Abstract
Bacteriophage T7 encodes a serine/threonine-specific protein kinase that phosphorylates multiple cellular proteins during infection of Escherichia coli. Recombinant T7 protein kinase (T7PK), normally purified in phosphorylated form, exhibits a modest level of phosphotransferase activity. A procedure is described that provides dephosphorylated T7PK with an enhanced ability to phosphorylate protein substrates, including translation initiation factor IF1 and the nuclease domain of ribonuclease III. Mass spectrometric analysis identified Thr12 as the site of IF1 phosphorylation in vitro. T7PK undergoes Mg2+-dependent autophosphorylation on Ser216 in vitro, which also is modified in vivo. The inability to isolate the presumptive autophosphorylation-resistant T7PK Ser216Ala mutant indicates a toxicity of the phosphotransferase activity and suggests a role for Ser216 modification in limiting T7PK activity during infection.
Keywords: Bacteriophage T7, T7 Protein Kinase, Autophosphorylation, IF1, Ribonuclease III
Introduction
Viral strategies of infection often involve expression of genes that are ordinarily nonessential, but confer a reproductive advantage under suboptimal growth conditions. Bacteriophage T7 is a lytic DNA phage that expresses a set of early genes that convert the infected Escherichia coli cell to an environment optimal for viral reproduction [1,2]. The T7 early gene 0.7 is dispensible under standard laboratory growth conditions, but is nearly essential for T7 growth at elevated temperatures, or in limited carbon/energy sources [3]. The 0.7 protein exhibits two distinct, biochemically separable functions: a serine/threonine-specific protein kinase (T7PK) activity, and a host transcription shut-off (SO) activity [4,5]. The N-terminal domain possesses T7PK activity, while the SO activity is associated with the C-terminal region, and represses transcription by an unknown mechanism that is independent of T7PK activity [6]. T7PK uses ATP as phosphate donor and requires Mg2+ as a cofactor [7]. The T7PK domain of the 0.7 gene has been separately cloned and the polypeptide expressed in recombinant form [8]. The full-length 0.7 protein has been purified from T7-infected cells [9]. However, neither the full-length 0.7 protein nor the SO domain has been obtained in purified recombinant form, presumably reflecting the toxicity of the 0.7 protein, and the SO activity in particular.
A specific set of cellular proteins is phosphorylated during T7 infection in a T7PK-dependent manner [5,10]. The β′ subunit of the host RNA polymerase is modified at Thr1068 [11,12], which sensitizes transcription to otherwise weak terminators [12]. The RNA degradosome subunits RNase E and RNA helicase RhlB are T7PK targets, and their modification stabilizes transcripts synthesized by T7 RNA polymerase [13]. The dsRNA-specific processing enzyme RNase III is phosphorylated on serine during T7 infection [14,15], and the enhanced catalytic activity observed in vitro may optimize the maturation of the late transcripts that contain multiple RNase III processing sites, and that are synthesized at high levels [2]. Translation initiation factors IF1, IF2, and IF3, ribosomal proteins S1 and S6, and elongation factor G are in vivo targets [10,15], and their modification may enhance T7 late protein production by promoting the preferential translation of the mRNAs. While the T7PK-dependent modification of these proteins serves to maximize T7 gene expression, phosphorylation of proteins involved in other cellular pathways is likely [3,16].
Understanding how T7PK supports T7 growth requires knowledge of how phosphorylation alters the activity of the target proteins. In this regard, analyzing changes in activity of proteins phosphorylated in vitro can provide important information. However, recombinant T7PK directly purified from bacterial cells exhibits only modest levels of activity that may reflect an inhibitory in vivo phosphorylation [8]. We describe here a procedure for the preparation of dephosphorylated T7PK and its use in the efficient phosphorylation of substrate in vitro. We also show that T7PK undergoes autophosphorylation, with the conserved serine 216 as the primary site of modification in vitro and in vivo, and the conserved serine 218 an in vivo modification target/
Materials and Methods
Water was deionized and distilled. Chemicals and reagents were molecular biology grade and were purchased from Sigma-Aldrich (St. Louis, MO, USA) or ThermoFisher Scientific (Chicago, IL, USA). Standardized 1 M solutions of MgCl2 and MnCl2 were obtained from Sigma-Aldrich. [γ–32P]ATP (3000 Ci/mmol) was purchased from Perkin–Elmer (Boston, MA, USA). Lambda protein phosphatase was purchased from New England BioLabs (Beverly, MA, USA). Ni2+-NTA affinity chromatography resin, biotinylated thrombin and streptavidin-agarose were purchased from Novagen (Madison, WI, USA). Protein assay kits and protein standards (low MW range) for SDS–PAGE were from Bio-Rad Laboratories (Hercules, CA, USA). ICON™ concentrators were obtained from Pierce (Rockford, IL, USA). Dialysis membranes (Spectra–Por CE 3500 and 10,000 MWCO) were purchased from ThermoFisher Scientific. Multisite-directed mutagenesis kits were from Agilent Technologies (Santa Clara, CA, USA). NuPAGE Precast Bis-Tris or Tricine gels (12% and 15%, respectively), agarose, and oligodeoxynucleotides were purchased from Invitrogen (Carlsbad, CA, USA). The oligodeoxynucleotides were obtained in deprotected form and were purified by denaturing gel electrophoresis, then stored at −20°C in TE buffer (pH 8.0).
