Abstract
Changes in phenotype and function of γδ T cells have been reported in inflammatory bowel disease (IBD), including Crohn's disease (CD) and ulcerative colitis (UC). Dysregulation of lymphocyte migration plays a key role in IBD pathogenesis; however, data on migratory properties of γδ T cells are scarce. Human circulating γδ T cells from healthy controls (n = 27), patients with active CD (n = 15), active UC (n = 14) or cutaneous manifestations of IBD (n = 2) were characterized by flow cytometry. Circulating γδ T cells in healthy controls were CD3hi and expressed CD45RO. They expressed gut-homing molecule β7 but not gut-homing molecule corresponding chemokine receptors (CCR)9, or skin-homing molecules cutaneous lymphocyte-associated antigen (CLA) and CCR4, despite conventional T cells containing populations expressing these molecules. CCR9 expression was increased on γδ T cells in CD and UC, while skin-homing CLA was expressed aberrantly on γδ T cells in patients with cutaneous manifestations of IBD. Lower levels of CD3 expression were found on γδ T cells in CD but not in UC, and a lower proportion of γδ T cells expressed CD45RO in CD and UC. Enhanced expression of gut-homing molecules on circulating γδ T cells in IBD and skin-homing molecules in cutaneous manifestations of IBD may be of clinical relevance.
Keywords: cell migration, cell trafficking, IBD, γδ T cells
Introduction
γδ T cells are innate lymphocytes that constitute only a minor T cell population in human peripheral blood, but are enriched at epithelial sites such as the skin, lung and gut [1]. Epithelial tissue is constantly exposed to pathogenic microorganisms, toxic substances and inflammatory molecules; one of the physiological functions of γδ T cells may be to control excessive inflammatory responses in epithelial tissues. Indeed, murine dendritic epidermal T cells expressing a γδ T cell receptor (TCR) are involved in the regulation of epidermal integrity and promote wound repair of the skin [2], whereas intestinal intraepithelial γδ T (γδ-IEL) cells regulate intestinal epithelial homeostasis [3,4]. γδ T cells can down-regulate αβ T cell responses which could otherwise result in severe immunopathology [5]. γδ T cells are also important in immune surveillance of the epithelium by providing a first line of defence against infectious invading pathogens, and regulating the bridge between innate and adaptive immunity [5,6].
Ulcerative colitis (UC) and Crohn's disease (CD), collectively termed inflammatory bowel disease (IBD), result from a dysregulated response of the mucosal immune system to components of the luminal microbiota, and breakdown of immune tolerance in individuals who are genetically predisposed to the disease [7–9]. Many pathways are involved in the induction of chronic inflammation in the gut mucosa in IBD. A role for γδ T cells in IBD was suggested originally by increased numbers of γδ T cells found in inflamed areas of the gut in humans with UC and CD [10–13], but there is also growing evidence that γδ T cells play a role in co-ordinating innate and acquired immune responses and maintaining the integrity of epithelial tissues [2,4–6,14]. Data from murine studies are conflicting, with some demonstrating a protective role for γδ T cells in models of colitis [15–18], but others suggesting that expansion and activation of γδ T cells is essential for the onset and aggravation of colitis [19–23].
Several mechanisms of immune dysregulation contribute to IBD, including disruption of lymphocyte homing and migration patterns. Effector T cells migrating to intestinal sites express high levels of the gut-homing molecule α4β7[24], with its ligand human mucosal addressin cell adhesion molecule-1 (MAdCAM-1) being expressed constitutively by post-capillary endothelial cells in the small intestine [25] and colonic lamina propria [26]. Interactions between α4β7 and MAdCAM-1 are key mediators of lymphocyte homing to intestinal sites [27]. In addition, interaction of chemokines such as CCL25 (TECK), with their corresponding chemokine receptors (CCR9), contribute to homing to the small intestine [28]. In animal models of colitis, expression of MAdCAM-1 is up-regulated in the intestinal lamina propria, and anti-MAdCAM-1 or α4β7 treatment results in reduced lymphocyte recruitment to the intestine [29–33]. MAdCAM-1, α4β7 and CCR9 expression are also dysregulated in humans with IBD [34–37].
Distribution patterns of circulating γδ T cells in peripheral tissues have not been well described, although the major blood subset of γδ T cells in macaques can enter the gut mucosa readily upon activation [38]. Although the mechanisms of specific recruitment of γδ T cells to the gut mucosa are unclear, human γδ T cells express CXCR3 [39], which binds chemokines CXCL9, CXCL10 and CXCL11, produced by local cells in inflammatory lesions. γδ T cells may be recruited to the gut mucosa by this method under inflammatory intestinal conditions; indeed, CXCR3 expression has been implicated in coeliac disease [40]. Similarly, human circulating γδ T cells can also express lymphocyte function-associated antigen (LFA)-1 [41], which recognizes intercellular adhesion molecule-1 (ICAM-1) [42], expressed by epithelial cells [43]. ICAM-1 expression is increased under inflammatory conditions [44] and tissue stress [45]. CCR2 expression has been implicated in γδ T cell trafficking during inflammation [46], although γδ T cells have yet to be defined for molecules involved in recruitment to the gut mucosa specifically.
IBD is associated with a variety of extra-intestinal manifestations (EIM), with up to a third of IBD patients developing cutaneous manifestations, including erythema nodosum (EN) and pyoderma gangrenosum (PG) [47]. The causes of EIM of IBD are poorly understood, but it has been suggested that compartmentalization of inflammatory processes to different organs (e.g. intestines, skin, liver) may be linked to homing and trafficking of immune cells. For example, CCL25, the ligand for gut-homing receptor CCR9, is expressed on epithelium in both the liver and the small intestine [48]. Skin T cells express E- and P-selectin ligands, including cutaneous lymphocyte-associated antigen (CLA) [49], thought to be involved in tissue-specific localization of cutaneous T cells within the skin [50]. Interaction between chemokine receptors CCR4 and CCR10 and their respective ligands CCL17 and CCL27 have also been implicated in skin-homing [51]; expression of skin-homing molecules may be dysregulated on immune cells in cutaneous manifestations of IBD.
