Abstract
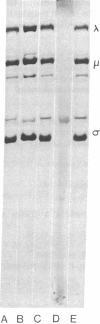
The translational apparatus in cell-free extracts prepared from L cells infected with reovirus undergoes a time-dependent transition from cap dependence to cap independence. Extracts from uninfected L cells translate capped reovirus mRNA at high efficiency and synthesize the expected three size classes of reovirus polypeptides, and the translation is sensitive to m7G(5')ppp. This same extract translates uncapped mRNA at a much lower efficiency. In contrast, extracts from infected L cells translate uncapped reovirus mRNA at high efficiency and synthesize the correct three size classes of polypeptides, and the translation is not sensitive to inhibition by m7G(5')ppp. Infected cell extracts translate capped mRNA at reduced efficiency (a,proximately 25%), the translation is not sensitive to inhibition by m7G(5')ppp, and the correct three size classes of viral polypeptides are not synthesized. These observations may explain how reovirus takes over the host translational apparatus.
Full text
PDF
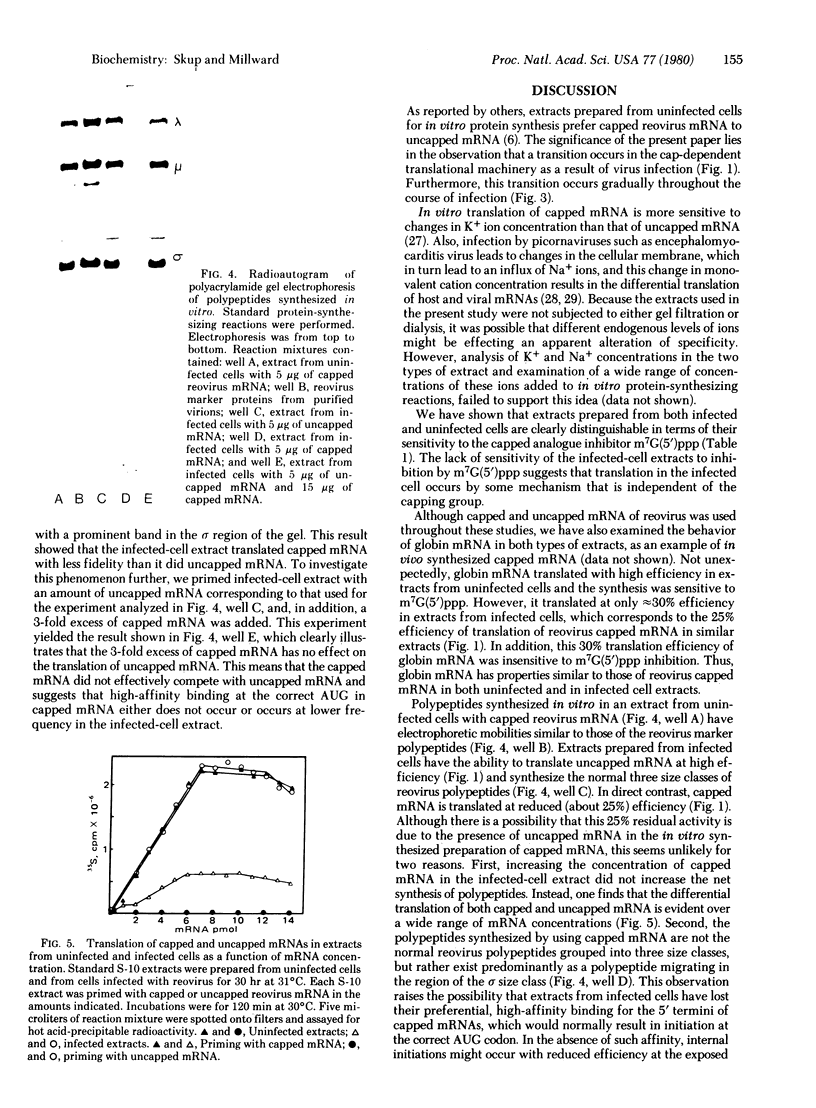

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Both G. W., Banerjee A. K., Shatkin A. J. Methylation-dependent translation of viral messenger RNAs in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1189–1193. doi: 10.1073/pnas.72.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani D., Revel M., Groner Y. Translational discrimination of 'capped' and 'non-capped' mRNAS: inhibition of a series of chemical analogs of m7GpppX. FEBS Lett. 1976 May 1;64(2):326–331. doi: 10.1016/0014-5793(76)80321-3. [DOI] [PubMed] [Google Scholar]
- Carrasco L. Membrane leakiness after viral infection and a new approach to the development of antiviral agents. Nature. 1978 Apr 20;272(5655):694–699. doi: 10.1038/272694a0. [DOI] [PubMed] [Google Scholar]
- Carrasco L., Smith A. E. Sodium ions and the shut-off of host cell protein synthesis by picornaviruses. Nature. 1976 Dec 23;264(5588):807–809. doi: 10.1038/264807a0. [DOI] [PubMed] [Google Scholar]
- Faust M., Hastings K. E., Millward S. m7G5'ppp5'GmptcpUp at the 5' terminus of reovirus messenger RNA. Nucleic Acids Res. 1975 Aug;2(8):1329–1343. doi: 10.1093/nar/2.8.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Munoz R., Darnell J. E. Structural difference between the 5' termini of viral and cellular mRNA in poliovirus-infected cells: possible basis for the inhibition of host protein synthesis. J Virol. 1976 May;18(2):719–726. doi: 10.1128/jvi.18.2.719-726.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Muthukrishnan S., Shatkin A. J. 5'-Terminal m-7G(5')ppp(5')G-m-p in vivo: identification in reovirus genome RNA. Proc Natl Acad Sci U S A. 1975 Feb;72(2):742–745. doi: 10.1073/pnas.72.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Shatkin A. J. A simple method for the preparation of [beta-32P]purine nucleoside triphosphase. Nucleic Acids Res. 1977 Oct;4(10):3341–3355. doi: 10.1093/nar/4.10.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Shatkin A. J. Differential synthesis of blocked and unblocked 5'-termini in reovirus mRNA: effect of pyrophosphate and pyrophosphatase. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3448–3452. doi: 10.1073/pnas.73.10.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedamu L., Dixon G. H. Effect of enzymatic "decapping" on protamine mRNA translation in wheat germ S-30. Biochem Biophys Res Commun. 1978 Nov 14;85(1):114–124. doi: 10.1016/s0006-291x(78)80018-7. [DOI] [PubMed] [Google Scholar]