Escherichia coli strains used included BL21(DE3) (Novagen), BL21(DE3)recA, rnc105 [17] and DH10B (Invitrogen). Recombinant plasmids included pET-15b(T7PK), which encodes a truncated version of the T7 protein kinase, having a C-terminus defined by a point mutation (JS78) [6] that changes the Gln243 codon to a UAG codon [8]; pET-15b(NucD), encoding the N-terminal nuclease domain of RNase III [18]; and pET-15b(IF1) encoding the gene for E. coli translation initiation factor IF1 (a gift of P.R. Cunningham, Wayne State University, Detroit, MI, USA). All proteins expressed from the recombinant pET-15b plasmids carried an N-terminal hexahistidine [(His)6] tag encoded by the vector.
Protein expression and purification
Protein purification followed a procedure described elsewhere [17], with some modification. A 5 ml overnight culture of LB broth containing Ampicillin (100 μg/ml) (LB-Amp) was prepared using a freshly-transformed colony of BL21(DE3) or BL21(DE3)recA, rnc105 cells containing pET-15b(T7PK). A portion of the overnight culture was used to inoculate 500 ml of LB-Amp, which was grown with vigorous aeration at 37 °C to an OD600 of ~0.4. IPTG was added (1 mM final concentration) followed by vigorous aeration for 3 hr at 37 °C. Aliquots were removed before and after IPTG addition and analyzed by 12% SDS-PAGE. Cells were collected by centrifugation (3500xg for 20 min at 4 °C) and stored at −20 °C until further use. The following steps were carried out at ~0–4 °C. Cells (~1 g wet weight) were resuspended in 30 ml of binding buffer (5 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH 7.9) and subjected to repeated sonication bursts in an ice bath. The cell disrupter (Misonix, Inc.) was used at the “4–5” setting, with each sonication burst (1 min) followed by a 1 min pause with cooling, and the cycle repeated 20 times, or until lysis was judged complete. The sonicated material was centrifuged at 3500xg for 20 min and the clarified lysate (~30 ml) passed through a 0.2 micron filter (Corning). The solution was applied (~1 ml/min) to a Ni2+-NTA (Ni2+-nitrilotriacetate) column (1 ml) prepared according to the supplier’s instructions. The column was washed with 10 column volumes of binding buffer, followed by 10 column volumes of washing buffer (60 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH 7.9). The protein was eluted with 6 column volumes of elution buffer (1 M imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH 7.9). Most of the protein was obtained in the first three eluted volumes, which were combined and dialyzed (Spectrapor 10,000 MWCO) against two liters of dialysis buffer 1 (100 mM NH4Cl, 60 mM Tris-HCl, pH 7.9) for 12 hr at 4 °C. The protein was further dialyzed against two liters of dialysis buffer 2 (100 mM NH4Cl, 60 mM Tris-HCl, pH 7.9, 2 mM EDTA-Na2, 2 mM DTT) for 12 hr at 4 °C. An equal volume of glycerol was added and the purified protein was stored at −20 °C.