The homing profile of γδ T cells has yet to be characterized in either healthy controls or IBD patients. We aimed to characterize the homing profile of human, circulating γδ T cells in healthy controls and to compare this profile to that of patients with active IBD.
Materials and methods
Human blood samples
Peripheral blood (20 ml) was collected into heparinized Vacutainer tubes (BD Biosciences, Oxford, UK) following informed consent (EC numbers 05/Q0405/71 and 08/H0717/24). Blood was obtained from healthy controls (n = 27; 12 female and 15 male; mean age 35 years) or from patients with active CD (n = 15; eight female and seven male; mean age 43 years) or UC (n = 14; six female and eight male; mean age 38 years). Diagnosis for patients with active CD and UC was made using clinical parameters, radiographic studies, endoscopic and histological criteria. Disease activity for UC was assessed using the UC disease activity index (UCDAI); all patients had a UCDAI > 4. Disease activity for CD was assessed using the CD activity index (CDAI); all patients had a CDAI > 220. All UC patients had pancolitis; CD patients were comprised of a mixture of Crohn's colitis (n = 4), small bowel CD only (n = 5), or both colonic and small bowel involvement (n = 6). Patients were either not receiving therapy or were on minimal treatment: 5-aminosalicylic acid (5-ASA) and/or azathioprine (AZA). Peripheral blood mononuclear cells (PBMC) were obtained by centrifugation over Ficoll-Paque Plus (Amersham Biosciences, Chalfont St Giles, UK).
Antibody labelling
Monoclonal antibodies with the following specificities and conjugations were used: CLA-fluorescein isothiocyanate (FITC) (HECA-452), CD103-FITC (Ber-ACT8), β7 integrin-phycoerythrin (PE) (FIB504), CD45RO-PE (UCHL1), CD3-peridinin chlorophyll cyanin 5·5 (PerCPCy5·5) (SK7), CD3-PECy5 (UCHT1), CLA-biotin (HECA-452) and strepavidin–allophycocyanin (APC) were purchased from BD Biosciences (Oxford, UK); CCR9 (either FITC- or APC-conjugated) (112509), CCR7-PE (150503), CCR10-APC (314315), CCR7-PE (150503) and CCR4-APC (205410) were purchased from R&D Systems (Abingdon, UK); appropriate isotype-matched control antibodies were purchased from the same manufacturers. After antibody labelling, cells were fixed with 1% paraformaldehyde in 0·85% saline and stored at 4°C prior to acquisition on the flow cytometer, within 48 h.
Flow cytometry and data analysis
Data were acquired on a fluorescence activated cell sorter (FACS)Calibur cytometer (BD Biosciences) and analysed using WinList 5·0 software (Verity, Topsham, ME, USA). T cells were identified as CD3+ lymphocytes within the PBMC population, and γδ T cells were identified as CD3+ lymphocytes expressing a γδ T cell receptor (TCR). The proportion of cells positive for a given marker was determined by reference to staining with an isotype-matched control antibody. WinList was used to subtract the normal cumulative histogram for isotype control staining from a similar histogram of staining with the test antibody using the superenhanced Dmax (SED) normalized subtraction.
Statistical analyses
Statistical analyses were carried out using GraphPad Prism software (GraphPad Software™, San Diego, CA, USA). Pooled data are expressed as mean values ± standard error; t-tests (paired and non-paired) were applied as stated in the figure legends. P < 0·05 was considered significant.
Results
Human circulating γδ T cells were CD3hi
Human circulating γδ T cells were identified by flow cytometry as CD3+ lymphocytes expressing a γδ TCR (Fig. 1a). Upon analysis of expression levels of CD3 per cell, measured via the mean fluorescence intensity (MFI) of CD3 staining, circulating γδ T cells were CD3hi in healthy controls, i.e. expressed a higher mean level of CD3 per cell than the total circulating T cell population (Fig. 1b).
Fig. 1.
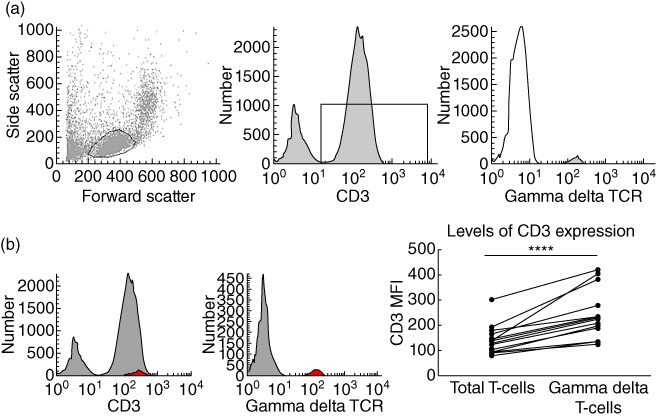
Identification of human circulating γδ T cells and levels of CD3 expression. (a) Identification of γδ T cells from peripheral blood mononuclear cells (PBMC) according to fluorescence activated cell sorter (FACS) plots of forward- and side-scatters, and subsequent CD3 histogram and γδ T cell receptor (TCR) histogram. This example is from a healthy control. γδ T cells comprised 5·2 ± 0·9% (n = 27) of the total circulating T cell population. (b) FACS histograms demonstrating γδ T cells (red) back-gated onto the CD3 peak using WinList™ software, and summary graphs demonstrating mean fluorescence intensity (MFI) of CD3 staining of total circulating T cells compared with γδ T cells (n = 27). Histograms are representative of 27 independent experiments and were compared to isotype-matched controls. Paired t-test was applied. A P-value < 0·05 was considered statistically significant (*P < 0·05; **P < 0·01; ***P < 0·001).
