Abstract
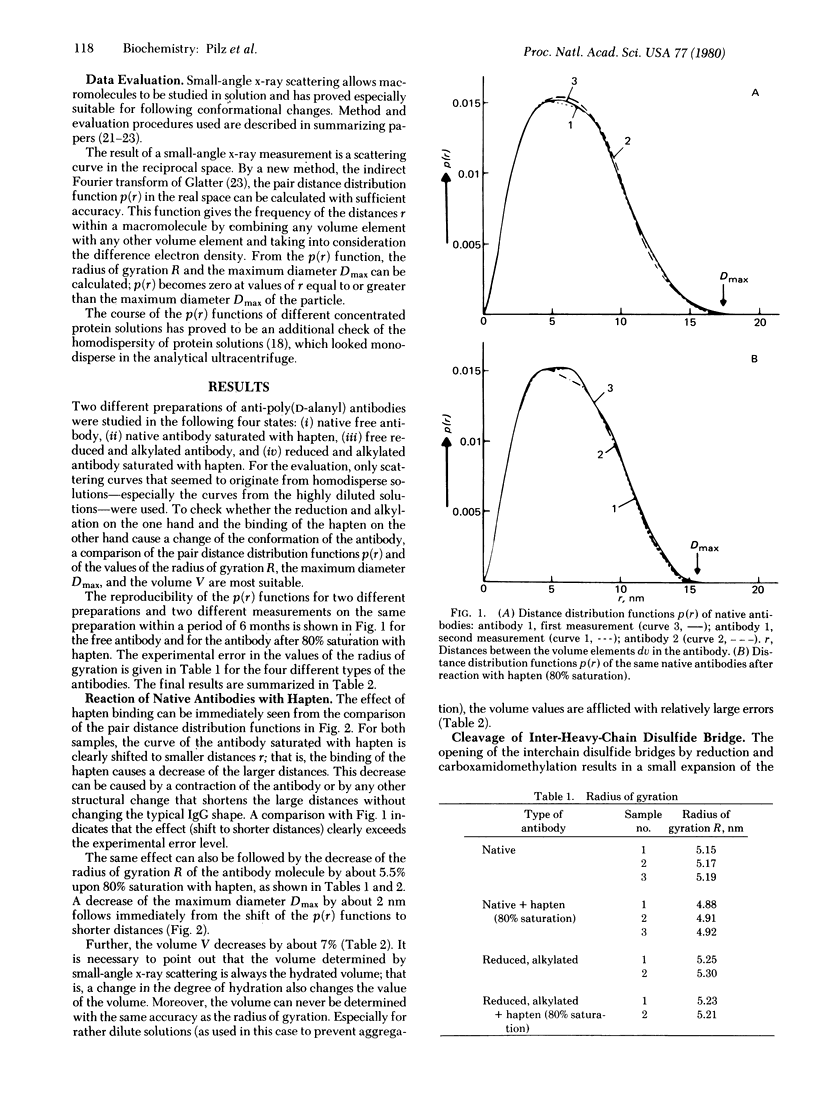
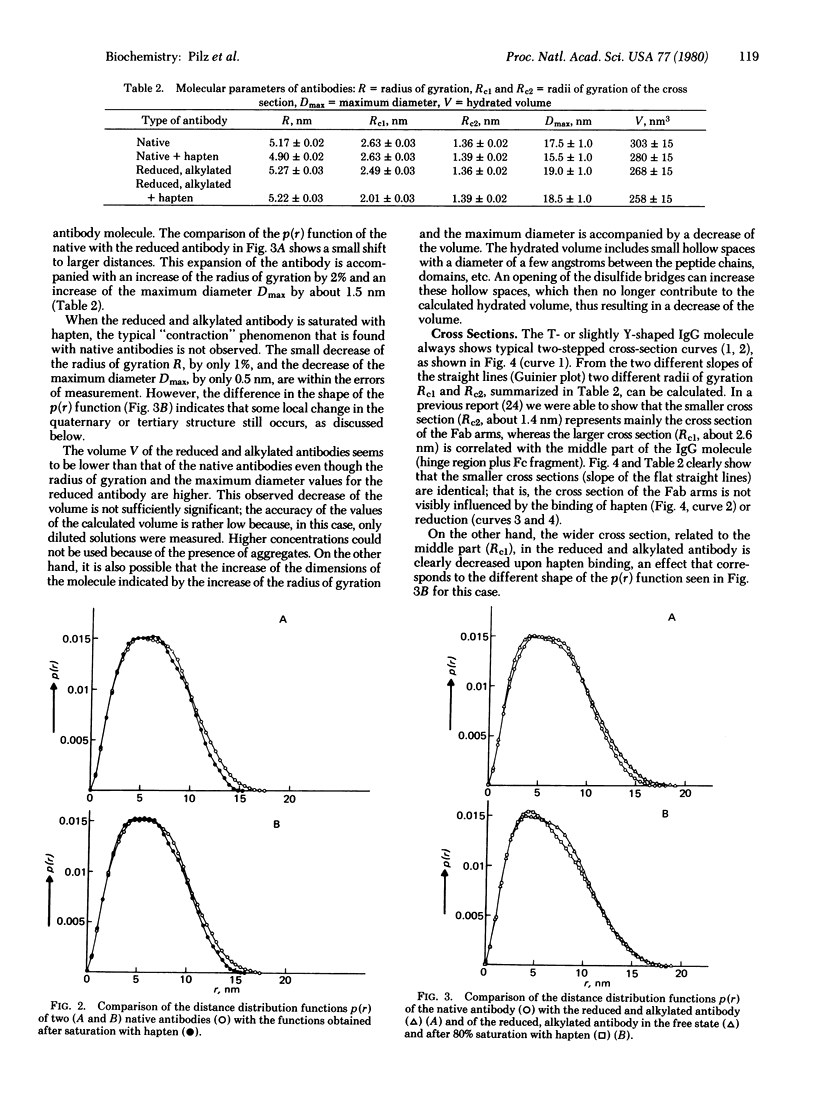
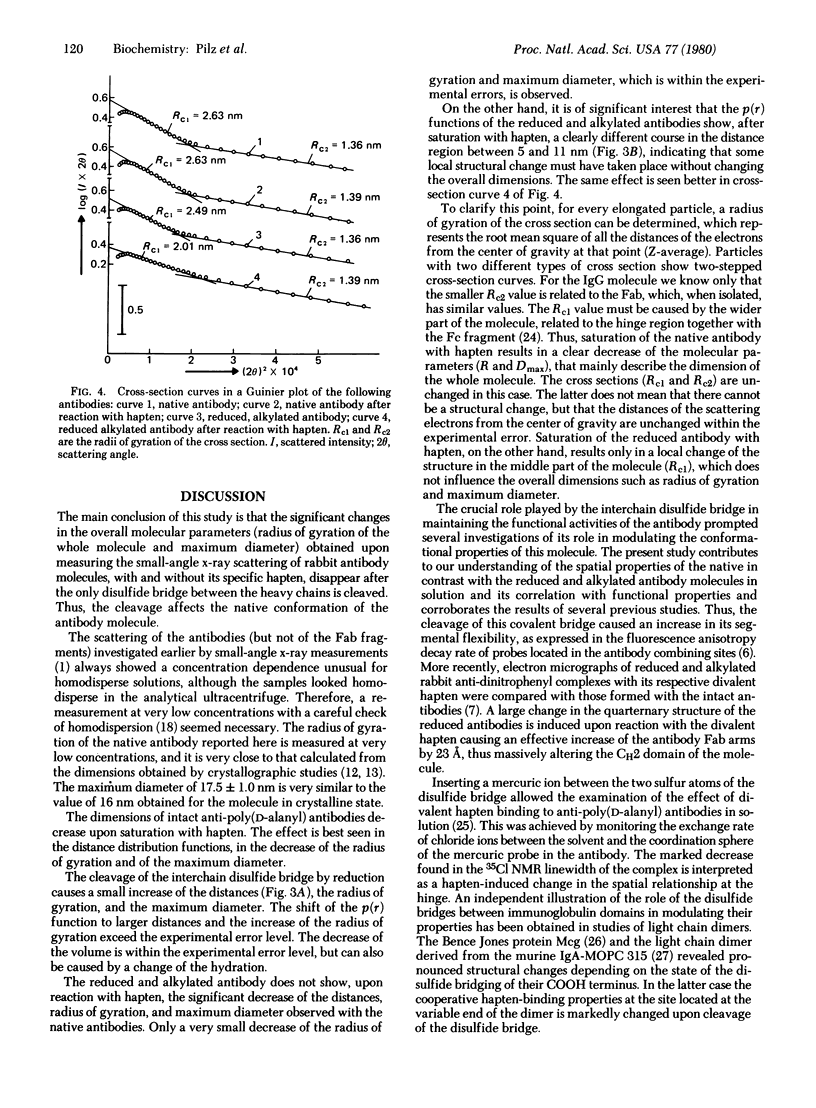
The small-angle x-ray scattering of solutions of rabbits IgG antibodies and their derivatives has been investigated. The reduction and alkylation of the native antibody cause a small increase of the molecular parameters, indicating a limited expansion of the molecule. Binding of native antipoly(D-alanyl) antibodies with hapten (80% saturation) causes a significant change of the quaternary structure, expressed by a decrease in the maximum diameter of about 2 nm, of the radius of gyration by 5.5%, and of the volume. The same antibodies, in which the single inter-heavy-chain disulfide bridge was opened by reduction and carboxamidomethylation, do not show any significant decrease in the overall molecular parameters upon reaction with hapten, except for a local structural change in a part of the molecule. These data lend further support to the notion that binding of hapten induces a conformational transition in its specific antibodies and suggest that the opening of the interchain disulfide bridges affects that transition. The dimensions of the intact antibodies calculated from measurements of small-angle x-ray scattering at low concentrations agree closely with those obtained from crystallographic studies.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan L. M., Cathou R. E. The role of the inter-heavy chain disulfide bond in modulating the flexibility of immunoglobulin G antibody. J Mol Biol. 1977 Jun 5;112(4):653–656. doi: 10.1016/s0022-2836(77)80170-8. [DOI] [PubMed] [Google Scholar]
