of M2 mAChRs was measured by quantifying the amount of cell surface receptors remaining with [3H]NMS following agonist treatment, except where indicated with an asterisk, where [3H]QNB was used to label the receptors. NA, not applicable, as no apparent agonist-mediated internalization was observed in time course experiments.
| Receptor construct | ACh | CCh | Arecoline | AC-42 | 77-LH-28−1 | NDMC |
|---|---|---|---|---|---|---|
| Wild type M2 | 6.68 ± 0.17 | 5.52 ± 0.08 | 5.62 ± 0.13 | NA | NA | <5 |
| Y802.61A | 5.78 ± 0.11a | 4.86 ± 0.14a | 5.08 ± 0.15a | <5 | <4.5 | NA |
| W993.28A | 5.80 ± 0.14a | 5.11 ± 0.19 | 5.58 ± 0.06 | 6.31 ± 0.14 | 7.79 ± 0.19 | <5 |
| Y1043.33A* | NA | NA | NA | 5.71 ± 0.10 | NA | 5.84 ± 0.12 |
| Y177A | 6.17 ± 0.07a | 5.46 ± 0.10 | 5.36 ± 0.17 | <4.5 | NA | NA |
| Y4036.51A* | NA | NA | NA | NA | NA | NA |
a Significantly different from wild type value; p < 0.05, one-way ANOVA, Dunnett's post test.
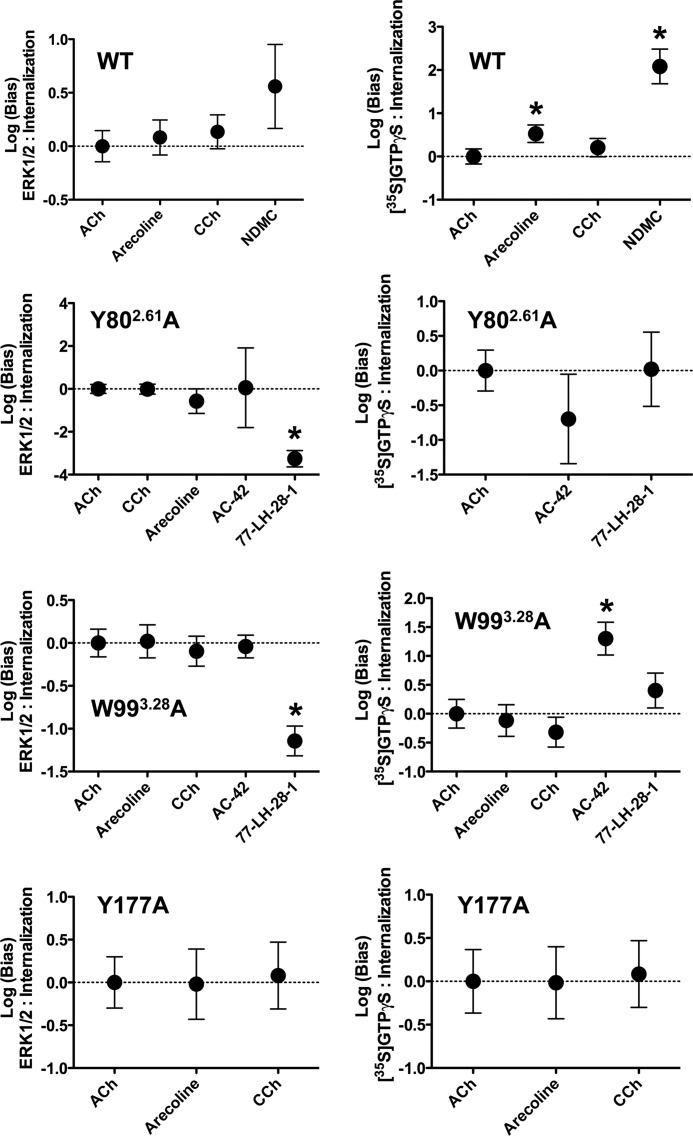
The concentration-response curves for internalization of the wild type (V5-tagged) M2 mAChR by ACh, CCh, arecoline, and NDMC were also fitted to an operational model of agonism to quantify agonist transduction coefficients (τ/KA ratios). The results of this analysis are reported in Table 4, together with those derived from re-analysis of data from our prior study (on the same receptor constructs and cell line) of acute signaling of these agonists, specifically in ERK1/2 phosphorylation and [35S]GTPγS binding assays (19). As outlined under “Experimental Procedures,” this analytical approach allows the quantification of the magnitude of the stimulus bias that each agonist has for each pathway relative to that of the endogenous agonist ACh. The log(bias) factors calculated for the comparison across the pathways indicate that, compared with ACh, arecoline is 2.8-fold biased toward promoting [35S]GTPγS binding relative to ERK1/2 phosphorylation (Table 4) and 3.4-fold biased toward promoting [35S]GTPγS binding relative to internalization (Table 4 and Fig. 5). For NDMC, the differences are more pronounced, showing a 33.1- and 120.2-fold greater bias toward [35S]GTPγS binding than toward ERK1/2 phosphorylation and receptor internalization, respectively (Table 4 and Fig. 5).
TABLE 4.