Human circulating γδ T cells expressed gut-homing marker β7 but not skin-homing markers
Expression of specific tissue-homing molecules on γδ T cells in peripheral blood, in particular skin- and gut-homing markers, were investigated. In healthy controls, the majority of circulating γδ T cells expressed gut-homing molecule β7 integrin, although expression of CCR9, a chemokine receptor involved in migration towards the small bowel, was minimal. β7 was expressed by a large fraction of the total T cell population, but a significantly greater proportion of cells expressed this gut-homing marker within the γδ T cell subset (Fig. 2a). Total T cells in healthy controls lacked CCR9+ cells (small bowel-homing) and included few CCR10+ cells (skin-associated migration), but incorporated significant numbers of CLA+ and CCR4+‘skin-homing’ cells. In contrast, circulating γδ T cells lacked putative ‘skin-homing’ subsets in healthy control blood (Fig. 2b).
Fig. 2.
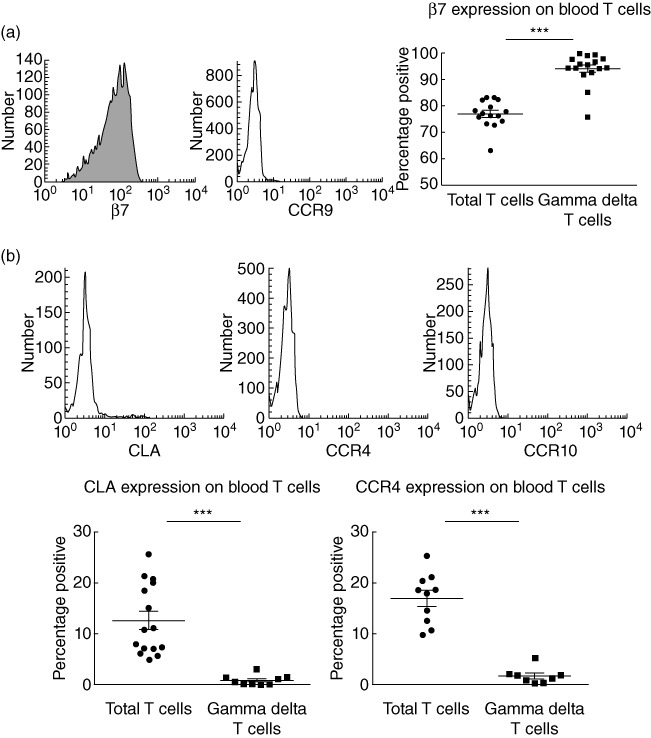
Expression of gut- and skin-homing markers on γδ T cells. (a) Fluorescence activated cell sorter (FACS) histograms demonstrating proportions of γδ T cells expressing β7 (93·9 ± 1·5%, n = 16) and corresponding chemokine receptor (CCR)9 (3·8 ± 1·4%, n = 11); comparison against total circulating T cells for expression of β7 (77·0 ± 1·3%, n = 16). (b) FACS histograms demonstrating proportions of γδ T cells expressing cutaneous lymphocyte-associated antigen (CLA) (0·8 ± 0·3%, n = 9), CCR4 (1·6 ± 0·6%, n = 8) and CCR10 (0·2 ± 0·1%, n = 5); comparison against total circulating T cells for expression of CLA (12·6 ± 1·8%, n = 15) and CCR4 (16·9 ± 1·6%, n = 10). Histograms are representative of several independent experiments performed with similar results and were compared to isotype-matched controls; t-test was applied. A P-value < 0·05 was considered statistically significant (*P < 0·05; **P < 0·01; ***P < 0·001). Error bars represent standard error of the mean, horizontal lines represent the mean.
Levels of CD3 were decreased on γδ T cells in Crohn's disease
Circulating γδ T cells in healthy controls were CD3hi, but circulating γδ T cells in patients with active CD expressed a significantly lower level of CD3 (CD3med cells; Fig. 3a,b), while γδ T cells from patients with active UC expressed comparable levels of CD3 to γδ T cells from healthy controls (Figs 1b and 3b).
Fig. 3.