Conversion of H-pT7PK to H-T7PK and T7PK
H-pT7PK (~0.8–1 mg) was dialyzed at 5 °C against binding buffer (5 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH 7.9), then incubated with 800 units of lambda protein phosphatase (2 hr, 37 °C) in a total volume of ~1 ml. An additional 800 units of phosphatase was added, followed by incubation for 2 hr at 37°C. The sample was loaded onto a His-bind column (1 ml), which was washed and protein recovered by elution with buffer containing 1 M imidazole as described above. H-T7PK was recovered in 10% yield; the source of the low yield is currently not known. To remove the affinity tag, H-T7PK was dialyzed against 40 mM imidazole, 500 mM NaCl, and 20 mM Tris-HCl (pH 8.4). The protein (~800 μg) was treated with ~10 units of biotinylated thrombin in a 1-ml reaction volume at room temperature for 16 hr. When the reaction was judged complete by SDS-PAGE and Coomassie staining, streptavidin agarose was added (32 μl per unit of biotinylated thrombin), followed by incubation for 30 min at room temperature with gentle mixing. The reaction was loaded onto a spin column provided with the thrombin kit and centrifuged (500xg, 5 min), and the filtrate dialyzed (Spectrapor, 3500 MWCO) against storage buffer. The cleaved affinity tag is eliminated in this step since its molecular mass is <2,000 Da. Purified T7PK was stored at a concentration of ~0.5 mg/ml in storage buffer containing 50% glycerol at −20 °C.
T7PK phosphorylation of protein in vitro
In a typical assay, ~1 μg of substrate was incubated with ~1 μg (33 pmol) of T7PK in reaction buffer (0.1 ml; 2 mM NH4Cl, 30 mM Tris-HCl, 1 mM DTT, 0.1 mM EDTA) for 5 min at 30 °C. MgCl2 was added (15 mM final concentration), followed by 1 mM [γ32-P]ATP (12 Ci/mol). The reaction was incubated for 10 min (30 °C), followed by a second addition of T7PK (0.5 μg) and incubation continued for 5 min at 30 °C. The reaction was stopped by adding excess EDTA (20 mM final concentration). Aliquots were combined with sample loading buffer, heated 100 °C for 3 min, and analyzed by SDS-PAGE. Proteins were visualized by Coomassie Brilliant Blue R staining. The 32P signal was detected by phosphorimaging (Typhoon 9400 System).
To determine the level of T7PK autophosphorylation, T7PK was incubated with 1 mM [γ-32P]ATP (12 Ci/mol) as described above, and subjected to SDS-PAGE. The polypeptide was located by Coomassie staining. The gel band was excised and 32P incorporation was measured by liquid scintillation counting of the gel slice usoing Scintiverse E (counting efficiency 99%). The incorporation of phosphate is reported as mol phosphate per mol protein, and assumes that 50% of the protein sample added to the gel lane was recovered in the excised gel band. This value is based on the observation (vide infra) that T7PK undergoes near-quantitative phosphorylation in vitro primarily on a single serine, providing a stoichiometry of ~1 mole phosphate per mole T7PK. As the T7PK autophosphorylation reaction provides an internal control in the protein phosphorylation reaction, the level of phosphorylation of the target protein recovered in the gel slice also was able to be accurately determined in comparison to the radioaactivity in the T7PK-containing gel slice. Protein phosphorylation experiments were performed in triplicate and the results were averaged.
Mass spectrometric analysis of phosphoproteins
Mass spectrometric analyses were performed at the Wistar Institute Proteomics Facility (Philadelphia, PA, USA). Briefly summarized, nonradioactive, phosphorylated samples of T7PK, IF1, or RNase III NucD were generated (see Results and Discussion) and subjected to SDS-PAGE, followed by Coomassie staining. The protein-containing gel band was excised, destained further, subjected to reductive alkylation of cysteine, then incubated in situ with trypsin overnight at 37 °C. The sample was eluted from the gel slice and chromatographed on a nanocapillary reverse-phase column (New Objective PicoFrit 75 μM column, terminating in a nanospray 15 μM tip self-packed with Microm Magic C18 AQ200A, 5 μM resin), directly coupled to a ThermoElectron Orbitrap XL mass spectrometer. The “top six” method was applied, wherein the six most intense peaks observed in the mass spectra were subjected to further analysis. A customized database was created using the E. coli database, with investigator-supplied sequence and common contaminants (tagged) added. The database was indexed for partial tryptic searching, and the resulting masses and MS/MS spectra were searched against the database using the SEQUEST search engine. Subsequent data were filtered using 5ppm, delta cN of 0.07. The data was further processed using ProteoIQ software (NuSep).
