Abstract
Background:
Osteoporosis is a major public health concern around the world. It has been shown that bone mineral density is correlated to anthropometric measures like height and weight, but this association may vary depending on ethnic and environmental factors. The aim of this study was to identify probable relations between anthropometric measures and bone mineral density.
Methods:
In this population-based study, we compiled the data collected from Iranian Multicenter Osteoporosis Study to assess the possible associations between different anthropometric indices and bone mineral density at femur and lumbar spine. The gathered data was analyzed using t-test and one way ANOVA.
Results:
Data was available for 4445 subjects, consisting 1900 males (42.7%) and 2545 females (57.3%). We observed statistically significant correlations between bone mineral density and height, weight, waist circumference, hip circumference, waist to hip ratio and body mass index (BMI). Based on the result of linear regression modeling studies, BMI could be considered an independent predictor of bone mineral density.
Conclusion:
Iranian population shows similar measures compared to analogous studies in other populations. Lower weight should be carefully considered as a predisposing factor for bone loss and osteoporosis.
Keywords: Osteoporosis, Anthropometric, Bone Mineral Density
Introduction
Osteoporosis is characterized by low bone mass (1). Bone mineral density (BMD) is a significant determinant of fracture risk (2). As a precursor of age related fractures, osteoporosis is a major public health problem. A previous study has showed that osteoporotic fractures imposed a burden of $13 billion to the United States in 1995 (3). With the growing life expectancy, one could expect this burden to increase as we moved to the 21st century. The prevalence of overweight and obesity is increasing in spite of public health efforts, even in the most developed nations (4). Several studies have attempted to discover associations between anthropometric measures such as weight, body surface area, height, and fat mass with BMD. A low body weight is shown to be associated with low bone mass and increase risk of fractures (5, 6). It has also been shown that body height is positively associated with higher calcium absorption (7). Body surface area, as a non-linear combination of weight and height, is consequently linked with osteoporotic fractures, while body mass index (BMI) is not (7). It has also been postulated that some anthropometric factors like patient’s weight could be used to increase the diagnostic value of BMD in women at risk of osteoporotic fractures (8). Recent studies have shown that obesity and osteoporosis share several common genetic and environmental factors (9). Obese osteoporotic patients are also affected by cardiac and metabolic problems more frequently than those with normal body mass are (10). On the other hand, weight loss regimens and surgeries put people at risk of osteoporosis, by causing calcium malabsorption (11). This is of particular importance when consultations are being made about weight loss in postmenopausal women. Previous studies have suggested that weight loss should occur at a moderate pace and be accompanied by higher calcium intake, to avoid bone loss (12). Interestingly, overweight women (BMI between 25 and 29.9 kg/m2) are prone to greater bone loss, compared to obese women (BMI between 30 and 40) (13). In other words, the association of anthropometric measures and bone mineral density is not only important at cross-sections, but also when they change over time, for example by weight loss regimens.
It has been shown that different ethnic groups may have different anthropometric measures and require population-specific cut-off points for these indices (14). This finding implicated similar studies to be undertaken in other countries. The initiation of Iranian Multicenter Osteoporosis Study (IMOS) in 2000 provided anthropometric and densitometry data for a random sample of Iranian population (15). In this study, we tried to identify probable relations between anthropometric measures like weight, height, BMI and WHR, and bone mineral density.
Materials and Methods
We based our study on the data gathered by IMOS, a national multicenter study which was conducted in duration of 3 mo in 2000. In this study, epidemiologic data were collected for the 4445 contributors who were sampled from healthy men and women with age 20–70 yr old residing in various regions of Iran using randomized clustered sampling.
Measured anthropometric indices included height, weight; waist circumference (WC) and hip circumference (HC) were measured by a trained person and a single method in all centers. Weight was measured using a digital electronic weighing scale without shoes with ±100 grams accuracy. Height was measured without shoes, in standing posture, by a tape meter stadiometer with 1 mm accuracy.