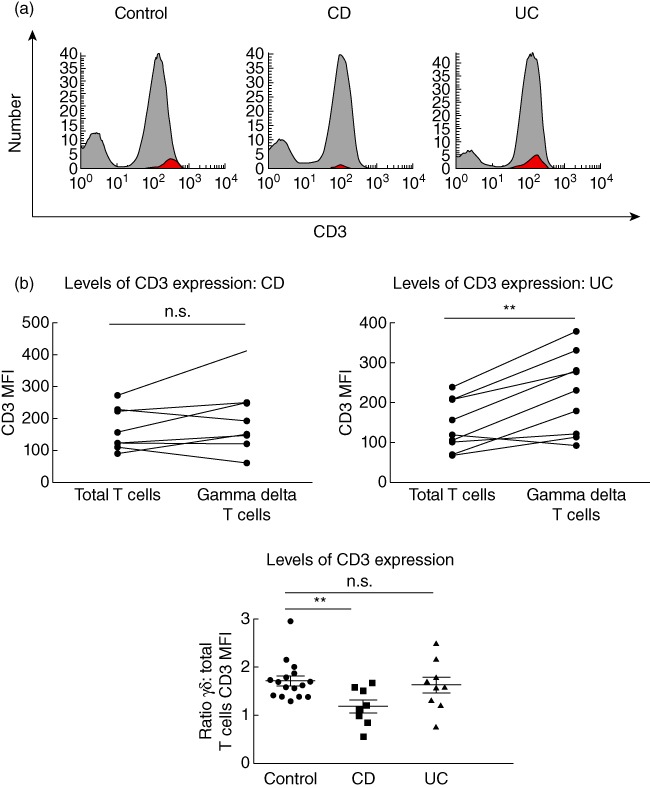
Reduced CD3 levels on γδ T cells in Crohn's disease but not ulcerative colitis. (a) Fluorescence activated cell sorter (FACS) histograms demonstrating levels of CD3 expression via γδ T cells (red) back-gated onto the CD3 peak using WinList™ software in healthy controls, active Crohn's disease (CD) patients and active ulcerative colitis (UC) patients. Histograms are representative of several independent experiments. (b) Summary graphs demonstrating mean fluorescence intensity (MFI) of CD3 staining of total circulating T cells compared with γδ T cells in active CD patients (n = 8) and active UC patients (n = 9), and summary graph demonstrating ratio of γδ T cell CD3 mean fluorescence intensity (MFI): total T cells CD3 MFI in healthy controls (1·6 ± 0·1, n = 16), active CD (1·2 ± 0·1, n = 8) and active UC (1·6 ± 0·2, n = 9). For comparison of total T cells versusγδ T cells CD3 MFI within the same individuals, paired t-tests were applied. For comparison of γδ T cell : total T cell CD3 MFI ratios between healthy controls, active CD and active UC patients, unpaired t-tests were applied. A P-value < 0·05 was considered statistically significant (*P < 0·05; **P < 0·01; ***P < 0·001; ***P < 0·001). Error bars represent standard error of the mean, horizontal line represents the mean.
Gut-homing marker CCR9 was increased on γδ T cells in Crohn's disease and ulcerative colitis
γδ T cells from healthy controls and patients with active CD, and active UC (no cutaneous manifestations) did not express skin-homing markers CLA, CCR4 or CCR10, and there were no differences in expression of gut-homing marker β7 between the two groups (data not shown). However, there was a significant increase in the proportion of γδ T cells expressing gut-homing marker CCR9 in both CD and UC, compared with γδ T cells from healthy controls (Fig. 4). This increase in CCR9 expression was not evident in the total circulating T cell population (data not shown).
Fig. 4.
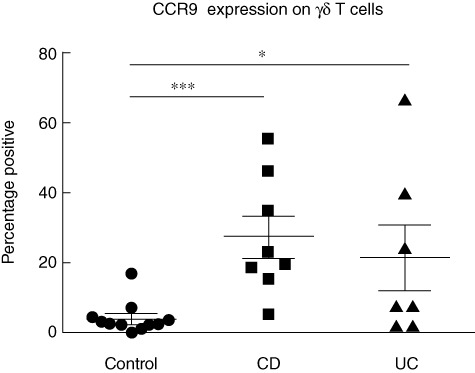
Increased proportion of corresponding chemokine receptors (CCR)9+γδ T cells in Crohn's disease (CD) and ulcerative colitis (UC). Proportions of circulating γδ T cells in healthy controls (3·8 ± 1·4%, n = 11), active CD patients (27·4 ± 5·9, n = 8) and active UC patients (21·4 ± 9·2%, n = 7) expressing gut-homing marker CCR9; t-test was applied. A P-value < 0·05 was considered statistically significant (*P < 0·05; **P < 0·01; ***P < 0·001). Error bars represent standard error of the mean, horizontal lines represent mean.
CD45RO expression was decreased on γδ T cells in Crohn's disease and ulcerative colitis
The majority of circulating γδ T cells display a pre-activated phenotype characterized by the expression of ‘memory marker’ CD45RO, allowing rapid induction of effector functions following detection of tissue stress [52]. We hypothesized that γδ T cell expression of CD45RO may be altered in IBD due to the chronic inflammation and tissue stress in the gut mucosa in IBD. Assessment of CD45RO expression on circulating T cells in healthy controls confirmed that the majority of γδ T cells in blood expressed CD45RO, but CD45RO expression was reduced significantly on γδ T cells from blood of patients with CD and UC without cutaneous manifestations, compared with healthy controls (Fig. 5). This change was not evident among the total circulating T cell pool (data not shown).
Fig. 5.
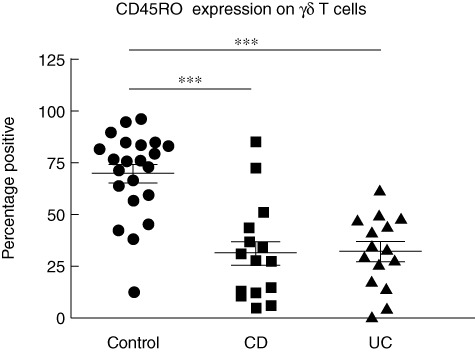
Reduced proportion of CD45RO+γδ T cells in Crohn's disease (CD) and ulcerative colitis (UC). Proportions of circulating γδ T cells in healthy controls (69·8 ± 4·4%, n = 22), active CD patients (31·4 ± 6·2%, n = 15) and active UC patients (32·1 ± 4·5%, n = 15) expressing CD45RO; t-test was applied. A P-value < 0·05 was considered statistically significant (***P < 0·001). Error bars represent standard error of the mean, horizontal lines represent mean.
Because γδ T cells can up-regulate expression of lymph-node homing marker CCR7 upon activation [53], we hypothesized that γδ T cell expression of CCR7 may also be altered in IBD. CCR7 expression was variable on γδ T cells from healthy controls (range 0–46·4%), but expression of CCR7 on γδ T cells was unaltered in CD or UC without cutaneous manifestations (data not shown).