RESULTS
Preparation of dephosphorylated recombinant T7PK
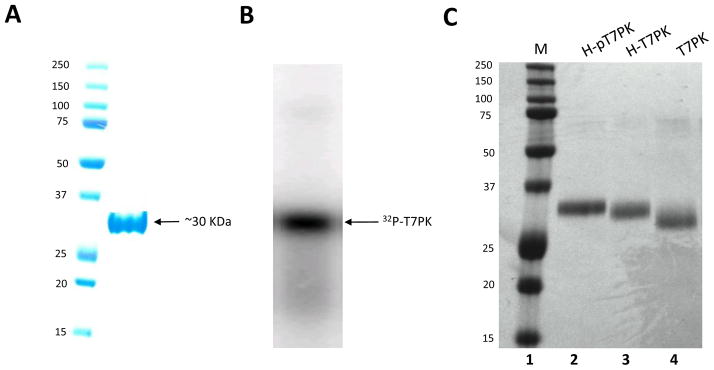
The N-terminal T7PK domain of the 0.7 protein has been cloned [8], allowing studies of T7PK function uncoupled from the SO activity [13,19]. To provide protein for in vitro analysis, T7PK was produced in BL21(DE3) cells carrying pET-15b(T7PK). The N-terminal His-tagged T7PK accumulated in the soluble and insoluble fractions of sonicated cell lysates, and purification was accomplished by Ni2+-NTA affinity chromatography of the soluble fraction, typically yielding ~2 mg protein from a 400 ml culture (Fig. 1A). Since T7PK obtained in this manner is phosphorylated ([8] and vide infra) it is referred to as H-pT7PK, with ‘H’ and ‘p’ referring to the His-tag and phosphate group(s), respectively. Since a previous study indicated that phosphorylation reduces T7PK catalytic activity [7], a method was developed to dephosphorylate H-pT7PK, as well as remove the His-tag, which also could affect activity. H-pT7PK was treated with lambda phage protein phosphatase [20], which has a broad specificity for protein phosphomonoester groups [21,22], then subjected to Ni2+-NTA affinity chromatography, providing H-T7PK in a yield of ~10%. Removal of phosphate causes a slight increase in mobility of H-pT7PK upon phosphatase treatment (Fig. 1C, compare lanes 2 and 3). The mobility difference between the dephosphorylated and phosphorylated forms is similar to that observed between T7PK and the T7PK Gly76Phe mutant, which is incapable of autophosphorylation due to an inability to bind ATP [19]. H-T7PK was treated with biotinylated thrombin to remove the His-tag, with completion of the reaction confirmed by SDS-PAGE and Coomassie staining (Fig. 1C, compare lanes 3 and 4). Streptavidin-agarose was added to remove the biotinylated thrombin, and dialysis using a 3500 MWCO membrane allowed removal of the released affinity tag. T7PK prepared in this manner carries three additional amino acids (GlySerHis) at the N-terminus, derived from the thrombin recognition sequence.
Figure 1.
Purification and dephosphorylation of T7PK. A. Analysis of purified H-pT7PK by 12% SDS-PAGE and Coomassie staining. Lane 1: protein standards (sizes in kDa). Lane 2. Purified H-pT7PK, with an apparent molecular mass of 30 kDa. B. Phosphorimage of T7PK autophosphorylated in the presence of [γ-32P]ATP, then analyzed by 12% SDS-PAGE as described in Materials and Methods. C. Electrophoretic mobility differences of H-pT7PK, H-T7PK, and T7PK. Shown is an image of a Coomassie-stained, 15% polyacrylamide-SDS gel. Lane 1: protein standards (sizes in kDa). Lane 2: H-pT7PK as directly purified from cells. Lane 3: H-T7PK, obtained by lambda phage protein phosphatase treatment of H-pT7PK. Lane 4: T7PK obtained by biotinylated thrombin treatment of H-T7PK. Note the greater mobility of T7PK compared to His-pT7PK or His-T7PK.