Body mass index was calculated by dividing weight to square of height, and subjects were categorized to four groups of underweight (BMI< 18.5 kg/m2), normal (BMI= 18.5–24.9 kg/m2), overweight (BMI= 25–29.9 kg/m2) and obese (BMI≥ 30 kg/m2) (16). Waist and hip circumferences were measured using the standard protocols and Waist-to-hip ratio (WHR) was simply calculated by dividing WC to HC.
Bone mineral density was measured in three different locations: neck of femur, total femur, and lumbar spines. Bone densitometry was performed using Dual-energy X-ray Absorptiometry (DXA) technique with a single protocol which was described previously (15) and a similar Lunar DPX device (Lunar 7164, GE, Madison, WI), in centers located in the cities of Tehran, Mashhad, Shiraz and Tabriz, and the results were reported in g/cm2. The machine was calibrated daily, and the coefficients of variation (CVs) of DEXA measurements at the lumbar spine and the hip were 1–1.5% and 2–2.5%. Several other variables were also measured which are beyond the interest of this study. Individuals taking drugs with known effect on bone mineral density were excluded from this study, as were those with conditions corresponding to secondary osteoporosis, such as thyroid and parathyroid diseases, malignancy, DM-1, CRF, CHF, adrenal insufficiency, metabolic bone disease, rheumatoid arthritis and history of amenorrhea or infertility. Details of design and methods of this study were explained previously (15).
Data were analyzed by means of SPSS version 13.0 (SPSS inc) using t-test and one way ANOVA (for comparing the mean of BMD in BMI categories). Variables were normally distributed and there was a linear relationship between the dependent and independent variables.
According to this fact that the epidemiological conditions and risk factors for osteoporosis in men and women are different, we examined the relations between anthropometric measures and BMD in both genders separately. Finally, linear regression analysis was done to determine the independent effect of variables related with BMD.
Results
Data were completely available for 4445 subjects, consisting of 1900 males (42.7%) and 2545 females (57.3%). A summary of demographic and anthropometric features of the population is presented in Table 1.
Table 1:
Demographic and anthropometric features of the population, separated by gender and city of residence
| Tehran n (%) | Mashhad n (%) | Tabriz n (%) | Shiraz n (%) | ||
|---|---|---|---|---|---|
| Male | Age | 44 (15) | 43 (14) | 41 (15) | 46 (15) |
| Height | 169 (8) | 170 (8) | 174 (7) | 167 (10) | |
| Weight | 75 (13) | 74 (12) | 75 (13) | 69 (13) | |
| WC | 90 (13) | 91 (12) | 93 (12) | ||
| HC | 98 (11) | 101 (9) | 105 (8) | ||
| Female | Age | 43 (12) | 41 (11) | 41 (15) | 44 (15) |
| Height | 156 (7) | 157 (6) | 159 (6) | 166 (10) | |
| Weight | 68 (14) | 67 (13) | 69 (13) | 67 (13) | |
| WC | 91 (13) | 86 (12) | 93 (14) | ||
| HC | 106 (9) | 105 (12) | 108 (10) |
Height is measured in centimeters, weight is measured in kilograms. Waist circumference (WC) and hip circumference (HC) were also measured in centimeters. Values are presented as mean (standard deviation).