Bias factors for agonist-mediated signaling at M2 mAChR constructs expressed in CHO-FlpIn cells
Unless otherwise indicated, data represent the mean ± S.E. for n = 3–4 experiments determined in triplicate. ND, not determined.
| Ligand | pERK1/2a | Log(τ/KA) [35S]GTPγSa | Internalization | pERK1/2:[35S]GTPγS | Log(Bias) pERK1/2:Internalization | [35S]GTPγS:Internalization |
|---|---|---|---|---|---|---|
| Wild type | ||||||
| ACh | 8.14 ± 0.03 | 7.07 ± 0.08 | 6.53 ± 0.09 | 0.00 ± 0.12 | 0.00 ± 0.15 | 0.00 ± 0.17 |
| CCh | 7.18 ± 0.06 | 6.17 ± 0.14 | 5.44 ± 0.09 | −0.07 ± 0.18 | 0.14 ± 0.16 | 0.20 ± 0.21 |
| Arecoline | 7.08 ± 0.06 | 6.46 ± 0.11 | 5.40 ± 0.11 | −0.44 ± 0.16b | 0.08 ± 0.16 | 0.53 ± 0.20b |
| NDMC | 6.29 ± 0.08 | 6.75 ± 0.14 | 4.13 ± 0.35 | −1.52 ± 0.21b | 0.56 ± 0.39 | 2.08 ± 0.40b |
| Y802.61A | ||||||
| ACh | 8.06 ± 0.04 | 6.65 ± 0.15 | 5.53 ± 0.14 | 0.00 ± 0.22 | 0.00 ± 021 | 0.00 ± 0.29 |
| CCh | 7.12 ± 0.05 | ND | 4.60 ± 0.17 | ND | −0.01 ± 0.23 | ND |
| Arecoline | 7.29 ± 0.06 | ND | 4.71 ± 0.17 | ND | −0.57 ± 0.57 | ND |
| AC-42 | 7.36 ± 1.85 | 5.20 ± 0.60 | 4.78 ± 0.10 | 0.75 ± 1.94 | 0.05 ± 1.86 | −0.69 ± 0.64 |
| 77-LH-28-1 | 3.79 ± 0.34 | 5.66 ± 0.49 | 4.52 ± 0.07 | −3.28 ± 0.62b | −3.26 ± 0.38b | 0.02 ± 0.54 |
| W993.28A | ||||||
| ACh | 7.42 ± 0.06 | 5.72 ± 0.15 | 5.45 ± 0.09 | 0.00 ± 0.22 | 0.00 ± 0.16 | 0.00 ± 0.25 |
| CCh | 6.62 ± 0.08 | 4.70 ± 0.16 | 4.75 ± 0.11 | 0.23 ± 0.23 | −0.09 ± 0.17 | −0.32 ± 0.26 |
| Arecoline | 7.13 ± 0.07 | 5.30 ± 0.16 | 5.15 ± 0.14 | 0.14 ± 0.23 | 0.02 ± 0.19 | −0.12 ± 0.27 |
| AC-42 | 7.99 ± 0.05 | 7.64 ± 0.22 | 6.08 ± 0.04 | −1.34 ± 0.27b | −0.04 ± 0.13 | 1.30 ± 0.28b |
| 77-LH-28−1 | 8.79 ± 0.06 | 8.65 ± 0.22 | 7.97 ± 0.12 | −1.54 ± 0.28b | −1.14 ± 0.17b | 0.40 ± 0.30 |
| Y177A | ||||||
| ACh | 8.08 ± 0.07 | 5.96 ± 0.16 | 6.06 ± 0.20 | 0.00 ± 0.25 | 0.00 ± 0.29 | 0.00 ± 0.37 |
| CCh | ND | 5.12 ± 0.22 | 5.18 ± 0.19 | ND | ND | 0.08 ± 0.39 |
| Arecoline | ND | 4.86 ± 0.21 | 4.97 ± 0.24 | ND | ND | −0.02 ± 0.41 |
a Data reanalyzed from Ref. 19.
b Significantly different from wild type value; p < 0.05, one-way ANOVA, Dunnett's post test.
FIGURE 5.
Determination of bias factors quantifying agonist functional selectivity at wild type and mutant M2 mAChRs. Agonist concentration-response data derived from this study and from our earlier study (19) were fitted to Equation 2 to derive log(τ/KA) values for each agonist at each pathway. These values were normalized to that derived for ACh at each pathway, and the resulting normalized values (Δlog(τ/KA)) were analyzed using Equation 3 under “Experimental Procedures” to yield the final log(bias) factor (ΔΔlog(τ/KA)) for each agonist between the two pathways. *, indicates statistical significance.
Effect of Mutations on the Time Course of Agonist-mediated M2 mAChR Internalization
Consistent with previous evidence that Tyr-802.61 at the top of TM2 has little or no impact on orthosteric ligand binding or efficacy (19, 30), the Y802.61A M2 mAChR behaved similarly to the wild type receptor in response to ACh and CCh, undergoing rapid internalization with a half-life of 2–4 min.6 In addition, arecoline-mediated internalization of the Y802.61A mutant was slower than ACh or CCh with a t½ of ∼6 min, and the agonist also displayed lower efficacy at mediating internalization (Fig. 2, A and B, and Tables 1 and 2). Also in line with results obtained with the wild type M2 mAChR, there was no change in the cell surface expression of the Y802.61A receptor after 30 min in response to pilocarpine, McN-A-343, or NDMC (Fig. 2, A and B). Interestingly, however, a rapid internalization (t½ < 1 min) was observed in response to AC-42 and 77-LH-28-1 at this mutant receptor, in contrast to the lack of effect of these atypical agonists at the wild type.
FIGURE 2.
