Abstract
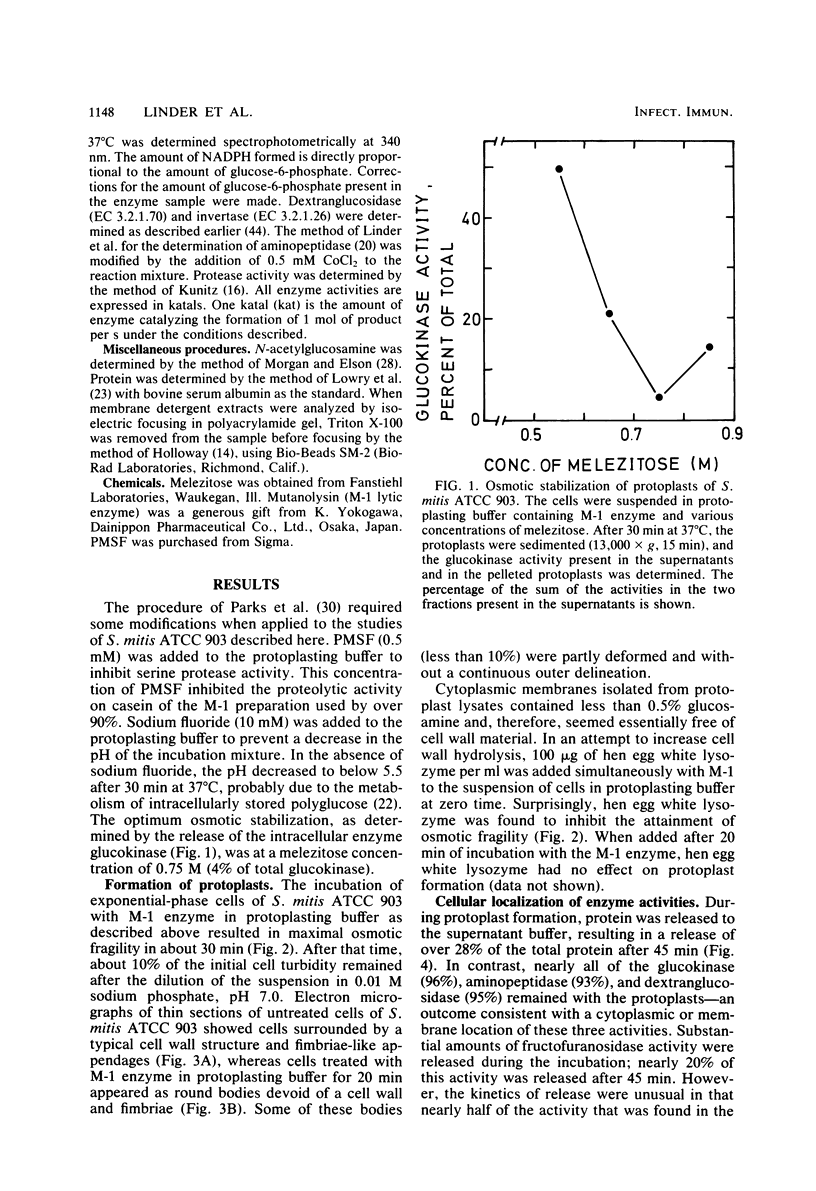
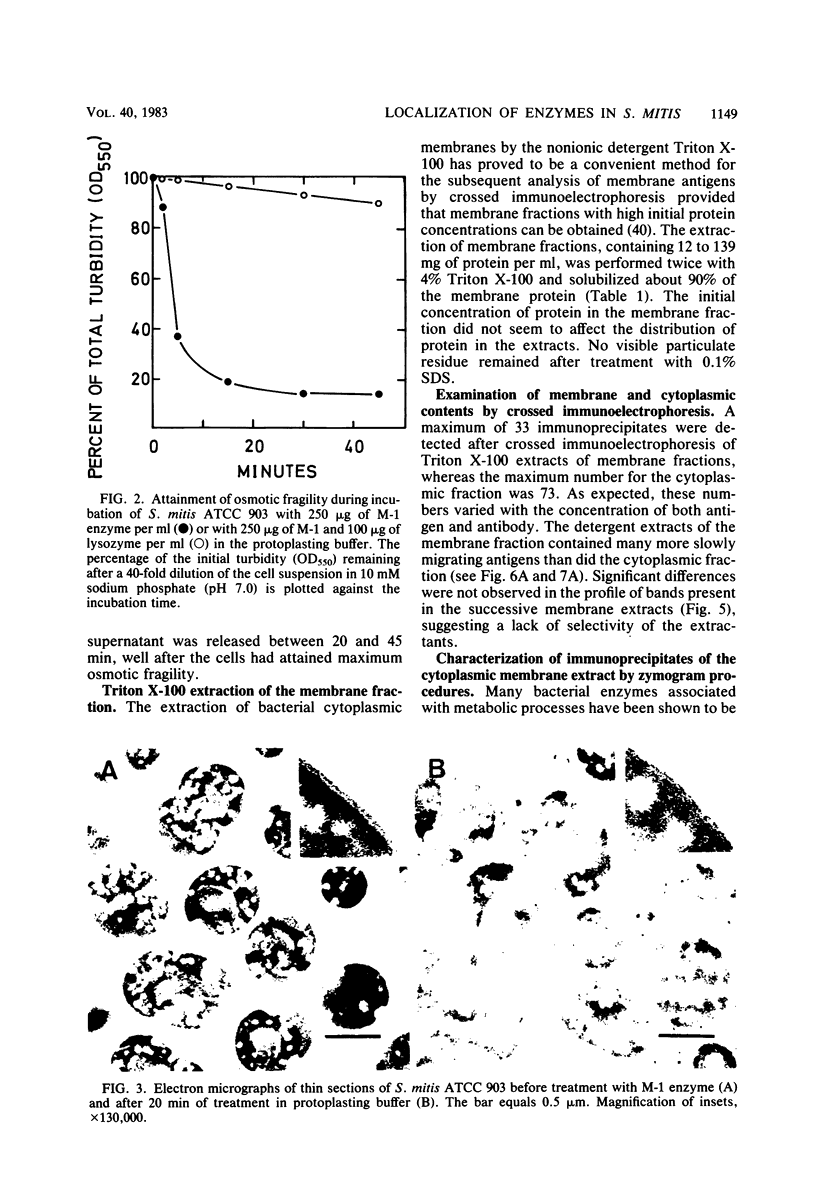
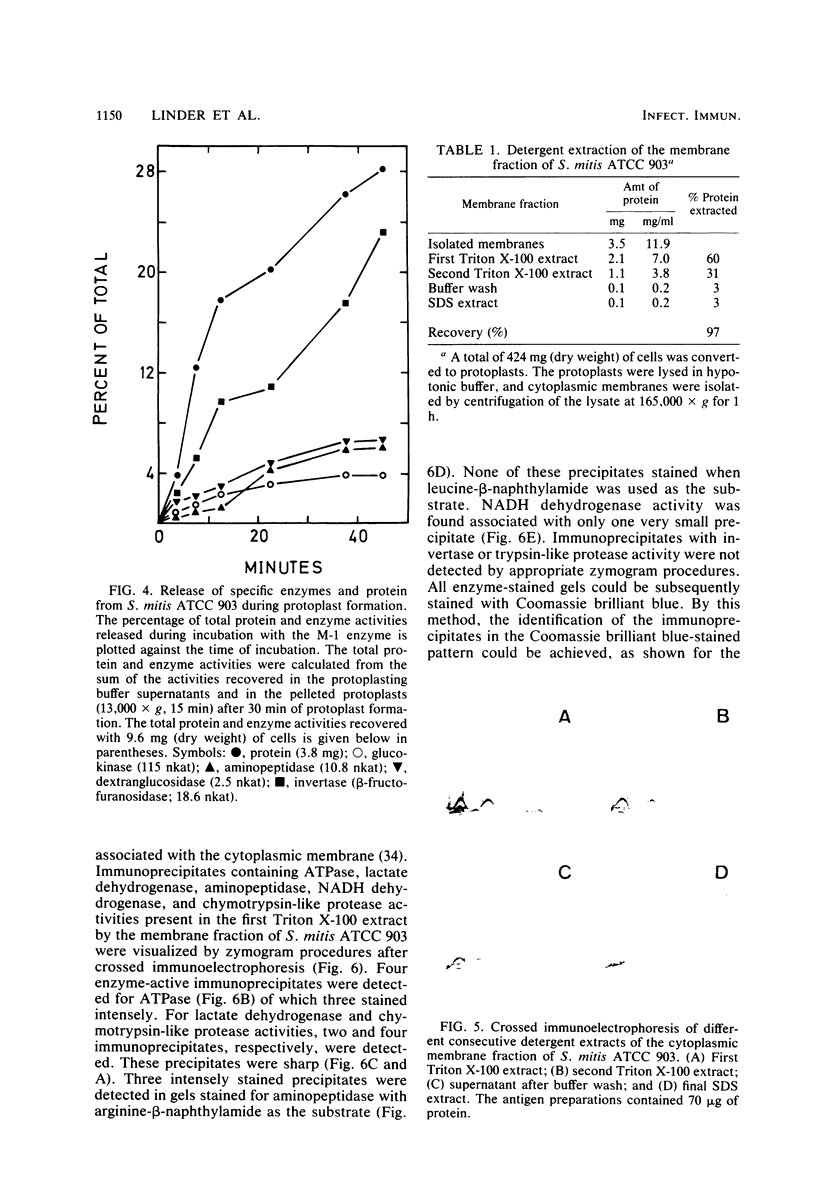
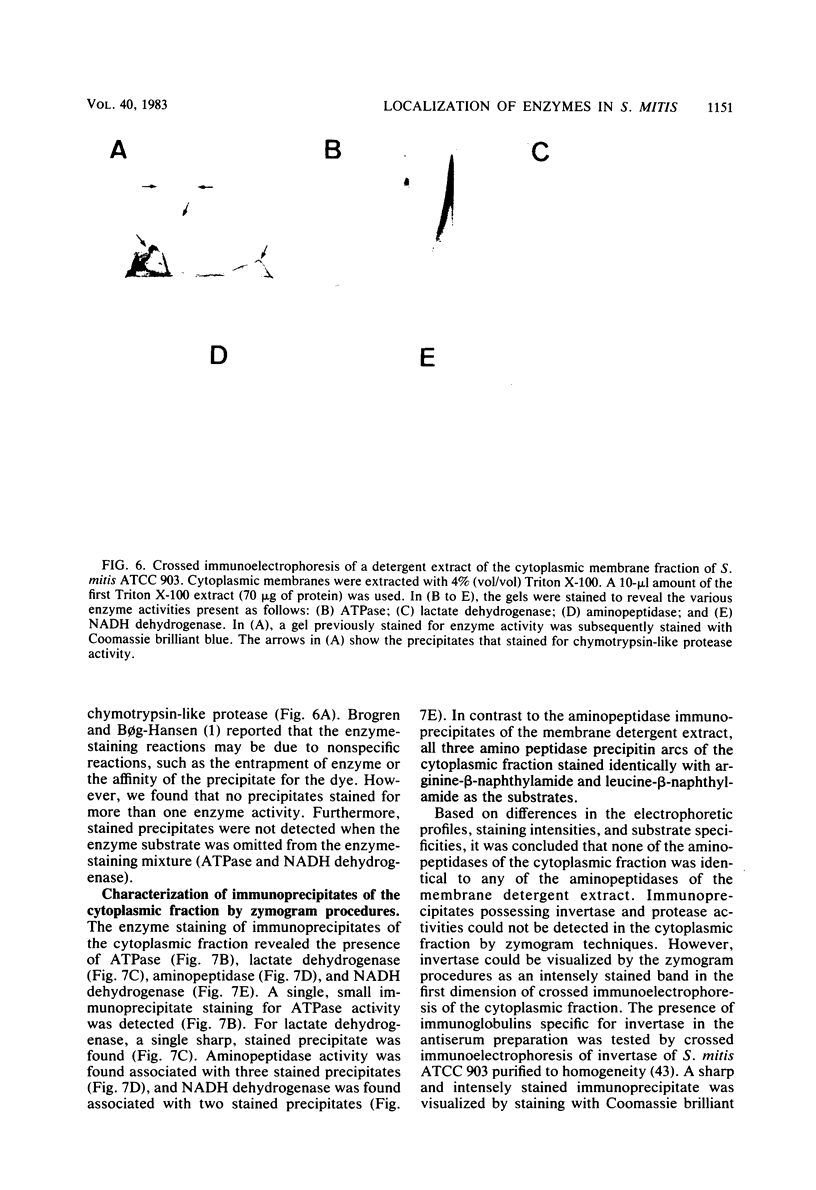
Cells of Streptococcus mitis ATCC 903 were converted to stable protoplasts by the cell wall-degrading M-1 enzyme of the mutanolysin complex isolated from Streptomyces globisporus. Over 90% of total glucokinase (EC 2.7.1.2), aminopeptidase (EC 3.4.11.1), and dextranglucosidase (EC 3.2.1.70) was recovered in the cytoplasmic fraction, whereas over 20% of total invertase (beta-fructofuranosidase: EC 3.2.1.26) was released during protoplast formation. ATPase (EC 3.6.1.3). chymotrypsin-like protease (EC 3.4.21.1), arginine aminopeptidase (EC 3.4.11.6), and lactate dehydrogenase (EC 1.1.1.27) were detected in Triton X-100 extracts of the cytoplasmic membrane fraction by crossed immunoelectrophoresis in combination with enzyme-staining procedures. By these methods, NADH dehydrogenase (EC 1.6.99.3), aminopeptidase, and lactate dehydrogenase were detected in the cytoplasmic fraction. Aminopeptidases in the cytoplasmic fraction differed from this activity in the membrane fractions in electrophoretic mobility and substrate specificity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calandra G. B., Cole R. M. Lysis and protoplast formation of group B streptococci by mutanolysin. Infect Immun. 1980 Jun;28(3):1033–1037. doi: 10.1128/iai.28.3.1033-1037.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Coleman S. E., van de Rijn I., Bleiweis A. S. Lysis of grouped and ungrouped streptococci by lysozyme. Infect Immun. 1970 Nov;2(5):563–569. doi: 10.1128/iai.2.5.563-569.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Lillmars K. A method for gentle lysis of Streptococcus sanguis and Streptococcus mutans. Biochem Biophys Res Commun. 