Unique population of skin-homing γδ T cells in cutaneous manifestations of inflammatory bowel disease
The homing profiles of γδ T cells in two patients with skin manifestations of IBD were also analysed. EN is one of the more common cutaneous manifestations of IBD, the causes of which are poorly understood, but the occurrence of EN is rare. In light of the extensive data collected in healthy controls and in IBD patients without EN with negligible expression of CLA and low standard errors, we considered two EN samples to be informative.
Although expression of skin-homing marker CLA was negligible on γδ T cells in both healthy controls and patients with active IBD without EN, aberrant expression of CLA on γδ T cells was detected in patients with IBD and EN. This unique population of CLA+γδ T cells in EN did not express the gut-homing marker β7 integrin, suggesting that this small population of cells were skin-homing γδ T cells (Fig. 6a). Expression of other tissue-homing markers on γδ T cells was unaffected, including gut-homing markers β7 and CCR9 and skin-homing markers CCR4 and CCR10 (data not shown).
Fig. 6.
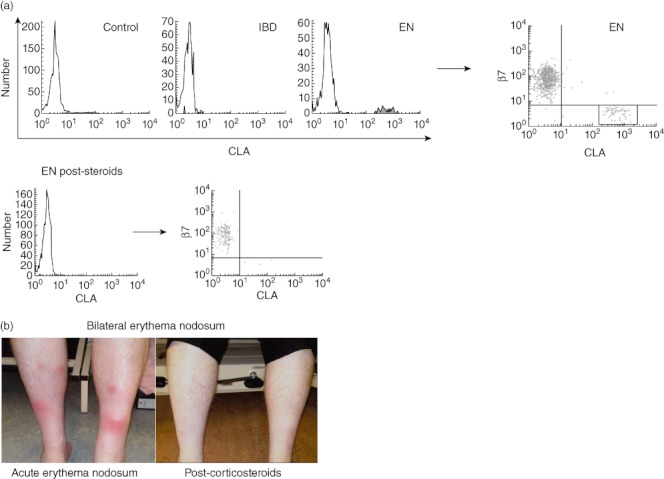
Aberrant expression of cutaneous lymphocyte-associated antigen (CLA) on γδ T cells in erythema nodosum. (a) Fluorescence activated cell sorter (FACS) histograms demonstrating proportions of circulating γδ T cells in healthy controls (0·8 ± 0·3%, n = 9), active inflammatory bowel disease (IBD) (1·2 ± 0·4%, n = 15) and erythema nodosum (EN) with inactive IBD (9·6% and 5·7%, n = 2). Bottom row: EN post-steroids (1·3%, n = 1; pre-steroids was 9·6%) expressing CLA. Histograms are representative of several independent experiments performed with similar results, and were compared to isotype-matched controls; FACS dot-plot demonstrating proportions of circulating γδ T cells in EN co-expressing gut-homing marker β7 and skin-homing marker CLA, expressing β7 only, or expressing CLA only. All plots were compared to isotype-matched controls. (b) Photographs of shins of EN patient pre- and post-corticosteroids.
Finally, an EN patient administered oral corticosteroids (prednisolone, 40 mg daily) underwent rapid resolution of the EN and improvement in general wellbeing (Fig. 6b). Following corticosteroid administration, the CLA+β7– abnormal skin-homing γδ T cell population was no longer detected in the circulation; circulating γδ T cells were CLA–β7+ (Fig. 6a).
Discussion
We demonstrate for the first time that circulating γδ T cells in the steady state express gut-homing marker β7 but do not express molecules enabling migration towards cutaneous sites. γδ T cells have not been analysed previously for expression of molecules involved in cell trafficking in the context of IBD. In this study, we identified and characterized γδ T cells exhibiting dysregulation of their homing properties; a subset of altered gut-homing γδ T cells was detected in both CD and UC, and a subset of skin-homing γδ T cells was present in EN (a cutaneous manifestation of IBD).
In contrast to rapid recruitment of effector lymphocytes to sites of inflammation [27], the mechanisms controlling steady state traffic of T cells and their maintenance within healthy tissue are not well understood [54–57]. Although human circulating γδ T cells did not express skin-homing markers or gut-homing marker CCR9 in the steady state, the majority expressed β7 gut-homing integrin. β7 combines with α4 integrin to allow leucocytes to enter intestinal tissue via interactions with MAdCAM-1 [25,26,58]. While circulating T cells can be either gut-homing or skin-homing [51,59], in the current report we demonstrate that circulating γδ T cells are mainly gut-homing. These data may reflect the suggested role for γδ T cells in intestinal homeostasis [3,4] and support studies demonstrating that the major blood subset of γδ T cells in primates (Vγ2 Vδ2 T cells) can enter the gut mucosa readily upon activation [38].
The current report is consistent with previous studies demonstrating changes in the phenotype and function of human γδ T cells in skin inflammation [60,61] and in IBD [11]. The increased expression of skin-homing molecule CLA on γδ T cells in EN and enhanced gut-homing receptor CCR9 expression on γδ T cells in CD and UC without cutaneous manifestations may reflect the recruitment of γδ T cells from the circulation to the inflamed skin and gut, respectively. Indeed, homing of γδ T cells to intestinal sites is impaired in CCR9-deficient mice [62] and a role for skin-homing γδ T cells has been reported in inflammatory skin disease [63].
The alterations in CCR9 expression in IBD without any observed changes in gut-homing β7 expression may be due to the inherently high expression of β7 on γδ T cells in healthy controls. Our previous studies have demonstrated that β7 is found not only on T cells from the human gut, but also from the blood and skin. The differences in T cell homing profiles between these tissues was due to co-expression with other homing molecules and chemokine receptors [59]. Although CCR9 contributes to leucocyte homing to the small bowel in particular [28], patients exhibiting enhanced CCR9 expression on γδ T cells in this study included those with UC and Crohn's colitis, suggesting either a role for CCR9 in the colon or the existence of immunological changes in the small bowel in these diseases, despite the lack of any presenting small bowel symptoms.
