Abstract
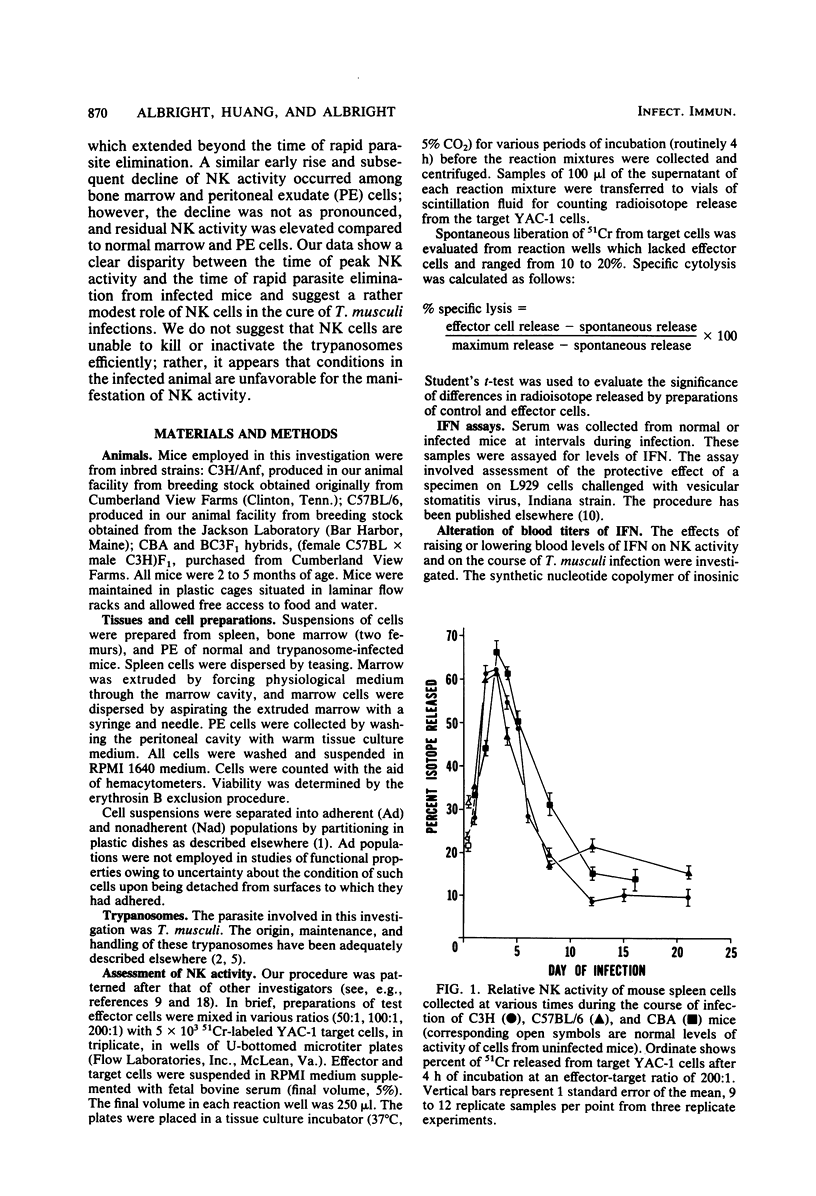
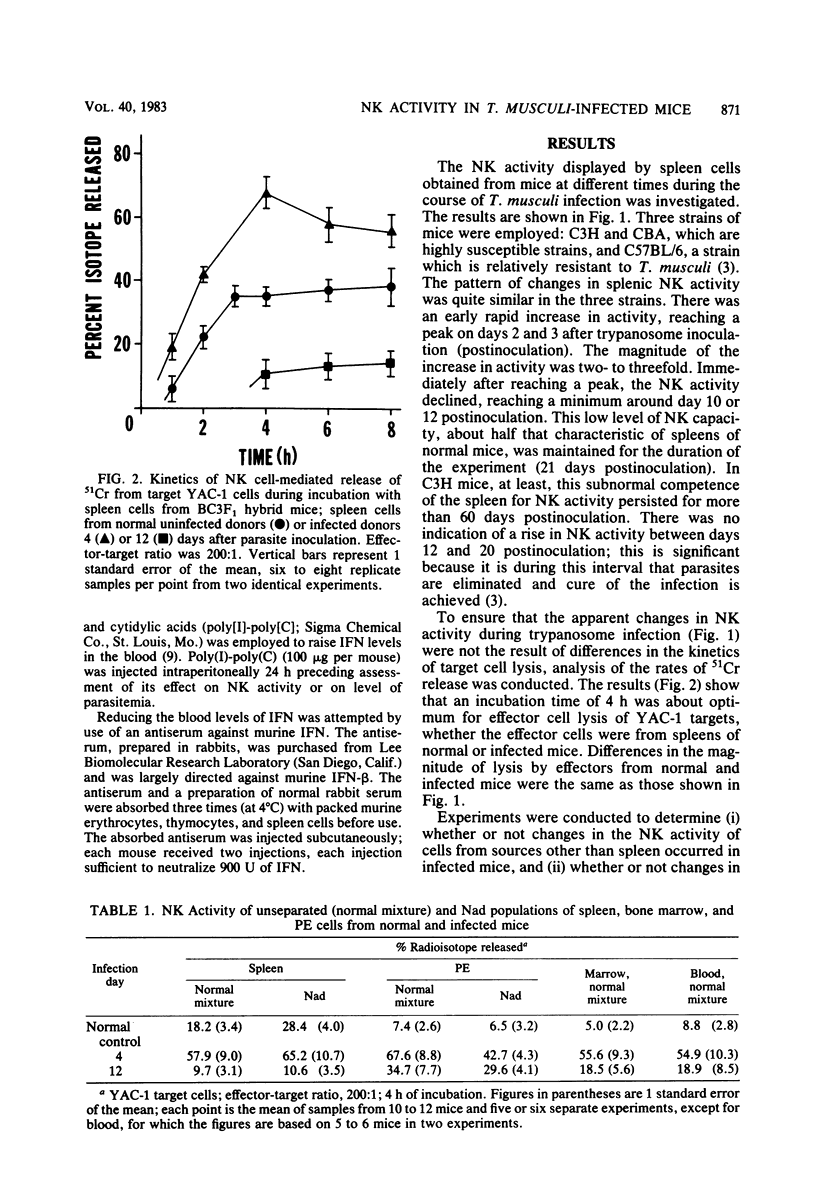
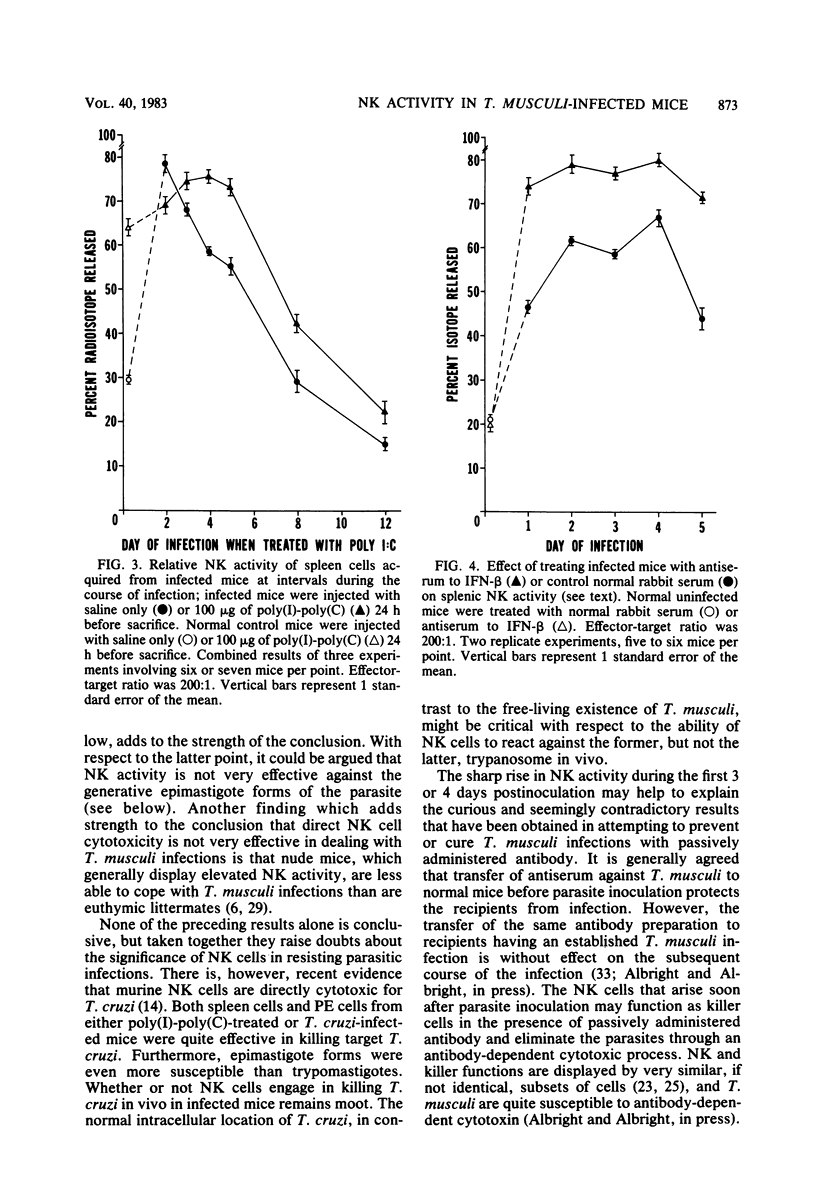
Trypanosoma musculi infection affected natural killer (NK) activity in mice. In the spleen, an increase of two to three times normal was displayed on days 2 to 4 after inoculation of parasites, followed by rapid decline to a subnormal level of activity that persisted for more than 3 weeks and included the phase of rapid parasite elimination. NK activity increased dramatically in peritoneal exudate and marrow early in infection, but the subsequent decline was more moderate than in the spleen. The subnormal splenic activity was not elevated by treating infected mice with an interferon inducer, polyinosinic acid-polycytidylic acid. Serum interferon levels were elevated early in infection, but by day 4 postinoculation, they had returned to undetectable. Injection of mice with antiserum to murine interferon-beta did not inhibit the early rise in NK activity or alter the course of trypanosome infection; in fact, the antiserum treatment enhanced splenic NK activity in infected mice. The early rise and subsequent decline of NK activity did not correlate with the course of T. musculi infection and subsequent cure. The cause of the dramatic decline in splenic NK activity is under investigation; it could result, for example, from arousal of suppressor cells, inhibition by prostaglandins, or inhibition by trypanosome-derived substances. Thus, NK cells may be prevented from fulfilling their potential of attacking the extracellular trypanosomes by the effects of inhibitory substances.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright J. F., Deitchman J. W., Hassell S. A., Ozato K. Differential antibody production of adherent and nonadherent spleen cells transferred to irradiated and cyclophosphamide-treated recipient mice. J Reticuloendothel Soc. 1975 Apr;17(4):195–209. [PubMed] [Google Scholar]