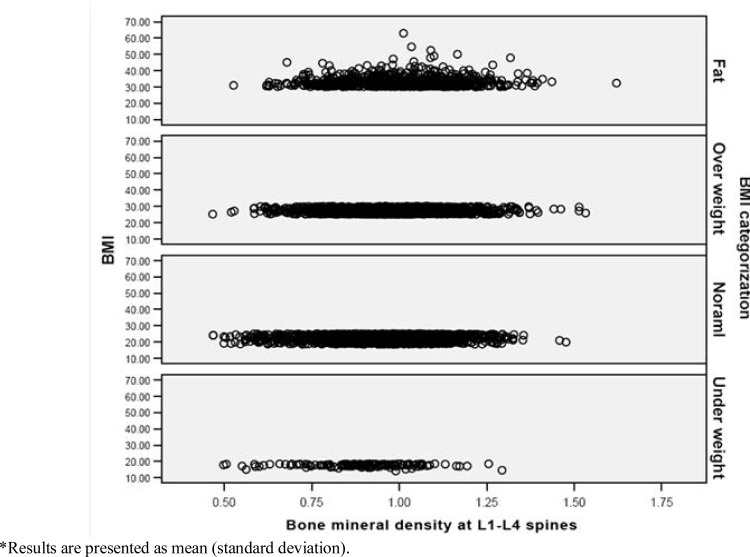
Correlation analysis was used to investigate the continuous variables related with BMD.BMD was significantly correlated to weight, height, body mass index and waist-to-hip ratio (P< 0.001, Table 2) in both males and females. This association existed regardless of the location of BMD measurement and it was generally stronger for WHR and weight (correlation coefficient was equal to 0.315 for WHR, 0.337 for weight and 0.191 for BMI, when bone mineral density of neck of femur was considered). With comparing the mean of BMD in BMI categories, there were significant differences between groups. Post hoc analysis showed that all groups were different from each other (P< 0.001, Table 3). Furthermore, there was a rising trend in BMD means when BMI raised in BMI categories. Distribution of bone mineral density results in different BMI categories in lumbar spines was shown in Fig. 1.
Table 2:
Correlations between different anthropometric measures and bone mineral density at different locations
| Neck of femur | Total femur | L1-L4 spines | ||
|---|---|---|---|---|
| Female | Height | 0.291 (<0.001) | 0.205 (<0.001) | 0.298 (<0.001) |
| Weight | 0.337 (<0.001) | 0.421 (<0.001) | 0.242 (<0.001) | |
| BMI | 0.191 (<0.001) | 0.323 (<0.001) | 0.128 (<0.001) | |
| WHR | 0.315 (<0.001) | 0.316 (<0.001) | 0.313 (<0.001) | |
| Male | Height | 0.387 (<0.001) | 0.299 (<0.001) | 0.235 (<0.001) |
| Weight | 0.306 (<0.001) | 0.382 (<0.001) | 0.367 (<0.001) | |
| BMI | 0.095 (<0.001) | 0.234 (<0.001) | 0.254 (<0.001) | |
| WHR | 0.367 (<0.001) | 0.355 (<0.001) | 0.403 (<0.001) |
BMI, body mass index; WHR, waist-to-hip ratio.
Data is presented as Pearson’s correlation coefficient (p-value). P-values lower than 0.05 are considered as statistically significant.
Table 3:
Bone mineral density (g/cm2) in different BMI categories
| Under weight Mean (SD) | Normal Mean (SD) | Over weight Mean (SD) | Obese Mean (SD) | P- Value | |
|---|---|---|---|---|---|
| Neck of femur | 0.715 (0.122) | 0.751 (0.136) | 0.767 (0.131) | 0.787 (0.127) | < 0.001 |
| Total femur | 0.821 (0.126) | 0.883 (0.144) | 0.926 (0.139) | 0.968 (0.138) | < 0.001 |
| L1-L4 spines | 0.891 (0.144) | 0.953 (0.151) | 0.978 (0.151) | 0.999 (0.154) | < 0.001 |
Fig. 1:
Distribution of spinal (L1-L4) mineral density in different BMI categories
Further analyses showed that a linear regression model fit for the effects of age, current smoking, current physical activity less than 3 times per week and BMI on BMD was statistically significant for all of the variables in different BMD sites (P< 0.001, Table 4), while a similar model fit for age, current smoking, current physical activity less than 3 times per week and WHR was statistically significant for WHR and physical activity in different sites of BMD (P< 0.001) and only in L1-L4 spines for age (P= 0.024, Table 5).