1975 Jul 8;65(1):378–384. doi: 10.1016/s0006-291x(75)80104-5. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., de Stoppelaar J. D., Harden L. Lysozyme insensitivity of bacteria indigenous to the oral cavity of man. J Dent Res. 1966 May-Jun;45(3):877–881. doi: 10.1177/00220345660450036201. [DOI] [PubMed] [Google Scholar]
- Goodman H., Pollock J. J., Iacono V. J., Wong W., Shockman G. D. Peptidoglycan loss during hen egg white lysozyme-inorganic salt lysis of Streptococcus mutans. J Bacteriol. 1981 May;146(2):755–763. doi: 10.1128/jb.146.2.755-763.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H., Pollock J. J., Katona L. I., Iacono V. J., Cho M. I., Thomas E. Lysis of Streptococcus mutans by hen egg white lysozyme and inorganic sodium salts. J Bacteriol. 1981 May;146(2):764–774. doi: 10.1128/jb.146.2.764-774.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- Heefner D. L., Kobayashi H., Harold F. M. ATP-linked sodium transport in Streptococcus faecalis. II. Energy coupling in everted membrane vesicles. J Biol Chem. 1980 Dec 10;255(23):11403–11407. [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Jacques N. A., Wittenberger C. L. Inactivation of cell-associated fructosyltransferase in Streptococcus salivarius. J Bacteriol. 1981 Dec;148(3):912–918. doi: 10.1128/jb.148.3.912-918.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linder L. Extraction of cell-bound hyaluronidase and aminopeptidase from Streptococcus mitis, ATCC 903. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Oct;82B(5):593–601. doi: 10.1111/j.1699-0463.1974.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Linder L., Holme T., Frostell G. Hyaluronidase and aminopeptidase activity in cultures of streptococcus mitis, ATCC 903. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Aug;82(4):521–526. doi: 10.1111/j.1699-0463.1974.tb02360.x. [DOI] [PubMed] [Google Scholar]