- Albright J. W., Albright J. F. Differences in resistance to Trypanosoma musculi infection among strains of inbred mice. Infect Immun. 1981 Aug;33(2):364–371. doi: 10.1128/iai.33.2.364-371.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright J. W., Albright J. F., Dusanic D. G. Mechanisms of trypanosome-mediated suppression of humoral immunity in mice. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3923–3927. doi: 10.1073/pnas.75.8.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright J. W., Albright J. F. Inhibition of murine humoral immune responses by substances derived from trypanosomes. J Immunol. 1981 Jan;126(1):300–303. [PubMed] [Google Scholar]
- Albright J. W., Albright J. F. The decline of immunological resistance of aging mice to Trypanosoma musculi. Mech Ageing Dev. 1982 Dec;20(4):315–330. doi: 10.1016/0047-6374(82)90099-9. [DOI] [PubMed] [Google Scholar]
- Albright J. W., Albright J. F. Trypanosome-mediated suppression of murine humoral immunity independent of typical suppressor cells. J Immunol. 1980 May;124(5):2481–2484. [PubMed] [Google Scholar]
- Brooks B. O., Reed N. D. Thymus dependency of Trypanosoma musculi elimination from mice. J Reticuloendothel Soc. 1977 Dec;22(6):605–608. [PubMed] [Google Scholar]
- Cudkowicz G., Hochman P. S. Do natural killer cells engage in regulated reactions against self to ensure homeostasis? Immunol Rev. 1979;44:13–41. doi: 10.1111/j.1600-065x.1979.tb00266.x. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Heinbaugh J. A., Holden H. T., Herberman R. B. Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J Immunol. 1979 Jan;122(1):175–181. [PubMed] [Google Scholar]
- Donahoe R. M., Huang K. Y. Interferon preparations enhance phagocytosis in vivo. Infect Immun. 1976 Apr;13(4):1250–1257. doi: 10.1128/iai.13.4.1250-1257.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugui E. M., Allison A. C. Differences in susceptibility of various mouse strains to haemoprotozoan infections: possible correlation with natural killer activity. Parasite Immunol. 