Time course for agonist-mediated internalization of cell surface mutant M2 mAChRs. A and B, CHO-FlpIn cells stably expressing the Y802.61A mutant M2 mAChR were exposed to 100 μm ACh (■), 1 mm CCh (●), 300 μm arecoline (▴), 300 μm pilocarpine (⧫), 1 μm 77-LH-28-1 (□), 10 μm AC-42 (△), 10 μm NDMC (◊), or 300 μm McN-A-343 (○) for the indicated times prior to quantification of cell surface receptors with [3H]NMS. C and D, CHO-FlpIn cells stably expressing the W993.28A mutant M2 mAChR were exposed to 300 μm ACh (■), 1 mm CCh (●), 100 μm arecoline (▴), 1 μm pilocarpine (⧫), 30 nm 77-LH-28-1 (□), 1 μm AC-42 (△), 30 μm NDMC (◊), or 300 μm McN-A-343 (○) for up to 30 min prior to quantification of cell surface receptors with [3H]NMS. E and F, CHO-FlpIn cells stably expressing the Y177A mutant M2 mAChR were exposed to 100 μm ACh (■), 1 mm CCh (●), 300 μm arecoline (▴), 300 μm pilocarpine (⧫), 10 μm 77-LH-28-1 (□), 30 μm AC-42 (△), 10 μm NDMC (◊), or 300 μm McN-A-343 (○) for up to 30 min prior to quantification of cell surface receptors with [3H]NMS. Data represent the mean ± S.E. for n = 3–4 experiments conducted in triplicate. Error bars not shown lie within the dimensions of the symbol.
Mutation of Trp3.28 in both the M1 and M2 mAChR subtypes influences the binding and acute signaling efficacy of both orthosteric and allosteric ligands (19, 30–32). ACh, CCh, and arecoline caused internalization of 35–45% of cell surface W993.28A M2 mAChRs, however the rates of internalization were slower than at the wild type (Fig. 2, C and D). Consistent with observations made at the wild type, McN-A-343, pilocarpine, and NDMC did not promote W993.28A receptor internalization. However, AC-42 and 77-LH-28-1 very rapidly internalized ∼90% of receptors with t½ values of less than 30 s (Tables 1 and 2).
Alanine substitution of Tyr-177 in the E2 loop has been reported to increase the acute signaling efficacy of McN-A-343 and decrease orthosteric agonist efficacy in a pathway-selective manner (19, 26). With respect to internalization of the Y177A M2 mAChR, the orthosteric agonists ACh, CCh, and arecoline caused a loss of cell surface receptors, with 40–55% of the [3H]NMS binding sites remaining after 30 min (Fig. 2, E and F, and Table 2). Similar to the wild type, the response to ACh and CCh was rapid, with t½ values of ∼4 min, whereas the response to arecoline was slower (t½ ∼ 8 min (Table 1)). AC-42 appeared to induce transient internalization of the Y177A M2 mAChR, whereas there was no apparent internalization in response to McN-A-343, pilocarpine, NDMC, or 77-LH-28-1.
The mutation of both Tyr3.33 and Tyr6.51 to Ala in the M1 and M2 mAChRs can drastically reduce the affinity (or potency) of orthosteric ligands while either increasing or having no effect on the affinity and efficacy of atypical agonists (19, 32–34). Because neither of these mutant receptors displayed appreciable levels of [3H]NMS binding, we utilized [3H]QNB as a structurally distinct orthosteric radioligand for investigating the regulation of these latter receptors. Of the agonists tested, only AC-42 and NDMC reduced the number of [3H]QNB-accessible binding sites for the Y1043.33A and Y4036.51A M2 mAChRs over 30 min, with a time course that was very rapid (t½ < 1 min) (Fig. 3 and Table 1).
FIGURE 3.
Time course for agonist-mediated reduction in [3H]QNB-accessible binding sites at mutant M2 mAChRs. A and B, CHO-FlpIn cells stably expressing the Y1043.33A mutant M2 mAChR were exposed to 10 mm ACh (■), 10 mm CCh (●), 10 mm arecoline (▴), 10 mm pilocarpine (⧫), 30 mm 77-LH-28-1 (□), 30 mm AC-42 (△), 30 mm NDMC (◊), or 300 mm McN-A-343 (○) for up to 30 min prior to measurement of [3H]QNB-accessible receptors. C and D, CHO-FlpIn-cells stably expressing the Y4036.51A mutant M2 mAChR were exposed to 10 mm ACh (■), 10 mm CCh (●), 10 mm arecoline (▴), 10 mm pilocarpine (⧫), 30 mm 77-LH-28-1 (□), 30 mm AC-42 (△), 30 mm NDMC (◊), or 300 mm McN-A-343 (○) for up to 30 min prior to measurement of [3H]QNB-accessible receptors. Data represent the mean ± S.E. for n = 3–4 experiments conducted in triplicate. Error bars not shown lie within the dimensions of the symbol.
Effect of Mutations on Agonist Potency and Stimulus Bias for M2 mAChR Internalization
Determination of complete concentration-response curves to ACh, CCh, and arecoline at the Y802.61A mutant yielded potencies that were 3–8-fold less than at the wild type (Fig. 4A and Table 3). Unfortunately, concentration-response curves to AC-42 and 77-LH-28-1 could not be defined in full individually because of the limited solubility of these compounds. However, application of the operational model (Equation 2) to the entire family of curves facilitated the ability to adequately fit the AC-42 and 77-LH-28-1 data sets (results summarized in Table 4). The most striking findings were noted with 77-LH-28-1, which had a significant 1900-fold bias toward [35S]GTPγS binding and a 1819-fold bias toward internalization relative to ERK1/2 phosphorylation; there was no bias between [35S]GTPγS and the internalization pathways (Table 4 and Fig. 5). These results indicate that mutations cannot only change the affinity and/or coupling efficiency of GPCR ligands but also promote or modify signaling bias between pathways.