T7PK phosphorylation of substrate in vitro: comparison with H-pT7PK and H-T7PK
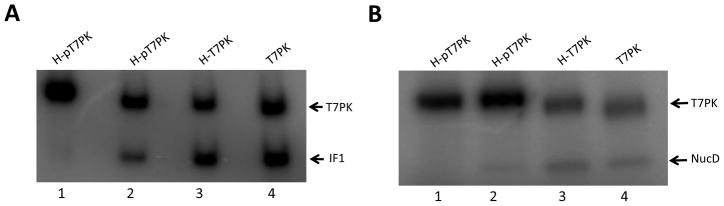
T7PK prepared as described above was tested for its ability to phosphorylate two substrates: translation initiation factor IF1 and the N-terminal nuclease domain (NucD) of RNase III. The latter polypeptide is a truncated version of RNase III that lacks the C-teminal dsRNA-binding domain [18]. The molecular masses of the IF1 and the RNase III NucD polypeptides (~13 and ~20 kDa, respectively) allow effective gel electrophoretic separation of each protein from H-pT7PK (~30 kDa). Both proteins were purified in His-tagged form and were separately treated with equimolar amounts of T7PK, and in the presence of [γ-32P]ATP. Phosphotransfer was initiated by adding Mg2+, followed by incubation at 30 °C. A second aliquot of T7PK was added, followed by incubation for 5 min. Excess EDTA was added to stop the reaction and the samples fractionated by SDS-PAGE. To assess the contribution of covalently attached phosphate and the His-tag on T7PK activity, parallel reactions used H-pT7PK or H-T7PK. The results are shown in Figure 2A, where it is seen that IF1 is phosphorylated by T7PK. Based on the intensity of the 32P signal in the IF1 band, the reaction with T7PK exhibits an apparently greater efficiency compared to the reaction involving either H-pT7PK or H-T7PK (Fig. 2A, compare lane 4 with lanes 3 and 2). Quantitation of phosphorylation (Table 1) reveals that T7PK is the most active form, and catalyzes the incorporation of 0.36 mole phosphate per mole of IF1. Phosphatase treatment of H-pT7PK provided the greatest enhancement of activity, with an additional but lesser increase achieved upon removal of the His-tag (Table 1). The site of IF1 phosphorylation in vitro was identified by LC-MS/MS analysis of the tryptic peptides (see Materials and Methods). The results (Supplemental Fig. 1) reveal that Thr12 (Fig. 3) is the site of IF1 phosphorylation. The RNase III NucD also is phosphorylated to the greatest extent (0.27 mole phosphate per mole polypeptide) with either H-T7PK or T7PK, both of which lack phosphate (Fig. 2B, compare lanes 3 and 4 with lane 2, and Table 1). For both IF1 and NucD, the level of phosphorylation was not significantly increased by a third round of T7PK addition and incubation (S. G. and A. W. N., data not shown).
Figure 2.
T7PK phosphorylation of IF1 and the RNase III Nuclease Domain (NucD). Purified E. coli IF1 or E. coli RNase III NucD (both lacking the His-Tag) was phosphorylated in the presence of [γ-32P]ATP using H-pT7PK, H-T7PK, or T7PK as described in Materials and Methods. Aliquots were fractionated by 12% SDS-PAGE and visualized by phosphorimaging as described in Materials and Methods. A. T7PK phosphorylation of IF1. Lane 1: Phosphorylation reaction lacking IF1. Lane 2: IF1 phosphorylated by H-pT7PK. Lane 3: IF1 phosphorylated by H-T7PK. Lane 4: IF1 phosphorylated by T7PK. B. Phosphorylation of RNase III NucD. Lane 1: Phosphorylation reaction lacking NucD. Lane 2: NucD phosphorylated by H-pT7PK. Lane 3: NucD phosphorylated by H-T7PK. Lane 4: NucD phosphorylation by T7PK.
Table 1.
Effect of phosphatase treatment and His-tag removal on T7PK phosphorylation of E. coli IF1 and the nuclease domain (NucD) of E. coli RNase III.
| H-pT7PK | H-T7PK | T7PK | |
|---|---|---|---|
|
| |||
| IF1 | 0.13±0.01 | 0.23±0.02 | 0.36±0.02 |
| NucD | 0.11±0.02 | 0.22±0.02 | 0.27±0.02 |
Purified IF1 and RNase III NucD (1 μg, both without His-tag) were incubated at 30 °C for 15 min with H-pT7PK, H-T7PK, or T7PK (two additions, 1.5 μg total amount) in the presence of 1 mM [γ-32P]ATP (12 Ci/mol) as described in Materials and Methods. Reactions were stopped with excess EDTA and were electrophoresed in a 10% Tricine NuPAGE precast gel (Invitrogen). The incorporation of 32P was determined as described in Materials and Methods.
Figure 3.
Thr12 is the site of IF1 phosphorylation by T7PK in vitro. Shown is the amino acid sequence of E. coli IF1, with Thr12 in bold face and underlined. The numbering of the amino acids does not include the N-terminal affinity tag sequence, which is given within the parentheses. The mass spectrometric (LC-MS/MS) data of the tryptic peptides of phosphorylated IF1 are provided in Supplemental Figure 1.