- Linder L., Lindquist L., Söder P. O., Holme T. Estimation of cell lysis. Determination of aminopeptidase in extracts of Streptococcus mitis, ATCC 903. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Oct;82B(5):602–607. doi: 10.1111/j.1699-0463.1974.tb00226.x. [DOI] [PubMed] [Google Scholar]
- Linder L., Sund M. L. Characterization of dextran glucosidase (1,6-a-D-glucan glucohydrolase) of Streptococcus mitis. Caries Res. 1981;15(5):436–444. doi: 10.1159/000260549. [DOI] [PubMed] [Google Scholar]
- Mauck J., Glaser L. Periplasmic nucleoside diphosphate sugar hydrolase from Bacillus subtilis. Biochemistry. 1970 Mar 3;9(5):1140–1147. doi: 10.1021/bi00807a014. [DOI] [PubMed] [Google Scholar]
- Miller R. V., Becker J. M. Peptide utilization in Pseudomonas aeruginosa: evidence for membrane-associated peptidase. J Bacteriol. 1978 Jan;133(1):165–171. doi: 10.1128/jb.133.1.165-171.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. T., Elson L. A. A colorimetric method for the determination of N-acetylglucosamine and N-acetylchrondrosamine. Biochem J. 1934;28(3):988–995. doi: 10.1042/bj0280988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G., Coutinho A., Persson U. Mechanism of b-lymphocyte activation: failure to obtain evidence of a direct role of the Ig receptors in the triggering process. Scand J Immunol. 1975;4(1):37–52. doi: 10.1111/j.1365-3083.1975.tb02598.x. [DOI] [PubMed] [Google Scholar]
- Owen P., Salton M. R. Antigenic and enzymatic architecture of Micrococcus lysodeikticus membranes established by crossed immunoelectrophoresis. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3711–3715. doi: 10.1073/pnas.72.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks L. C., Shockman G. D., Higgins M. L. Growth of Streptococcus mutans protoplasts is not inhibited by penicillin. J Bacteriol. 