The CD3hi phenotype of γδ T cells in healthy controls may reflect the pre-activated status of circulating γδ T cells to allow rapid effector functions; indeed, CD45RO expression on γδ T cells enables rapid effector functions following tissue stress and bacterial infections [52]. The reduction of CD3 levels in CD and reduced CD45RO expression in CD and UC may be a result of chronic activation of the immune system; chronic infections can reduce expression of TCR chains [64–66] likely to affect associated co-receptors such as CD3 [67]. γδ T cells lost CD45RO expression following viral infection in cattle [68,69].
The population of γδ T cells that we have studied, circulating γδ T cells, are comprised mainly of Vγ2 Vδ2 T cells that exist only in primates and humans [70]. Vγ2 Vδ2 T cells are linked typically with anti-microbial immune responses [70,71] and IBD results from a dysregulated immune response to components of the luminal microbiota [7–9]. As Vγ2 Vδ2 cells can enter the gut mucosa readily upon activation [38], it is therefore possible that a dysregulated response of circulating Vγ2 Vδ2 T cells to the microbiota occurs in the gut and the skin in IBD and EN, respectively, or alternatively that γδ T cells are recruited from the circulation to sites of inflammation to promote homeostasis at these sites.
Conclusions
Further work will be necessary to determine the functional role of γδ T cells in IBD and cutaneous manifestations of IBD, but the dysregulation of γδ T cell homing profiles in these diseases is likely to be clinically relevant. Oral corticosteroids led to rapid resolution of EN, accompanied by removal of the unique skin-homing population of γδ T cells from the circulation. Targeting tissue-homing pathways provides a more specific approach to IBD therapy, and allows for selective anti-inflammatory treatments. For instance, α4β7 inhibitor MLN-O2 [72] and CCR9 inhibitor Traficet-EN [73] have efficacy in IBD. These data suggest that homing profiles could potentially be used as markers for active inflammation at particular sites in IBD and in cutaneous manifestations of IBD.
Acknowledgments
Grant support was from BBSRC, Unilever, FP7-people-IEF-2008-235993, St Mark's Foundation.
Disclosure
Authors have no disclosures to report.
References
- 1.Tonegawa S, Berns A, Bonneville M, et al. Diversity, development, ligands, and probable functions of gamma delta T cells. Cold Spring Harbor Symposium. Quant Biol. 1989;54:31–44. doi: 10.1101/sqb.1989.054.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Jameson J, Ugarte K, Chen N, et al. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–9. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 3.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994;266:1253–5. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 4.Komano H, Fujiura Y, Kawaguchi M, et al. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc Natl Acad Sci USA. 1995;92:6147–51. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–42. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 6.Mak TW, Ferrick DA. The gammadelta T-cell bridge: linking innate and acquired immunity. Nat Med. 1998;4:764–5. doi: 10.1038/nm0798-764. [DOI] [PubMed] [Google Scholar]
- 7.Bamias G, Cominelli F. Immunopathogenesis of inflammatory bowel disease: current concepts. Curr Opin Gastroenterol. 2007;23:365–9. doi: 10.1097/MOG.0b013e3281c55eb2. [DOI] [PubMed] [Google Scholar]
- 8.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–40. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 9.Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 10.Fukushima K, Masuda T, Ohtani H, et al. Immunohistochemical characterization, distribution, and ultrastructure of lymphocytes bearing T-cell receptor gamma/delta in inflammatory bowel disease. Gastroenterology. 1991;101:670–8. doi: 10.1016/0016-5085(91)90524-o. [DOI] [PubMed] [Google Scholar]
- 11.McVay LD, Li B, Biancaniello R, et al. Changes in human mucosal gamma delta T cell repertoire and function associated with the disease process in inflammatory bowel disease. Mol Med. 1997;3:183–203. [PMC free article] [PubMed] [Google Scholar]
- 12.Soderstrom K, Bucht A, Halapi E, Gronberg A, Magnusson I, Kiessling R. Increased frequency of abnormal gamma delta T cells in blood of patients with inflammatory bowel diseases. J Immunol. 1996;156:2331–9. [PubMed] [Google Scholar]
- 13.Giacomelli R, Parzanese I, Frieri G, et al. Increase of circulating gamma/delta T lymphocytes in the peripheral blood of patients affected by active inflammatory bowel disease. Clin Exp Immunol. 1994;98:83–8. doi: 10.1111/j.1365-2249.1994.tb06611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiohara T, Moriya N, Hayakawa J, Itohara S, Ishikawa H. Resistance to cutaneous graft-vs.-host disease is not induced in T cell receptor delta gene-mutant mice. J Exp Med. 1996;183:1483–9. doi: 10.1084/jem.183.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holtmeier W, Hennemann A, May E, Duchmann R, Caspary WF. T cell receptor delta repertoire in inflamed and noninflamed colon of patients with IBD analyzed by CDR3 spectratyping. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1024–G1034. doi: 10.1152/ajpgi.00224.2001. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann JC, Peters K, Henschke S, et al. Role of T lymphocytes in rat 2,4,6-trinitrobenzene sulphonic acid (TNBS) induced colitis: increased mortality after gammadelta T cell depletion and no effect of alphabeta T cell depletion. Gut. 2001;48:489–95. doi: 10.1136/gut.48.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuchiya T, Fukuda S, Hamada H, et al. Role of gamma delta T cells in the inflammatory response of experimental colitis mice. J Immunol. 2003;171:5507–13. doi: 10.4049/jimmunol.171.10.5507. [DOI] [PubMed] [Google Scholar]