- Groner Y., Grosfeld H., Littauer U. Z. 5'-Capping structures of Artemia salina mRNA and the translational inhibition by cap analogs. Eur J Biochem. 1976 Dec;71(1):281–293. doi: 10.1111/j.1432-1033.1976.tb11114.x. [DOI] [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A., Baglioni C. Inhibition of initiation of protein synthesis by 7-methylguanosine-5'-monophosphate. Proc Natl Acad Sci U S A. 1976 Jan;73(1):19–23. doi: 10.1073/pnas.73.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klootwijk J., Klein I., Zabel P., van Kammen A. Cowpea mosaic virus RNAs have neither m7GpppN ... nor mono-, di- or triphosphates at their 5' ends. Cell. 1977 May;11(1):73–82. doi: 10.1016/0092-8674(77)90318-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Rose J. K. Relative importance of 7-methylguanosine in ribosome binding and translation of vesicular stomatitis virus mRNA in wheat germ and reticulocyte cell-free systems. J Biol Chem. 1977 Feb 25;252(4):1181–1188. [PubMed] [Google Scholar]
- Lodish H. F. Translational control of protein synthesis. Annu Rev Biochem. 1976;45:39–72. doi: 10.1146/annurev.bi.45.070176.000351. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Both G. W., Furuichi Y., Shatkin A. J. 5'-Terminal 7-methylguanosine in eukaryotic mRNA is required for translation. Nature. 1975 May 1;255(5503):33–37. doi: 10.1038/255033a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Lodish H. F. Translation in vitro of vesicular stomatitis virus mRNA lacking 5'-terminal 7-methylguanosine. Nature. 1976 Jul 1;262(5563):32–37. doi: 10.1038/262032a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Trachsel H., Leong K., Baltimore D. Inhibition of translation by poliovirus: inactivation of a specific initiation factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2732–2736. doi: 10.1073/pnas.75.6.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg M., Silverstein S. C., Levin D. H., Acs G. Asynchronous synthesis of the complementary strands of the reovirus genome. Proc Natl Acad Sci U S A. 1971 Feb;68(2):505–508. doi: 10.1073/pnas.68.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz D. A., Weinstein J. A., Safer B., Merrick W. C., Weber L. A., Hickey E. D., Baglioni C. Evidence for role of m7G5'-phosphate group in recognition of eukaryotic mRNA by initiation factor IF-M3. Nature. 1976 May 27;261(5558):291–294. doi: 10.1038/261291a0. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Dasgupta R., Kaesberg P. 7-Methyl-guanosine and efficiency of RNA translation. J Virol. 1976 Aug;19(2):637–642. doi: 10.1128/jvi.19.2.637-642.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Kodama Y., Hashimoto J., Miura K. I. Importance of 5'-terminal blocking structure to stabilize mRNA in eukaryotic protein synthesis. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2734–2738. doi: 10.1073/pnas.74.7.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skup D., Millward S. Highly efficient translation of messenger RNA in cell-free extracts prepared from L-cells. Nucleic Acids Res. 1977 Oct;4(10):3581–3587. doi: 10.1093/nar/4.10.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Krystal G., Graham A. F. Regulated transcription of the genomes of defective virions and temperature-sensitive mutants of reovirus. J Virol. 1976 Apr;18(1):7–19. doi: 10.1128/jvi.18.1.7-19.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber L. A., Feman E. R., Hickey E. D., Williams M. C., Baglioni C. Inhibition of HeLa cell messenger RNA translation by 7-methylguanosine 5'-monophosphate. J Biol Chem. 1976 Sep 25;251(18):5657–5662. [PubMed] [Google Scholar]
- Weber L. A., Hickey E. D., Nuss D. L., Baglioni C. 5'-Terminal 7-methylguanosine and mRNA function: influence of potassium concentration on translation in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3254–3258. doi: 10.1073/pnas.74.8.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]