1980 Sep;143(3):1491–1497. doi: 10.1128/jb.143.3.1491-1497.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S., Doak C. W., Kirk G. A novel mechanism for group translocation: substrate-product reutilization by gamma-glutamyl transpeptidase in peptide and amino acid transport. J Cell Physiol. 1976 Dec;89(4):853–863. doi: 10.1002/jcp.1040890453. [DOI] [PubMed] [Google Scholar]
- Roberts K. R., Linder L., Sund M. L. Glucose incorporation by human dental plaque bacteria. Caries Res. 1981;15(6):492–500. doi: 10.1159/000260557. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D., LAMPEN J. O. Inhibition by antibiotics of the growth of bacterial and yeast protoplasts. J Bacteriol. 1962 Sep;84:508–512. doi: 10.1128/jb.84.3.508-512.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Phillips P. M., Riley L. S., Toennies G. LYSIS OF STREPTOCOCCUS FAECALIS. J Bacteriol. 1961 Jan;81(1):36–43. doi: 10.1128/jb.81.1.36-43.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. L., Hurst S. F., Liberman E. S., Coleman S. E., Bleiweis A. S. Mutanolysin-induced spheroplasts of Streptococcus mutants are true protoplasts. Infect Immun. 1981 Feb;31(2):808–815. doi: 10.1128/iai.31.2.808-815.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Friedman-Kien A. E., Salton M. R. Antigenic analysis of Neisseria gonorrhoeae by crossed immunoelectrophoresis. Infect Immun. 1976 Apr;13(4):1273–1288. doi: 10.1128/iai.13.4.1273-1288.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Siegel J., Salton M. R., Owen P. Immunochemical analysis of inner and outer membranes of Escherichia coli by crossed immunoelectrophoresis. J Bacteriol. 1978 Jan;133(1):306–319. doi: 10.1128/jb.133.1.306-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sund M. L., Linder L., Andersson C. Isoelectric focusing studies on the beta-fructofuranosidases and alpha-glucosidases of Streptococcus mitis. J Gen Microbiol. 1978 Jun;106(2):337–342. doi: 10.1099/00221287-106-2-337. [DOI] [PubMed] [Google Scholar]