Table 4:
Linear regression model A fit for different demographic measures and BMI, to predict bone mineral density
| Neck of femur* | Total femur** | L1-L4 spines † | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Parameter | coefficient | P-value | coefficient | P-value | coefficient | P-value |
| Age | −0.006 | < 0.001 | −0.004 | 0.007 | −0.005 | < 0.001 |
| Current smoking | −0.031 | < 0.001 | −0.032 | 0.001 | −0.035 | < 0.001 |
| Current Physical activity (< 3/week) | −0.064 | < 0.001 | −0.066 | < 0.001 | −0.051 | < 0.001 |
| BMI | 0.007 | < 0.001 | 0.011 | < 0.001 | 0.007 | < 0.001 |
BMI, body mass index
R2 = 0.21
R2 = 0.25
R2 = 0.26
Table 5:
Linear regression model B fit for different demographic measures and WHR, to predict bone mineral density
| Neck of femur* | Total femur** | L1-L4 spines † | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Parameter | Coefficient | P-value | Coefficient | P-value | Coefficient | P-value |
| Age | −0.002 | 0.51 | −0.002 | 0.242 | −0.003 | 0.024 |
| Current smoking | −0.012 | 0.187 | −0.006 | 0.521 | −0.012 | 0.228 |
| Current Physical activity (< 3 /week) | −0.049 | < 0.001 | −0.052 | < 0.001 | −0.037 | 0.001 |
| WHR | 0.314 | < 0.001 | 0.352 | < 0.001 | 0.342 | < 0.001 |
WHR, waist-to-hip ratio
R2 = 0.29
R2 = 0.21
R2 = 0.26
Discussion
In this population-based study, we found a relatively mild correlation between WHR and BMD in the first place; and regarding the results of the regression analysis, it is likely that the association is not confounded by the other variables. Cigarette smoking, age and low physical activity have been implicated as risk factors for low bone mass and osteoporotic fractures in several reports (17, 18). Furthermore, the association between BMI and BMD, although not as strong, remained eminently significant when reassessed through a regression model indicating that the association is not being confounded, at least by the variables incorporated in the model.
Our study is similar to Johnell et al’s by design (2). They focused on the fact that the connection between lower body mass and higher risk of hip fractures may be partly due to the higher vulnerability of people with lean bodies to the effects of falling. To investigate if there is a direct connection between lower body mass and osteoporotic fractures, they focused on spine fractures. Their conclusion was that lower body weight is associated with presence of vertebral deformities. We noted that the correlation between different anthropometric factors and bone mineral density was of similar strength in different measures, particularly in spine. We believe this fortifies the findings of Johnell et al, and suggests that lower vertebral mineral density can plays a role in the pathophysiology of fractures in those with lower body mass. Furthermore, bone mineral density is under strong genetic control, for example polymorphism of vitamin D receptor has been suggested to account for some of the genetic variation in bone mass (19). Zhao et al took a different approach in their research on the relationship of obesity and osteoporosis (20). They divided their subjects into the two groups of Chinese and Caucasians, looking for the role of ethnicity and genetics in this association. They concluded that fat mass is inversely correlated with bone mass when the mechanical loading effects of body weight on bone mass were controlled. A common limitation of their study and ours is the cross-sectional design; the relationship between bone mass and obesity (or anthropometric measures used to describe obesity, e.g. body mass index) might be confounded by cohort effects. This implies that longitudinal studies should be carried out to investigate further the strength and pathophysiology of these relationships.