T7PK is phosphorylated primarily on Ser216 in vitro and in vivo
Incubation of T7PK with [γ-32P]ATP and Mg2+ yielded a single radiolabeled species with a similar electrophoretic mobility as the Coomassie-stained polypeptide (see Fig. 1B and Fig. 2A, B). To identify the site(s) of autophosphorylation, the tryptic peptides derived from T7PK reacted with ATP and Mg2+ were analyzed by LC-MS/MS. The results (Supplemental Figure 2) show that Ser216 (Fig. 4) is the major target, with a lesser level of phosphorylation occurring at Ser227. We conclude that T7PK undergoes autophosphorylation in vitro in a site-specific manner. The tryptic peptides derived from H-pT7PK directly purified from cells also were subjected to mass spectrometric analysis (Supplemental Fig. 4), which revealed Ser216 as the primary site of phosphorylation (Fig. 4), with a lesser level of phosphorylation of Ser227. The conserved residue Ser218 also is modified in vivo (Supplemental Fig. 4). We conclude that Ser216 is the primary site of T7PK phosphorylation in vivo and in vitro, with lesser levels of Ser227, and that Ser218 is modified only in vivo. Several attempts were made to generate the Ser216Ala mutation, with the expectation that the presumptive autophosphorylation-resistant T7PK mutant would afford greater levels of target protein phosphorylation. However, the mutant gene was not obtained, despite the use of several different mutagenic oligonucleotides. This may reflect the toxicity of the autophosphorylation-resistant protein, even when produced in minor amounts.
Figure 4.
Sites of phosphorylation of T7PK in vivo and in vitro. Shown is the predicted amino acid sequence of the protein kinase (PK) domain of the T7 0.7 protein. The amino acid numbering scheme does not include the N-terminal affinity tag sequence, which is given within the parentheses. The serines that are phosphorylated (S216, S218, S227) are shown in bold face; S216 and S227 are phosphorylated in vivo and in vitro, as denoted by the solid underlining, while S218 is phosphorylated only in vivo, as denoted by the dotted underlining (see also Results and Discussion, and Supplemental Figures 2 and 3 for tryptic peptide analysis by LC-MS/MS.
DISCUSSION
We have shown that T7PK - a C-terminal truncated form of the T7 early protein 0.7 - is able to phosphorylate protein in vitro. The most active form of T7PK is obtained by phosphatase treatment, with a lesser enhancement afforded by removal of the His-tag. The inability to achieve near-stoichiometric phosphorylation of target proteins in vitro may reflect a number of factors, including a competing autophosphorylation reaction, and that not all of the substrate may be in a form competent for phosphorylation. T7PK activity in T7-infected cells also may not be fully efficient for these reasons. However, nonstoichiometric phosphorylation of substrate is not necessarily a limitation, as it will be shown elsewhere that a biochemical analysis of T7PK phosphorylation of RNase III has afforded insight on the regulation of double-stranded RNA processing (S.G. and A.W.N., in preparation). It is not known whether in vitro phosphorylation sites are identical to the in vivo sites, but we note that for IF1 and RNase III there is a concordance of target site identity (Thr and Ser, respectively) in vivo and in vitro [10,14,15]. How IF1 function may be altered by phosphorylation is unclear. However, since T7PK action enhances translation of the T7 late mRNAs [3] and other transcripts synthesized by T7 RNA polymerase [13], it has been proposed that modification of IF1 as well as other ribosomal factors allows preferential translation of these mRNAs [9,12]. However, the mechanism of preferential translation is not known.
T7PK undergoes autophosphorylation in vitro in a Mg2+-dependent reaction, with Ser216 as the primary target. Phosphopeptide analysis of H-pT7PK directly purified from cells shows that Ser216 is a primary target in vivo, and that T7PK phosphorylation is a consequence of autophosphorylation rather than modification by another protein. Ser227, also is phosphorylated in vivo and in vitro, but is not conserved. The functional importance of Ser216 phosphorylation is suggested by the conservation of this residue, as seen in an alignment of 0.7 polypeptide sequences from six T7 phage group members (Fig. 4). Interestingly, the conserved residue Ser218 is phosphorylated in vivo, but not in vitro. This may reflect subtle differences between the in vivo and in vitro environments, and an involvement of Ser218 in T7PK regulation therefore is possible. However, if Ser218 phosphorylation is functionally important, it is insufficient to suppress the apparent toxicity of the Ser216Ala mutant (assuming that the Ser216Ala mutation also does not inhibit Ser218 phosphorylation). It is not known whether T7PK autophosphorylation is intramolecular or intermolecular, and the mechanism by which serine phosphorylation reduces phosphotransferase activity also remains to be determined. The phosphotransferase activity most likely is the toxic feature of T7PK, since the Gly76Phe T7PK mutant that is catalytically inactive, due to an inability to bind ATP, is not toxic [19]. A primary function of autophosphorylation in downregulating T7PK activity is indicated by the enhancement of phosphotransferase activity upon removal of the phosphate group. Autophosphorylation may serve to limit T7PK activity to a narrow window during the T7 reproductive cycle. In fact, a burst of T7PK phosphotransferase activity is observed in T7-infected cells, prior to the onset of T7 late protein synthesis and phage particle assembly, and which ceases upon appearance of the phosphorylated form of the 0.7 protein [5].