FIGURE 4.
Agonist concentration-response curves for internalization of mutant M2 mAChRs. A–C, after agonist stimulation for 30 min, concentration-response curves were constructed as follows before determination of cell surface expression with [3H]NMS: A, ACh (■), CCh (●), arecoline (▴), 77-LH-28-1 (□), and AC-42 (△) at the Y802.61A; B, ACh (■), CCh (●), arecoline (▴), 77-LH-28-1 (□), AC-42 (△), and NDMC (◊) at the W993.28A; C, ACh (■), CCh (●), arecoline (▴), and AC-42 (△) at the Y177A. D and E, after agonist stimulation for 30 min, concentration-response curves were constructed as follows before determination of [3H]QNB binding levels: D, NDMC (◊) and AC-42 (△) at the Y1043.33A; E, NDMC (◊) and AC-42 (△) at the Y4036.51A. Data represent the mean ± S.E. for n = 3–4 experiments conducted in triplicate. Error bars not shown lie within the dimensions of the symbol.
At the W993.28A mutant, ACh potency was also significantly reduced (∼7-fold) compared with the wild type M2 mAChR, whereas the potencies of CCh and arecoline were not significantly different. Internalization of the W993.28A receptor in response to NDMC could not be fully defined because of the limited solubility of the compound. The rank order of agonist potencies for W993.28A M2 mAChR internalization was 77-LH-28-1 > AC-42 > ACh > arecoline ≥ CCh (Fig. 4B). Of note, both AC-42 and 77-LH-28-1 displayed significant bias (22- and 35-fold, respectively) toward [35S]GTPγS binding relative to ERK1/2 phosphorylation (Table 4), whereas differences were noted between the two compounds when their effects on internalization were considered. Specifically, AC-42 was biased toward internalization when compared with [35S]GTPγS binding, whereas 77-LH-28-1 was biased toward internalization when compared with ERK1/2 phosphorylation (Table 4 and Fig. 5).
Mutation of Y177A reduced the potency of ACh (∼3-fold) but had little or no effect on the potencies of CCh and arecoline. Interestingly, no significant bias was noted for either CCh or arecoline between the [35S]GTPγS binding and internalization pathways at this mutant (Table 4), despite the fact that arecoline displayed such bias at the wild type receptor. Internalization of Y177A receptors in response to AC-42 was apparent only at the highest concentration applied. Similarly, at the Y4036.51A mutant receptor, a concentration-response relationship could not be defined for either AC-42 or NDMC (Fig. 4D). However, at the Y1043.33A receptor, AC-42 and NDMC had similar potencies, causing 70–80% of receptors to be inaccessible to [3H]QNB after 30 min (Fig. 4E).
Characterization of Agonist-dependent M2 mAChR Phosphorylation
Immunoprecipitation of wild type M2 mAChRs treated with 100 μm ACh was performed using an anti-V5 antibody, yielding a single band on the autoradiograph (Fig. 6A). Immunoblotting with an anti-M2 antibody confirmed that this single band was the phosphorylated M2 mAChR (Fig. 6C). The reverse was also true, in that the M2 mAChR could be immunoprecipitated with anti-M2 antibody, with the anti-V5 antibody identifying the same band on the membrane (Fig. 6, B and D). No bands were present on the autoradiographs for non-transfected (nt) CHO-FlpIn cells treated in parallel; similarly immunoblotting revealed only nonspecific bands (Fig. 6). The unstimulated wild type receptor showed minimal levels of basal phosphorylation. For all subsequent studies of receptor phosphorylation, only the endogenous agonist, ACh, and the partial agonist, pilocarpine, were included as reference orthosteric ligands for comparison with NDMC, McN-A-343, 77-LH-28-1, and AC-42. As shown in Fig. 7A, ACh caused robust phosphorylation of the wild type M2 mAChR. Pilocarpine caused an intermediate level of phosphorylation, whereas NDMC, 77-LH-28-1, AC-42, and McN-A-343 had little or no effect on wild type M2 mAChR phosphorylation.
FIGURE 6.
Validation of 32P labeling and immunoprecipitation of M2 mAChRs. After 32P labeling, either non-transfected CHO-FlpIn cells (CHO-nt) or those stably expressing the wild type M2 mAChR were immunoprecipitated with either anti-M2 antibody (A) or anti-V5 antibody (B), resolved by SDS-PAGE, and transferred to nitrocellulose membranes before autoradiography was performed as described under “Experimental Procedures.” The nitrocellulose membrane corresponding to the autoradiograph in A was then reprobed with anti-V5 antibody (C). The nitrocellulose membrane corresponding to the autoradiograph in B was then reprobed with anti-M2 antibody (D).
FIGURE 7.
Agonist-mediated phosphorylation of wild type and allosteric site-mutated M2 mAChRs. After 32P labeling, CHO-FlpIn cells stably expressing M2 mAChR constructs were exposed to agonists for 5 min and then immunoprecipitated with anti-V5, resolved by SDS-PAGE, transferred to nitrocellulose, and visualized by autoradiography. The panels on the right are representative autoradiographs, and those on the left are grouped autoradiography data. A, phosphorylation of wild type M2 mAChR after treatment with 100 μm ACh, 100 μm pilocarpine, 30 μm NDMC, 1 mm McN-A-343, or 30 μm 77-LH-28-1. B, phosphorylation of Y802.61A receptors after treatment with 100 μm ACh, 300 μm pilocarpine, 30 μm NDMC, 1 mm McN-A-343, or 10 μm 77-LH-28-1. C, phosphorylation of Y177A receptors after treatment with 100 μm ACh, 300 μm pilocarpine, 30 μm NDMC, 1 mm McN-A-343, or 30 μm 77-LH-28-1. Data represent n = 3–4 experiments performed in duplicate and normalized to the percent of the ACh-stimulated response (itself designated as 100%). *, denotes a significant difference from basal, as determined by one-way ANOVA and Dunnett's post-test. In all instances, the ACh response was also significantly different from basal.