1980 Winter;2(4):277–292. doi: 10.1111/j.1365-3024.1980.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Gidlund M., Orn A., Wigzell H., Senik A., Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978 Jun 29;273(5665):759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- Hatcher F. M., Kuhn R. E., Cerrone M. C., Burton R. C. Increased natural killer cell activity in experimental American trypanosomiasis. J Immunol. 1981 Sep;127(3):1126–1130. [PubMed] [Google Scholar]
- Hatcher F. M., Kuhn R. E. Destruction of Trypanosoma cruzi by Natural killer cells. Science. 1982 Oct 15;218(4569):295–296. doi: 10.1126/science.6812218. [DOI] [PubMed] [Google Scholar]
- Hatcher F. M., Kuhn R. E. Spontaneous lytic activity against allogeneic tumor cells and depression of specific cytotoxic responses in mice infected with Trypanosoma cruzi. J Immunol. 1981 Jun;126(6):2436–2442. [PubMed] [Google Scholar]
- Hauser W. E., Jr, Sharma S. D., Remington J. S. Natural killer cells induced by acute and chronic toxoplasma infection. Cell Immunol. 1982 May 15;69(2):330–346. doi: 10.1016/0008-8749(82)90076-4. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Melder R. J., Ho M. Modulation of natural killer cell activity in mice after interferon induction: depression of activity and depression of in vitro enhancement by interferon. Infect Immun. 1982 Jun;36(3):990–995. doi: 10.1128/iai.36.3.990-995.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo-Amaize E. A., Salimonu L. S., Williams A. I., Akinwolere O. A., Shabo R., Alm G. V., Wigzell H. Positive correlation between degree of parasitemia, interferon titers, and natural killer cell activity in Plasmodium falciparum-infected children. J Immunol. 1981 Dec;127(6):2296–2300. [PubMed] [Google Scholar]