One clinical implication from our work relates to the use of simple weight adjustments for bone mass measurements. The International Society of Clinical Densitometry (ISCD) has showed that weight adjustments should not be applied to bone density measurements as a single concern about the validity of the corrections (21). Different ethnic groups have different fat distributions and assessment of soft tissue composition such as lean body mass or fat mass and adjustment for these differences can provide a more complete picture of ethnic differences in BMD (22). Although weight and body composition exert important effects on BMD, sex-specific differences have been shown in some studies. In the study on a group of healthy older men and women, fat mass has a positive effect on femoral neck BMD in white and black women but has no effect on men (23). In our study there was a positive relation between all anthropometric measures and BMD in both genders that can be explained by genetic determinants. However, our findings provide the best available data to date, which could be applied to the Iranian population.
Morin et al had previously confirmed that higher weight is accompanied by higher mineral density and lower risk of fractures in women aged 40 to 59 yr (24). Weight loss regimens and surgeries are in wide use around the world. Weight loss could result loss in bone mineral density, especially in obese women. This effect may vary depending on the method used for weight loss (25). We also noted a positive correlation between weight and bone mineral density in our study sample. Our data could not be used for further investigation about the effect of weight loss one BMD, due to the cross-sectional design of the study. Another limitation of this study is that nutritional state of this population was not evaluated exactly and these parameters may have impact on bone density.
In summary, our study confirms the presence of a positive association between anthropometric measures like weight and derivative measures like body mass index and waist-to-hip ratio with bone mineral density in different locations of bone mineral densitometry in a large random sample of Iranian population.
It should be noted that the present study has not adjusted the data for clustering effect; further studies are therefore needed to overcome this limitation.
Ethical Considerations
Ethical issues including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc. have been completely observed by the authors.
Acknowledgments
The authors of this study would like to thank the staff of the laboratory and BMD department (particularly Ms Shirazi and Ms Zare) of the Endocrinology and Metabolism Research Center, as without their help we would not be able to finish this job. The authors declare that there is no conflict of interests.
References
- 1.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367(9527):2010–18. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 2.Johnell O, O'Neill T, Felsenberg D, Kanis J, Cooper C, Silman AJ. Anthropometric measurements and vertebral deformities. European Vertebral Osteoporosis Study (EVOS) Group. Am J Epidemiol. 1997;146(4):287–93. doi: 10.1093/oxfordjournals.aje.a009269. [DOI] [PubMed] [Google Scholar]
- 3.Morales-Torres J, Gutierrez-Urena S. The burden of osteoporosis in Latin America. Osteoporos Int. 2004;15(8):625–32. doi: 10.1007/s00198-004-1596-3. [DOI] [PubMed] [Google Scholar]
- 4.Hopman WM, Leroux C, Berger C, Joseph L, Barr SI, Prior JC, et al. Changes in body mass index in Canadians over a five-year period: results of a prospective, population-based study. BMC Public Health. 2007;7:150. doi: 10.1186/1471-2458-7-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espallargues M, Sampietro-Colom L, Estrada MD, Sola M, del Rio L, Setoain J, et al. Identifying bone-mass-related risk factors for fracture to guide bone densitometry measurements: a systematic review of the literature. Osteoporos Int. 2001;12(10):811–22. doi: 10.1007/s001980170031. [DOI] [PubMed] [Google Scholar]