- Cser L., Franek F., Gladkikh I. A., Nezlin R. S., Novotny J., Ostanevich Y. M. Neutron small-angle scattering study on two different precipitin types of pig anti-Dnp antibodies. FEBS Lett. 1977 Aug 15;80(2):329–331. doi: 10.1016/0014-5793(77)80468-7. [DOI] [PubMed] [Google Scholar]
- Dorrington K. J. The structural basis for the functional versatility of immunoglobulin G1. Can J Biochem. 1978 Dec;56(12):1087–1101. doi: 10.1139/o78-172. [DOI] [PubMed] [Google Scholar]
- Firca J. R., Ely K. R., Kremser P., Westholm F. A., Dorrington K. J., Edmundson A. B. Interconversion of conformational isomers of light chains in the Mcg immunoglobulins. Biochemistry. 1978 Jan 10;17(1):148–158. doi: 10.1021/bi00594a022. [DOI] [PubMed] [Google Scholar]
- Huber R., Deisenhofer J., Colman P. M., Matsushima M., Palm W. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976 Dec 2;264(5585):415–420. doi: 10.1038/264415a0. [DOI] [PubMed] [Google Scholar]
- Isenman D. E., Dorrington K. J., Painter R. H. The structure and function of immunoglobulin domains. II. The importance of interchain disulfide bonds and the possible role of molecular flexibility in the interaction between immunoglobulin G and complement. J Immunol. 1975 Jun;114(6):1726–1729. [PubMed] [Google Scholar]
- KRATKY O. X-RAY SMALL ANGLE SCATTERING WITH SUBSTANCES OF BIOLOGICAL INTEREST IN DILUTED SOLUTIONS. Prog Biophys Mol Biol. 1963;13:105–173. doi: 10.1016/s0079-6107(63)80015-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pilz I., Glatter O., Kratky O. Small-angle X-ray scattering. Methods Enzymol. 1979;61:148–249. doi: 10.1016/0076-6879(79)61013-3. [DOI] [PubMed] [Google Scholar]