Effect of Mutations on Agonist-dependent M2 mAChR Phosphorylation
At the Y802.61A and Y177A M2 mAChR mutants, the agonist profile of receptor phosphorylation was similar to the wild type (Fig. 7, B and C). Both of these mutant receptors had low levels of constitutive phosphorylation, with significant induction of phosphorylation following exposure to ACh and an intermediate level of phosphorylation induced by pilocarpine. Despite the fact that these two mutations have been found to increase the affinity and/or the signaling and internalization efficacy of different agonists, there was no detectable induction of receptor phosphorylation by NDMC, McN-A-343, AC-42, or 77-LH-28-1. The W993.28A M2 mAChR was robustly phosphorylated in response to ACh, with AC-42 and 77-LH-28-1 also inducing small but detectable increases in phosphorylation (Fig. 8A). This is consistent with the increased internalization efficacy for these two compounds at this mutant. ACh significantly increased the level of Y4036.51A receptor phosphorylation, and NDMC also increased phosphorylation above basal levels (Fig. 8C). Pilocarpine, 77-LH-28-1, AC-42, and McN-A-343 had little or no effect on the phosphorylation status of the Y4036.51A M2 mAChR. ACh alone caused a small but detectable increase in phosphorylation of the Y1043.33A receptor. Interestingly, the basal level of phosphorylation appeared elevated compared with wild type (Fig. 8B).
FIGURE 8.
Agonist-mediated phosphorylation of orthosteric site-mutated M2 mAChRs. After 32P labeling, CHO-FlpIn cells stably expressing M2 mAChR constructs were exposed to agonists for 5 min and then immunoprecipitated with anti-V5 antibody, resolved by SDS-PAGE, transferred to nitrocellulose, and visualized by autoradiography. The panels on the right are representative autoradiographs, and those on the left are grouped autoradiography data. A, phosphorylation of W993.28A receptors after treatment with 300 μm ACh, 300 μm pilocarpine, 30 μm NDMC, 1 mm McN-A-343, or 3 μm 77-LH-28-1. B, phosphorylation of Y1043.33A receptors after treatment with 10 mm ACh, 10 mm pilocarpine, 30 mm NDMC, 1 mm McN-A-343, or 100 mm 77-LH-28-1. C, phosphorylation of Y4036.51A receptors after treatment with 10 mm ACh, 10 mm pilocarpine, 30 μm NDMC, 1 mm McN-A-343, or 30 μm 77-LH-28-1. Data represent n = 3 experiments performed in duplicate and normalized to the percent of the ACh-stimulated response (itself designated as 100%). *, denotes a significant difference from basal, as determined by one-way ANOVA and Dunnett's post-test. In all instances, the ACh response was also significantly different from basal.
DISCUSSION
This study has utilized a novel method of quantitatively determining the stimulus bias of GPCR ligands to probe the effects that mutations within orthosteric and allosteric binding pockets of the M2 mAChR receptor have on the signaling profiles of prototypical and atypical ligands. The mutations were found to induce significant changes in the transduction efficiency of ligands in a manner that revealed evidence not only of residues that regulate the nature of the activate conformation of the receptor but also, unexpectedly, that signaling pathways originally thought to have a linear relationship (for example receptor phosphorylation and receptor internalization) are in fact able to operate independently of each other.
Determination of Stimulus Bias
It is now clear that GPCRs have at least two fundamental signaling modes (4, 6). The first is via heterotrimeric G proteins and a second is independent of heterotrimeric G proteins but, rather, is driven by the recruitment of arrestin adaptor proteins (4, 6). Thus, it is now widely accepted that the agonist-bound active conformation of receptors is able to promote the interaction with, and activation of, both heterotrimeric G proteins and arrestins (7). Importantly, the active conformation of a receptor bound to the natural ligand may not activate both arms of the bimodal pathway equally. This “natural bias” is the physiologically relevant state of the active receptor and as such cannot, by definition, be considered as reflecting stimulus bias associated with surrogate agonists. Rather, the relative coupling efficiencies of the receptor occupied by the natural ligand reflect the state(s) with which the signaling induced by synthetic ligands should be compared (19). If the balance between the bimodal coupling efficiencies of the receptor stimulated by synthetic ligands is different from that mediated by the natural ligand, then this can be considered as true ligand stimulus bias.
An important issue we addressed is how best to quantify stimulus bias. In extreme cases, synthetic ligands can show agonist properties for one arm of the bimodal pathway and antagonist/inverse agonist properties for the other arm (8, 35). This is seen for the ligand carvedilol, for example, at the β2-adrenoreceptor, which is a partial agonist for arrestin signaling and an inverse agonist at Gs protein signaling (8). Where there is such a case of reversal of efficacy, then the ligand is biased by definition. However, in most instances there is a more subtle difference in the transduction efficiency of ligands at two pathways. It is under such more common circumstances that our approach for determining the transduction coefficient (log(τ/KA)) of an agonist (20–22) can prove particularly useful. By including the natural ligand (ACh in this instance) in our analyses, this method simultaneously nullifies the impact of cell background and natural bias and facilitates the determination of true bias factors that describe the ability of the test ligands to promote distinct receptor conformational states (21).