- 18.Inagaki-Ohara K, Chinen T, Matsuzaki G, et al. Mucosal T cells bearing TCRgammadelta play a protective role in intestinal inflammation. J Immunol. 2004;173:1390–8. doi: 10.4049/jimmunol.173.2.1390. [DOI] [PubMed] [Google Scholar]
- 19.Kohyama M, Nanno M, Kawaguchi-Miyashita M, et al. Cytolytic and IFN-gamma-producing activities of gamma delta T cells in the mouse intestinal epithelium are T cell receptor-beta-chain dependent. Proc Natl Acad Sci USA. 1999;96:7451–5. doi: 10.1073/pnas.96.13.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dianda L, Hanby AM, Wright NA, Sebesteny A, Hayday AC, Owen MJ. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol. 1997;150:91–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Kawaguchi-Miyashita M, Shimada S, Kurosu H, et al. An accessory role of TCRgammadelta (+) cells in the exacerbation of inflammatory bowel disease in TCRalpha mutant mice. Eur J Immunol. 2001;31:980–8. doi: 10.1002/1521-4141(200104)31:4<980::aid-immu980>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Nanno M, Matsumoto S, Koike R, et al. Development of intestinal intraepithelial T lymphocytes is independent of Peyer's patches and lymph nodes in aly mutant mice. J Immunol. 1994;153:2014–20. [PubMed] [Google Scholar]
- 23.Kawaguchi-Miyashita M, Shimada S, Matsuoka Y, Ohwaki M, Nanno M. Activation of T-cell receptor-gammadelta+ cells in the intestinal epithelia of KN6 transgenic mice. Immunology. 2000;101:38–45. doi: 10.1046/j.1365-2567.2000.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefrancois L, Parker CM, Olson S, et al. The role of beta7 integrins in CD8 T cell trafficking during an antiviral immune response. J Exp Med. 1999;189:1631–8. doi: 10.1084/jem.189.10.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berlin C, Berg EL, Briskin MJ, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–95. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 26.Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature. 1993;366:695–8. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- 27.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–53. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 28.Zabel BA, Agace WW, Campbell JJ, et al. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med. 1999;190:1241–56. doi: 10.1084/jem.190.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farkas S, Hornung M, Sattler C, et al. Blocking MAdCAM-1 in vivo reduces leukocyte extravasation and reverses chronic inflammation in experimental colitis. Int J Colorect Dis. 2006;21:71–8. doi: 10.1007/s00384-004-0709-y. [DOI] [PubMed] [Google Scholar]
- 30.Goto A, Arimura Y, Shinomura Y, Imai K, Hinoda Y. Antisense therapy of MAdCAM-1 for trinitrobenzenesulfonic acid-induced murine colitis. Inflamm Bowel Dis. 2006;12:758–65. doi: 10.1097/00054725-200608000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Kato S, Hokari R, Matsuzaki K, et al. Amelioration of murine experimental colitis by inhibition of mucosal addressin cell adhesion molecule-1. J Pharmacol Exp Ther. 2000;295:183–9. [PubMed] [Google Scholar]
- 32.Matsuzaki K, Tsuzuki Y, Matsunaga H, et al. In vivo demonstration of T lymphocyte migration and amelioration of ileitis in intestinal mucosa of SAMP1/Yit mice by the inhibition of MAdCAM-1. Clin Exp Immunol. 2005;140:22–31. doi: 10.1111/j.1365-2249.2005.02742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Picarella D, Hurlbut P, Rottman J, Shi X, Butcher E, Ringler DJ. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of SCID mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol. 1997;158:2099–106. [PubMed] [Google Scholar]
- 34.Arihiro S, Ohtani H, Suzuki M, et al. Differential expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in ulcerative colitis and Crohn's disease. Pathol Int. 2002;52:367–74. doi: 10.1046/j.1440-1827.2002.01365.x. [DOI] [PubMed] [Google Scholar]
- 35.Souza HS, Elia CC, Spencer J, MacDonald TT. Expression of lymphocyte–endothelial receptor–ligand pairs, alpha4beta7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 1999;45:856–63. doi: 10.1136/gut.45.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hart AL, Kamm MA, Knight SC, Stagg AJ. Quantitative and functional characteristics of intestinal-homing memory T cells: analysis of Crohn's disease patients and healthy controls. Clin Exp Immunol. 2004;135:137–45. doi: 10.1111/j.1365-2249.2004.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 38.Huang D, Shen Y, Qiu L, et al. Immune distribution and localization of phosphoantigen-specific Vgamma2Vdelta2 T cells in lymphoid and nonlymphoid tissues in Mycobacterium tuberculosis infection. Infect Immun. 2008;76:426–36. doi: 10.1128/IAI.01008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poggi A, Zancolli M, Catellani S, Borsellino G, Battistini L, Zocchi MR. Migratory pathways of gammadelta T cells and response to CXCR3 and CXCR4 ligands: adhesion molecules involved and implications for multiple sclerosis pathogenesis. Ann NY Acad Sci. 2007;1107:68–78. doi: 10.1196/annals.1381.008. [DOI] [PubMed] [Google Scholar]
- 40.Lammers KM, Lu R, Brownley J, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194–204. doi: 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byeseda SE, Burns AR, Dieffenbaugher S, Rumbaut RE, Smith CW, Li Z. ICAM-1 is necessary for epithelial recruitment of gammadelta T cells and efficient corneal wound healing. Am J Pathol. 2009;175:571–9. doi: 10.2353/ajpath.2009.090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staunton DE, Dustin ML, Erickson HP, Springer TA. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990;61:243–54. doi: 10.1016/0092-8674(90)90805-o. [DOI] [PubMed] [Google Scholar]
- 43.Iwata M, Sawada S, Sawa M, Thoft RA. Mechanisms of lymphocyte adhesion to cultured human corneal epithelial cells. Curr Eye Res. 1997;16:751–60. doi: 10.1076/ceyr.16.8.751.8983. [DOI] [PubMed] [Google Scholar]