- Ojo E., Wigzell H. Natural killer cells may be the only cells in normal mouse lymphoid cell populations endowed with cytolytic ability for antibody-coated tumour target cells. Scand J Immunol. 1978 Apr;7(4):297–306. doi: 10.1111/j.1365-3083.1978.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Opremcak E. M., Bakenhaster K., Whisler R. L. NK and K cell characteristics of human lymphocytes enriched for subpopulations isolated from NK tumor cell conjugates. Cell Immunol. 1981 Mar 1;58(2):415–425. doi: 10.1016/0008-8749(81)90234-3. [DOI] [PubMed] [Google Scholar]
- Paige C. J., Figarella E. F., Cuttito M. J., Cahan A., Stutman O. Natural cytotoxic cells against solid tumors in mice. II. Some characteristics of the effector cells. J Immunol. 1978 Nov;121(5):1827–1835. [PubMed] [Google Scholar]
- Quan P. C., Kolb J. P., Lespinats G. NK activity in carrageenan-treated mice. Immunology. 1980 Aug;40(4):495–503. [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Roberts D. W., Weidanz W. P. Chronic infection with Trypanosoma musculi in congenitally athymic nude mice. Infect Immun. 1977 May;16(2):715–716. doi: 10.1128/iai.16.2.715-716.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savary C. A., Lotzová E. Suppression of natural killer cell cytotoxicity by splenocytes from Corynebacterium parvum-injected, bone marrow-tolerant, and infant mice. J Immunol. 1978 Jan;120(1):239–243. [PubMed] [Google Scholar]
- Stutman O., Paige C. J., Figarella E. F. Natural cytotoxic cells against solid tumors in mice. I. Strain and age distribution and target cell susceptibility. J Immunol. 1978 Nov;121(5):1819–1826. [PubMed] [Google Scholar]
- Targett G. A., Viens P. The immunological response of CBA mice to Trypanosoma musculi: elimination of the parasite from the blood. Int J Parasitol. 1975 Apr;5(2):231–234. doi: 10.1016/0020-7519(75)90034-x. [DOI] [PubMed] [Google Scholar]
- Wolfe S. A., Tracey D. E., Henney C. S. BCG-induced murine effector cells. II. Characterization of natural killer cells in peritoneal exudates. J Immunol. 1977 Sep;119(3):1152–1158. [PubMed] [Google Scholar]