- 6.Shapses SA, Riedt CS. Bone, body weight, and weight reduction: what are the concerns? J Nutr. 2006;136(6):1453–56. doi: 10.1093/jn/136.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barger-Lux MJ, Heaney RP. Calcium absorptive efficiency is positively related to body size. J Clin Endocrinol Metab. 2005;90(9):5118–120. doi: 10.1210/jc.2005-0636. [DOI] [PubMed] [Google Scholar]
- 8.Dargent-Molina P, Douchin MN, Cormier C, Meunier PJ, Breart G. Use of clinical risk factors in elderly women with low bone mineral density to identify women at higher risk of hip fracture: The EPIDOS prospective study. Osteoporos Int. 2002;13(7):593–99. doi: 10.1007/s001980200078. [DOI] [PubMed] [Google Scholar]
- 9.Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, et al. Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res. 2008;23(1):17–29. doi: 10.1359/JBMR.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izmozherova NV, Popov AA. Postmenopausal osteoporosis in obese women. Klin Med (Mosk) 2008;86(3):44–46. [PubMed] [Google Scholar]
- 11.Hogan SL. The effects of weight loss on calcium and bone. Crit Care Nurs Q. 2005;28(3):269–275. doi: 10.1097/00002727-200507000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Riedt CS, Schlussel Y, von TN, Ambia-Sobhan H, Stahl T, Field MP, et al. Premenopausal overweight women do not lose bone during moderate weight loss with adequate or higher calcium intake. Am J Clin Nutr. 2007;85(4):972–80. doi: 10.1093/ajcn/85.4.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen TV, Sambrook PN, Eisman JA. Bone loss, physical activity, and weight change in elderly women: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res. 1998;13(9):1458–1467. doi: 10.1359/jbmr.1998.13.9.1458. [DOI] [PubMed] [Google Scholar]
- 14.Kagawa M, Binns CB, Hills AP. Body composition and anthropometry in Japanese and Australian Caucasian males and Japanese females. Asia Pac J Clin Nutr. 2007;16(Suppl 1):31–36. [PubMed] [Google Scholar]
- 15.Aghaei Meybodi HR, Heshmat R. Iranian osteoporosis research network: Back ground, misson and its role in osteoporosis management. Iranian J Public Health. 2008;1:1–7. [Google Scholar]
- 16.National Institute of Health, National. Heart, Lung, and Blood Institute Clinical Guidlines on the Identification, Evaluation, and diabetes risk in Japanese -Americans. Am J Clin Nutr. 2001;74:101–7. [Google Scholar]
- 17.Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315(7112):841–46. doi: 10.1136/bmj.315.7112.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyun-Ju S, Soo-Geun K, Chong-Soon K. Risk factors for bone mineral density at the calcaneus in 40–59 year-old male workers: A cross-sectional study in Korea. BMC Public Health. 2008;8:253–58. doi: 10.1186/1471-2458-8-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houston LA, Grant SFA, Reid DM, Ralston SH. Vitamin D Receptor Polymorphism, Bone Mineral Density, and Osteoporotic Vertebral Fracture: Studies in a UK Population. Bone. 1996;18(3):249–52. doi: 10.1016/8756-3282(95)00483-1. [DOI] [PubMed] [Google Scholar]
- 20.Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab. 2007;92(5):1640–46. doi: 10.1210/jc.2006-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leslie WD, Metge CJ, Weiler HA, Doupe M, Wood SP, O'Neil JD. Bone density and bone area in Canadian Aboriginal women: the First Nations Bone Health Study. Osteoporos Int. 2006;17(12):1755–62. doi: 10.1007/s00198-006-0184-0. [DOI] [PubMed] [Google Scholar]
- 22.Rush E, Plank L, Chandu V, Laulu M, Simmons D, Swinburn B, et al. Body size, body composition, and fat distribution: a comparison of young New Zealand men of European, Pacific Island, and Asian Indian ethnicities. N Z Med J. 2004;117(1207):U1203. [PubMed] [Google Scholar]
- 23.Taaffe DR, Cauley JA, Danielson M, Nevitt MC, Lang TF, Bauer DC, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001;16:1343–52. doi: 10.1359/jbmr.2001.16.7.1343. [DOI] [PubMed] [Google Scholar]
- 24.Morin S, Tsang JF, Leslie WD. Weight and body mass index predict bone mineral density and fractures in women aged 40 to 59 years. Osteoporos Int. 2009;20(3):363–70. doi: 10.1007/s00198-008-0688-x. [DOI] [PubMed] [Google Scholar]
- 25.Pritchard JE, Nowson CA, Wark JD. Bone loss accompanying diet-induced or exercise-induced weight loss: a randomised controlled study. Int J Obes Relat Metab Disord. 1996;20(6):513–520. [PubMed] [Google Scholar]