- Sund M. L., Linder L. Autolysis in strains of viridans streptococci. J Gen Microbiol. 1976 Sep;96(1):87–94. doi: 10.1099/00221287-96-1-87. [DOI] [PubMed] [Google Scholar]
- Sund M. L., Linder L. Cell-surface bound beta-fructofuranosidase (invertase) of the oral bacterium Streptococcus mitis. Arch Oral Biol. 1980;25(8-9):573–578. doi: 10.1016/0003-9969(80)90070-9. [DOI] [PubMed] [Google Scholar]
- Sund M. L., Linder L. Purification and characterization of beta-fructofuranosidase (invertase) from an oral strain of Streptococcus mitis. Arch Oral Biol. 1979;24(6):439–447. doi: 10.1016/0003-9969(79)90006-2. [DOI] [PubMed] [Google Scholar]
- Sund M. L., Linder L. Regulation of synthesis of beta-fructofuranosidase (invertase) in Streptococcus mitis. J Gen Microbiol. 1980 May;118(1):85–94. doi: 10.1099/00221287-118-1-85. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevithick J. R., Metzenberg R. L. Molecular sieving by Neurospora cell walls during secretion of invertase isozymes. J Bacteriol. 1966 Oct;92(4):1010–1015. doi: 10.1128/jb.92.4.1010-1015.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke B. A manual of quantitative immunoelectrophoresis. Methods and applications. 1. General remarks on principles, equipment, reagents and procedures. Scand J Immunol Suppl. 1973;1:15–35. doi: 10.1111/j.1365-3083.1973.tb03776.x. [DOI] [PubMed] [Google Scholar]
- Yokogawa K., Kawata S., Nishimura S., Ikeda Y., Yoshimura Y. Mutanolysin, bacteriolytic agent for cariogenic Streptococci: partial purification and properties. Antimicrob Agents Chemother. 1974 Aug;6(2):156–165. doi: 10.1128/aac.6.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]