- Pilz I., Kratky O., Karush F. Changes of the conformation of rabbit IgG antibody caused by the specific binding of a hapten. X-ray small-angle studies. Eur J Biochem. 1974 Jan 3;41(1):91–96. doi: 10.1111/j.1432-1033.1974.tb03247.x. [DOI] [PubMed] [Google Scholar]
- Pilz I., Kratky O., Licht A., Sela M. Shape and volume of fragments Fab' and (Fab')2 of anti-poly(D-alanyl) antibodies in the presence and absence of tetra-D-alanine as determined by small-angle x-ray scattering. Biochemistry. 1975 Mar 25;14(6):1326–1333. doi: 10.1021/bi00677a035. [DOI] [PubMed] [Google Scholar]
- Pilz I., Puchwein G., Kratky O., Herbst M., Haager O., Gall W. E., Edelman G. M. Small angle x-ray scattering of a homogeneous gamm G-1 immunoglobulin. Biochemistry. 1970 Jan 20;9(2):211–219. doi: 10.1021/bi00804a004. [DOI] [PubMed] [Google Scholar]
- Press E. M. Fixation of the first component of complement by immune complexes: effect of reduction and fragmentation of antibody. Biochem J. 1975 Jul;149(1):285–288. doi: 10.1042/bj1490285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUR P. H., CHRISTIAN G. D. THE ROLE OF DISULFIDE BONDS IN THE COMPLEMENT-FIXING AND PRECIPITATING PROPERTIES OF 7S RABBIT AND SHEEP ANTIBODIES. J Exp Med. 1964 Oct 1;120:531–545. doi: 10.1084/jem.120.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma V. R., Silverton E. W., Davies D. R., Terry W. D. The three-dimensional structure at 6 A resolution of a human gamma Gl immunoglobulin molecule. J Biol Chem. 1971 Jun 10;246(11):3753–3759. [PubMed] [Google Scholar]