- 44.Mickelson JK, Kukielka G, Bravenec JS, et al. Differential expression and release of CD54 induced by cytokines. Hepatology. 1995;22:866–75. [PubMed] [Google Scholar]
- 45.Hu X, Zhang Y, Cheng D, et al. Mechanical stress upregulates intercellular adhesion molecule-1 in pulmonary epithelial cells. Respiration. 2008;76:344–50. doi: 10.1159/000137509. [DOI] [PubMed] [Google Scholar]
- 46.Penido C, Costa MF, Souza MC, et al. Involvement of CC chemokines in gammadelta T lymphocyte trafficking during allergic inflammation: the role of CCL2/CCR2 pathway. Int Immunol. 2008;20:129–39. doi: 10.1093/intimm/dxm128. [DOI] [PubMed] [Google Scholar]
- 47.Requena L, Requena C. Erythema nodosum. Dermatol Online J. 2002;8:4. [PubMed] [Google Scholar]
- 48.Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006;6:244–51. doi: 10.1038/nri1784. [DOI] [PubMed] [Google Scholar]
- 49.Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–81. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 50.Picker LJ, Kishimoto TK, Smith CW, Warnock RA, Butcher EC. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991;349:796–9. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- 51.Campbell JJ, Haraldsen G, Pan J, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–80. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 52.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–96. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–8. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 54.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 55.Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- 56.Moser B, Willimann K. Chemokines: role in inflammation and immune surveillance. Ann Rheum Dis. 2004;63(Suppl. 2):ii84–9. doi: 10.1136/ard.2004.028316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaerli P, Moser B. Chemokines: control of primary and memory T-cell traffic. Immunol Res. 2005;31:57–74. doi: 10.1385/IR:31:1:57. [DOI] [PubMed] [Google Scholar]
- 58.Berlin C, Bargatze RF, Campbell JJ, et al. alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–22. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 59.Mann ER, Bernardo D, Al-Hassi HO, et al. Human gut-specific homeostatic dendritic cells are generated from blood precursors by the gut microenvironment. Inflamm Bowel Dis. 2012;18:1275–86. doi: 10.1002/ibd.21893. [DOI] [PubMed] [Google Scholar]
- 60.Cairo C, Arabito E, Landi F, et al. Analysis of circulating gammadelta T cells in children affected by IgE-associated and non-IgE-associated allergic atopic eczema/dermatitis syndrome. Clin Exp Immunol. 2005;141:116–21. doi: 10.1111/j.1365-2249.2005.02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katsuta M, Takigawa Y, Kimishima M, Inaoka M, Takahashi R, Shiohara T. NK cells and gamma delta+ T cells are phenotypically and functionally defective due to preferential apoptosis in patients with atopic dermatitis. J Immunol. 2006;176:7736–44. doi: 10.4049/jimmunol.176.12.7736. [DOI] [PubMed] [Google Scholar]
- 62.Chennupati V, Worbs T, Liu X, et al. Intra- and intercompartmental movement of gammadelta T cells: intestinal intraepithelial and peripheral gammadelta T cells represent exclusive nonoverlapping populations with distinct migration characteristics. J Immunol. 2010;185:5160–8. doi: 10.4049/jimmunol.1001652. [DOI] [PubMed] [Google Scholar]
- 63.Laggner U, Di MP, Perera GK, et al. Identification of a novel proinflammatory human skin-homing Vgamma9Vdelta2 T cell subset with a potential role in psoriasis. J Immunol. 2011;187:2783–93. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zea AH, Ochoa MT, Ghosh P, et al. Changes in expression of signal transduction proteins in T lymphocytes of patients with leprosy. Infect Immun. 1998;66:499–504. doi: 10.1128/iai.66.2.499-504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stefanova I, Saville MW, Peters C, et al. HIV infection-induced posttranslational modification of T cell signaling molecules associated with disease progression. J Clin Invest. 1996;98:1290–7. doi: 10.1172/JCI118915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liossis SN, Ding XZ, Dennis GJ, Tsokos GC. Altered pattern of TCR/CD3-mediated protein–tyrosyl phosphorylation in T cells from patients with systemic lupus erythematosus. Deficient expression of the T cell receptor zeta chain. J Clin Invest. 1998;101:1448–57. doi: 10.1172/JCI1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valitutti S, Muller S, Salio M, Lanzavecchia A. Degradation of T cell receptor (TCR)–CD3–zeta complexes after antigenic stimulation. J Exp Med. 1997;185:1859–64. doi: 10.1084/jem.185.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silflow RM, Degel PM, Harmsen AG. Bronchoalveolar immune defense in cattle exposed to primary and secondary challenge with bovine viral diarrhea virus. Vet Immunol Immunopathol. 2005;103:129–39. doi: 10.1016/j.vetimm.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 69.Toka FN, Kenney MA, Golde WT. Rapid and transient activation of gammadelta T cells to IFN-gamma production, NK cell-like killing, and antigen processing during acute virus infection. J Immunol. 2011;186:4853–61. doi: 10.4049/jimmunol.1003599. [DOI] [PubMed] [Google Scholar]
- 70.Shen Y, Zhou D, Qiu L, et al. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science. 2002;295:2255–8. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen ZW, Letvin NL. Vgamma2Vdelta2+ T cells and anti-microbial immune responses. Microbes Infect. 2003;5:491–8. doi: 10.1016/s1286-4579(03)00074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feagan BG, Greenberg GR, Wild G, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499–507. doi: 10.1056/NEJMoa042982. [DOI] [PubMed] [Google Scholar]
- 73.Eksteen B, Adams DH. GSK-1605786, a selective small-molecule antagonist of the CCR9 chemokine receptor for the treatment of Crohn's disease. IDrugs. 2010;13:472–781. [PubMed] [Google Scholar]