Based on the high amounts of T7PK that can be produced in E. coli from a single copy gene under control of a T7 promoter [19], T7PK has been used as a fusion tag to enhance the production of soluble recombinant proteins [23,24]. If combined with prior phosphatase treatment, autophosphorylation can provide a convenient route to the 32P-labeling of T7PK-tagged proteins. An alternative radiolabeling route is revealed by the observation that the N-terminal His-tag sequence encoded by the pET-15 vector is phosphorylated to a limited extent on two of the five serine residues, as seen with IF1 (Supplemental Fig. 1) and T7PK phosphorylated in vivo (Supplemental Fig. 4). However, this phosphorylation may be dependent on the identity of the attached polypeptide, as it was not observed with H-T7PK phosphorylated in vitro (Supplemental Fig. 2). Such variability notwithstanding, the occurrence of this modification necessitates removal of the His-tag to accurately assess substrate phosphorylation.
Supplementary Material
Mass spectrometric analysis of tryptic peptides derived from E. coli IF1 phosphorylated by T7PK in vitro.
Mass spectrometric analysis of tryptic peptides derived from T7PK autophosphorylated in vitro.
Mass spectrometric analysis of tryptic peptides derived from H-pT7PK, directly isolated from E. coli.
Alignment of 0.7 protein sequences of six T7 group phages. The authors thank Dr. Kaye Speicher of the Wistar Institute Proteomics Facility for performing the mass spectrometric analyses of phosphorylated IF1 and autophosphorylated T7PK. The authors also thank Dr. Philip R. Cunningham (Wayne State University) for providing the pET-15b plasmid encoding E. coli IF1 gene, and Dr. Rhonda Nicholson for input on the manuscript. This research was supported in part by the National Institutes of Health (RO1 GM56772).
Highlights.
T7 Protein Kinase Autophosphorylation
Preparation and Use of Dephosphorylated T7 Protein Kinase
T7PK Phosphorylation of IF1
T7PK Phosphorylation of RNase III Nuclease Domain
Abbreviations
- T7PK
T7 protein kinase
- H-pT7PK
T7PK with the His-tag (H), and covalently attached phosphate (p)
- H-T7PK
T7PK with the His-tag, without covalently incorporated phosphate
- IF1
Translation Initiation Factor 1
- NucD
Nuclease Domain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Molineux IJ. The T7 Group. In: Calendar R, editor. The Bacteriophages. Oxford University Press; New York: 2006. pp. 277–301. [Google Scholar]
- 2.Dunn JJ, Studier FW. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983;166:477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch-Kaufmann M, Herrlich P, Ponta H, Schweiger M. Helper function of T7 protein kinase in virus propagation. Nature. 1975;255:508–510. doi: 10.1038/255508a0. [DOI] [PubMed] [Google Scholar]
- 4.Brunovskis I, Summers WC. The process of infection with coliphage T7. VI. A phage gene controlling shutoff of host RNA synthesis. Virol. 1972;50:322–327. doi: 10.1016/0042-6822(72)90383-2. [DOI] [PubMed] [Google Scholar]
- 5.Rahmsdorf HJ, Pai SH, Ponta H, Herrlich P, Roskoski R, Schweiger M, Studier FW. Protein kinase induction in Escherichia coli by bacteriophage T7. Proc Natl Acad Sci USA. 1974;71:586–589. doi: 10.1073/pnas.71.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon MN, Studier FW. Physical mapping of the early region of bacteriophage T7 DNA. J Mol Biol. 1973;79:249–265. doi: 10.1016/0022-2836(73)90004-1. [DOI] [PubMed] [Google Scholar]
- 7.Pai SH, Rahmsdorf HJ, Ponta H, Hirsch-Kaufmann M, Herrlich P, Schweiger M. Protein kinase of bacteriophage T7. 2. Properties, enzyme synthesis in vitro and regulation of enzyme synthesis and activity in vivo. Eur J Biochem. 1975;55:305–314. doi: 10.1111/j.1432-1033.1975.tb02164.x. [DOI] [PubMed] [Google Scholar]