One example of the power of this method of determining stimulus bias was illustrated in the analysis of the partial agonist arecoline acting at the wild type M2 mAChR. Experimentally, pathways that have greater amplification or receptor reserve, such as ERK1/2, will be more readily measured than pathways with lower amplification such as [35S]GTPγS binding (5, 36). This, together with the partial agonism associated with arecoline, means that cursory analysis of the concentration-response data might lead to the erroneous conclusion that arecoline is biased toward ERK1/2 signaling (compare the non-normalized transduction coefficient values in Table 4, i.e. log(τ/KA) for arecoline at ERK1/2 and [35S]GTPγS binding = 7.08 and 6.46, respectively). However, analysis using the method described here reveals the opposite, that in fact arecoline has a 2.8-fold bias toward [35S]GTPγS binding over ERK1/2.
Evidence for Molecular Constraints in the Structure of GPCRs
Mutating tyrosine at the top of transmembrane domain two of the M2 mAChR (Tyr-802.61) to alanine affected the binding of the natural ligand ACh only slightly, resulting in a small reduction in the transduction efficiency toward ERK1/2 and [35S]GTPγS binding and a larger reduction in coupling to internalization. Hence, this mutation appears to have an overall slightly negative effect on receptor signaling stimulated by the endogenous ligand. In contrast, the mutation had a substantial effect on the signaling mediated by the atypical ligands 77-LH-28-1 and AC-42. Neither of these two ligands stimulated ERK1/2 phosphorylation and [35S]GTPγS binding or receptor internalization at the wild type receptor, but they both gave robust responses at the Y802.61A mutant. A simplistic explanation for this observation might be that the mutation of Tyr-802.61 results in an increase in the affinity of the receptor for atypical ligands and a subsequent increase in the responsiveness of the receptor. Certainly, in the case of 77-LH-28-1, mutating Tyr-802.61 to alanine resulted in a 31-fold increase in 77-LH-28-1 affinity (19). However, the important finding here is that the increase in signaling transduction efficiency seen in the Y802.61A mutant in response to 77-LH-28-1 is not equivalent for all three pathways. This argues strongly against a simple explanation based on affinity change but suggests that Tyr-802.61 may act as a molecular switch or, alternatively, a gatekeeper residue that can regulate the conformation adopted by the receptor in a way that introduces signaling bias. Thus, at the Y802.61A mutant, 77-LH-28-1 showed a ∼2000-fold bias toward [35S]GTPγS binding compared with ERK1/2 signaling. Similarly, there was a ∼2000-fold bias toward internalization compared with ERK1/2 signaling. Hence, for 77-LH-28-1 acting at the Y802.61A mutant, there is an equivalent increase in transduction efficiency in [35S]GTPγS binding and receptor internalization but a much smaller increase in ERK1/2 signaling.
Analysis of AC-42 at the Y802.61A mutant similarly revealed non-equivalent changes in transduction efficiency between the signaling pathways measured. Together with the 77-LH-28-1 data, this suggests that Tyr-802.61 not only regulates the binding affinity of atypical ligands but that this residue also regulates the conformational states adopted following atypical ligand binding that in turn impacts on signal transduction. This residue in the wild type receptor can thus be seen as offering a constraint on the receptor conformation. Mutating Tyr-802.61 appears to remove this constraint, allowing the receptor to adopt an active conformational state that increases signaling efficacy but not in a manner that is equal for all pathways.
In addition, analysis of the Y177A mutant indicates that this tyrosine residue in the second extracellular loop is important for the ability of the receptor to transition between states; this point was made previously by us and adds to the body of literature suggesting a role for the extracellular loops in contributing to state transitions. This residue increases McN-A-343 (bitopic ligand) efficacy for ERK signaling (19, 26), yet with regards to receptor internalization and phosphorylation, the mutation is indistinguishable from wild type. Thus, through quantitative analysis of stimulus bias introduced to the M2 mAChR via point mutations, we have identified residues in the structure of the receptor that introduce constraints that restrict the number of conformational states adopted by the wild type receptor. One way of introducing stimulus bias, therefore, is mutating these residues (as we have done here), or alternatively, it may be possible to target these constraining residues with selective ligands and in this way generate novel chemical entities that mediate stimulus bias.
Mutant Receptors Reveal the Nonlinear Nature of Receptor Signaling Pathways
As discussed above, it is widely accepted that GPCRs show bimodal signaling, a concept that has led to a very linear view of GPCR signaling where, for example, receptor internalization is downstream of receptor phosphorylation and arrestin recruitment (4, 37). However, analysis of the stimulus bias introduced in mutant M2 mAChR described in the present study challenges this notion and indicates that the four signaling events monitored in our studies (receptor phosphorylation, receptor internalization, [35S]GTPγS binding, and ERK1/2 signaling) do not necessarily have a linear relationship but rather can be independent of each other. Mutation of W993.28A, for example, did not make any apparent impact on receptor phosphorylation in response to the full agonists ACh and CCh but did reduce the ability of these agonists to mediate receptor internalization. More profound was the impact of this mutation on the atypical ligands AC-42 and 77-LH-28-1. These ligands showed an increase in affinity for the W993.28A mutant and a corresponding robust internalization response that was not associated with any detectable receptor phosphorylation. Similarly, the internalization response seen in another mutant, Y802.61A, in response to AC-42 and 77-LH-28-1 was not associated with any detectable receptor phosphorylation. It appears therefore that the receptor conformation adopted in these mutants was able to promote internalization in a manner that was independent of receptor phosphorylation, indicating that these two processes are not necessarily connected. Hence, quantitative analysis of the ligand bias between the four receptor responses observed in mutant M2 mAChRs indicates that receptor conformations can be adopted that bias one pathway over another, highlighting the fact that these signaling responses cannot be directly related to each other in a linear fashion.