- Schechter I. Mapping of the combining sites of antibodies specific to polyalanine chains. Ann N Y Acad Sci. 1971 Dec 31;190:394–419. doi: 10.1111/j.1749-6632.1971.tb13551.x. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Steinberg I. Z., Givol D., Hochman J., Pecht I. Antigen-induced conformational changes in antibodies and their Fab fragments studied by circular polarization of fluorescence. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2775–2779. doi: 10.1073/pnas.72.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegan G. W., Smith C. A., Schumaker V. N. Changes in quaternary structure of IgG upon reduction of the interheavy-chain disulfide bond. Proc Natl Acad Sci U S A. 1979 Feb;76(2):907–911. doi: 10.1073/pnas.76.2.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuk-Pavlović S., Isenman D. E., Elgavish G. A., Gafni A., Licht A., Pecht I. Hapten-induced structural changes in rabbit immunoglobulin G with specifically mercuriated inter-heavy-chain disulfide. Biochemistry. 1979 Apr 3;18(7):1125–1129. doi: 10.1021/bi00574a001. [DOI] [PubMed] [Google Scholar]
- Zidovetski R., Licht A., Pecht I. Effect of interchain disulfide bond on hapten binding properties of light chain dimer of protein 315. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5848–5852. doi: 10.1073/pnas.76.11.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]