Acknowledgment
We are grateful to GlaxoSmithKline UK for the generous gifts of 77-LH-28-1 and AC-42.
This work was supported by Program Grant 519461 from the National Health and Medical Research Council (NHMRC) of Australia.
Ballesteros and Weinstein numbers are provided (in superscript) to indicate the relative position of residues within the transmembrane domain.
- GPCR
- G protein-coupled receptor
- mAChR
- muscarinic acetylcholine receptor
- GTPγS
- guanosine 5′-O-(thiotriphosphate)
- NMS
- [3H]-N-methylscopolamine
- QNB
- [3H]quinuclidinyl benzilate
- NDMC
- N-desmethylclozapine
- ANOVA
- analysis of variance
- CCh
- carbachol.
REFERENCES
- 1. M. C., H. B. (2008) Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 7, 339–357 [DOI] [PubMed] [Google Scholar]
- 2. Overington J. P., Al-Lazikani B., Hopkins A. L. (2006) How many drug targets are there? Nat. Rev. Drug Discov. 5, 993–996 [DOI] [PubMed] [Google Scholar]
- 3. Lefkowitz R. J. (2004) Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol. Sci. 25, 413–422 [DOI] [PubMed] [Google Scholar]
- 4. Shenoy S. K., Lefkowitz R. J. (2011) β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol. Sci. 32, 521–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kenakin T. (2005) New concepts in drug discovery: collateral efficacy and permissive antagonism. Nat. Rev. Drug Discov. 4, 919–927 [DOI] [PubMed] [Google Scholar]
- 6. Violin J. D., Lefkowitz R. J. (2007) β-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol. Sci. 28, 416–422 [DOI] [PubMed] [Google Scholar]
- 7. Reiter E., Ahn S., Shukla A. K., Lefkowitz R. J. (2012) Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu. Rev. Pharmacol. Toxicol. 52, 179–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wisler J. W., DeWire S. M., Whalen E. J., Violin J. D., Drake M. T., Ahn S., Shenoy S. K., Lefkowitz R. J. (2007) A unique mechanism of beta-blocker action: carvedilol stimulates β-arrestin signaling. Proc. Natl. Acad. Sci. U.S.A. 104, 16657–16662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gesty-Palmer D., Luttrell L. M. (2011) “Biasing” the parathyroid hormone receptor: a novel anabolic approach to increasing bone mass? Br. J. Pharmacol. 164, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gesty-Palmer D., Chen M., Reiter E., Ahn S., Nelson C. D., Wang S., Eckhardt A. E., Cowan C. L., Spurney R. F., Luttrell L. M., Lefkowitz R. J. (2006) Distinct β-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J. Biol. Chem. 281, 10856–10864 [DOI] [PubMed] [Google Scholar]
- 11. Violin J. D., DeWire S. M., Yamashita D., Rominger D. H., Nguyen L., Schiller K., Whalen E. J., Gowen M., Lark M. W. (2010) Selectively engaging β-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J. Pharmacol. Exp. Ther. 335, 572–579 [DOI] [PubMed] [Google Scholar]
- 12. Barker E. L., Westphal R. S., Schmidt D., Sanders-Bush E. (1994) Constitutively active 5-hydroxytryptamine 2C receptors reveal novel inverse agonist activity of receptor ligands. J. Biol. Chem. 269, 11687–11690 [PubMed] [Google Scholar]
- 13. Blanpain C., Vanderwinden J. M., Cihak J., Wittamer V., Le Poul E., Issafras H., Stangassinger M., Vassart G., Marullo S., Schlndorff D., Parmentier M., Mack M. (2002) Multiple active states and oligomerization of CCR5 revealed by functional properties of monoclonal antibodies. Mol. Biol. Cell 13, 723–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Labrecque J., Fargin A., Bouvier M., Chidiac P., Dennis M. (1995) Serotonergic antagonists differentially inhibit spontaneous activity and decrease ligand binding capacity of the rat 5-hydroxytryptamine type 2C receptor in Sf9 cells. Mol. Pharmacol. 48, 150–159 [PubMed] [Google Scholar]
- 15. Roettger B. F., Ghanekar D., Rao R., Toledo C., Yingling J., Pinon D., Miller L. J. (1997) Antagonist-stimulated internalization of the G protein-coupled cholecystokinin receptor. Mol. Pharmacol. 51, 357–362 [PubMed] [Google Scholar]
- 16. Wei H., Ahn S., Shenoy S. K., Karnik S. S., Hunyady L., Luttrell L. M., Lefkowitz R. J. (2003) Independent β-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc. Natl. Acad. Sci. U.S.A. 100, 10782–10787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whistler J. L., von Zastrow M. (1998) Morphine-activated opioid receptors elude desensitization by β-arrestin. Proc. Natl. Acad. Sci. U.S.A. 95, 9914–9919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kenakin T., Miller L. J. (2010) Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 62, 265–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gregory K. J., Hall N. E., Tobin A. B., Sexton P. M., Christopoulos A. (2010) Identification of orthosteric and allosteric site mutations in M2 muscarinic acetylcholine receptors that contribute to ligand-selective signaling bias. J. Biol. Chem. 285, 7459–7474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Evans B. A., Broxton N., Merlin J., Sato M., Hutchinson D. S., Christopoulos A., Summers R. J. (2011) Quantification of functional selectivity at the human α(1A)-adrenoceptor. Mol. Pharmacol. 79, 298–307 [DOI] [PubMed] [Google Scholar]
- 21. Kenakin T., Watson C., Muniz-Medina V., Christopoulos A., Novick S. (2012) A simple method for quantifying functional selectivity and agonist bias. ACS Chem. Neurosci. 3, 193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koole C., Wootten D., Simms J., Valant C., Sridhar R., Woodman O. L., Miller L. J., Summers R. J., Christopoulos A., Sexton P. M. (2010) Allosteric ligands of the glucagon-like peptide 1 receptor (GLP-1R) differentially modulate endogenous and exogenous peptide responses in a pathway-selective manner: implications for drug screening. Mol. Pharmacol. 78, 456–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Avlani V. A., Gregory K. J., Morton C. J., Parker M. W., Sexton P. M., Christopoulos A. (2007) Critical role for the second extracellular loop in the binding of both orthosteric and allosteric G protein-coupled receptor ligands. J. Biol. Chem. 282, 25677–25686 [DOI] [PubMed] [Google Scholar]
- 24. May L. T., Avlani V. A., Langmead C. J., Herdon H. J., Wood M. D., Sexton P. M., Christopoulos A. (2007) Structure-function studies of allosteric agonism at M2 muscarinic acetylcholine receptors. Mol. Pharmacol. 72, 463–476 [DOI] [PubMed] [Google Scholar]
- 25. Avlani V., May L. T., Sexton P. M., Christopoulos A. (2004) Application of a kinetic model to the apparently complex behavior of negative and positive allosteric modulators of muscarinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 308, 1062–1072 [DOI] [PubMed] [Google Scholar]
- 26. Valant C., Gregory K. J., Hall N. E., Scammells P. J., Lew M. J., Sexton P. M., Christopoulos A. (2008) A novel mechanism of G protein-coupled receptor functional selectivity. Muscarinic partial agonist McN-A-343 as a bitopic orthosteric/allosteric ligand. J. Biol. Chem. 283, 29312–29321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Black J. W., Leff P. (1983) Operational models of pharmacological agonism. Proc. R. Soc. Lond. B Biol. Sci. 220, 141–162 [DOI] [PubMed] [Google Scholar]
- 28. Christopoulos A. (1998) Assessing the distribution of parameters in models of ligand-receptor interaction: to log or not to log. Trends Pharmacol. Sci. 19, 351–357 [DOI] [PubMed] [Google Scholar]
- 29. Avlani V. A., Langmead C. J., Guida E., Wood M. D., Tehan B. G., Herdon H. J., Watson J. M., Sexton P. M., Christopoulos A. (2010) Orthosteric and allosteric modes of interaction of novel selective agonists of the M1 muscarinic acetylcholine receptor. Mol. Pharmacol. 78, 94–104 [DOI] [PubMed] [Google Scholar]
- 30. Lebon G., Langmead C. J., Tehan B. G., Hulme E. C. (2009) Mutagenic mapping suggests a novel binding mode for selective agonists of M1 muscarinic acetylcholine receptors. Mol. Pharmacol. 75, 331–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsui H., Lazareno S., Birdsall N. J. (1995) Probing of the location of the allosteric site on m1 muscarinic receptors by site-directed mutagenesis. Mol. Pharmacol. 47, 88–98 [PubMed] [Google Scholar]
- 32. Spalding T. A., Ma J. N., Ott T. R., Friberg M., Bajpai A., Bradley S. R., Davis R. E., Brann M. R., Burstein E. S. (2006) Structural requirements of transmembrane domain 3 for activation by the M1 muscarinic receptor agonists AC-42, AC-260584, clozapine, and N-desmethylclozapine: evidence for three distinct modes of receptor activation. Mol. Pharmacol. 70, 1974–1983 [DOI] [PubMed] [Google Scholar]
- 33. Spalding T. A., Trotter C., Skjaerbaek N., Messier T. L., Currier E. A., Burstein E. S., Li D., Hacksell U., Brann M. R. (2002) Discovery of an ectopic activation site on the M(1) muscarinic receptor. Mol. Pharmacol. 61, 1297–1302 [DOI] [PubMed] [Google Scholar]
- 34. Sur C., Mallorga P. J., Wittmann M., Jacobson M. A., Pascarella D., Williams J. B., Brandish P. E., Pettibone D. J., Scolnick E. M., Conn P. J. (2003) N-Desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-d-aspartate receptor activity. Proc. Natl. Acad. Sci. U.S.A. 100, 13674–13679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosethorne E. M., Charlton S. J. (2011) Agonist-biased signaling at the histamine H4 receptor: JNJ7777120 recruits β-arrestin without activating G proteins. Mol. Pharmacol. 79, 749–757 [DOI] [PubMed] [Google Scholar]
- 36. Rajagopal S., Ahn S., Rominger D. H., Gowen-MacDonald W., Lam C. M., Dewire S. M., Violin J. D., Lefkowitz R. J. (2011) Quantifying ligand bias at seven-transmembrane receptors. Mol. Pharmacol. 80, 367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reiter E., Lefkowitz R. J. (2006) GRKs and β-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol. Metab. 17, 159–165 [DOI] [PubMed] [Google Scholar]