- 8.Michalewicz J, Nicholson AW. Molecular cloning and expression of the bacteriophage T7 0.7(protein kinase) gene. Virol. 1992;186:452–462. doi: 10.1016/0042-6822(92)90010-m. [DOI] [PubMed] [Google Scholar]
- 9.Pai SH, Ponta H, Rahmsdorf HJ, Hirsch-Kauffmann M, Herrlich P, Schweiger M. Protein kinase of bacteriophage T7. 1. Purification. Eur J Biochem. 1975;55:299–304. doi: 10.1111/j.1432-1033.1975.tb02163.x. [DOI] [PubMed] [Google Scholar]
- 10.Robertson ES, Nicholson AW. Phosphorylation of Escherichia coli translation initiation factors by the bacteriophage T7 protein kinase. Biochem. 1992;31:4822–4827. doi: 10.1021/bi00135a012. [DOI] [PubMed] [Google Scholar]
- 11.Zillig W, Fujiki H, Blum W, Janekovic D, Schweiger M, Rahmsdorf HJ, Ponta H, Hirsch-Kauffmann M. In vivo and in vitro phosphorylation of DNA-dependent RNA polymerase of Escherichia coli by bacteriophage T7-induced protein kinase. Proc Natl Acad Sci USA. 1975;72:2506–2510. doi: 10.1073/pnas.72.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Severinova E, Severinov K. Localization of the Escherichia coli RNA polymerase β′ subunit residue phosphorylated by bacteriophage T7 kinase gp0.7. J Bacteriol. 2006;188:3470–3476. doi: 10.1128/JB.188.10.3470-3476.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchand I, Nicholson AW, Dreyfus M. Bacteriophage T7 protein kinase phosphorylates RNase E and stabilizes mRNAs synthesized by T7 RNA polymerase. Mol Microbiol. 2001;42:767–776. doi: 10.1046/j.1365-2958.2001.02668.x. [DOI] [PubMed] [Google Scholar]
- 14.Mayer JE, Schweiger M. RNase III is positively regulated by T7 protein kinase. J Biol Chem. 1983;258:5340–5343. [PubMed] [Google Scholar]
- 15.Robertson ES, Aggison LA, Nicholson AW. Phosphorylation of elongation factor G and ribosomal protein S6 in bacteriophage T7-infected Escherichia coli. Mol Microbiol. 1994;11:1045–1057. doi: 10.1111/j.1365-2958.1994.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 16.Qimron U, Tabor S, Richardson CC. New details about bacteriophage T7-host interactions. Microbe. 2010;5:117–122. [Google Scholar]
- 17.Amarasinghe AK, Calin-Jageman I, Harmouch A, Sun W, Nicholson AW. Escherichia coli ribonuclease III. Affintiy purification of hexahistidine-tagged enzyme and assays for substrate binding and cleavage. Methods Enzymol. 2001;342:143–158. doi: 10.1016/s0076-6879(01)42542-0. [DOI] [PubMed] [Google Scholar]
- 18.Sun W, Jun EJ, Nicholson AW. Intrinsic double-stranded-RNA processing activity of Escherichia coli ribonuclease III lacking the dsRNA-binding domain. Biochem. 2001;40:14976–14984. doi: 10.1021/bi011570u. [DOI] [PubMed] [Google Scholar]
- 19.Marchand I, Nicholson AW, Dreyfus M. High-level autoenhanced expression of a single-copy gene in Escherichia coli: overproduction of bacteriophage T7 protein kinase directed by T7 late genetic elements. Gene. 2001;262:231–238. doi: 10.1016/s0378-1119(00)00526-6. [DOI] [PubMed] [Google Scholar]
- 20.Barik S. Expression and biochemical properties of a protein serine/threonine phosphatase encoded by bacteriophage λ. Proc Natl Acad Sci USA. 1993;90:10633–10637. doi: 10.1073/pnas.90.22.10633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsui T, Tanihara K, Date T. Expression of unphosphorylated form of human double-stranded RNA-activated protein kinase in Escherichia coli. Biochem Biophys Res Comm. 2001;284:798–807. doi: 10.1006/bbrc.2001.5039. [DOI] [PubMed] [Google Scholar]
- 22.Budini M, Jacob G, Jedlicki A, Pérez C, Allende CC, Allende JE. Autophosphorylation of carboxy-terminal residues inhibits the activity of protein kinase CK1α. J Cell Biochem. 2009;106:399–408. doi: 10.1002/jcb.22019. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee DK, Esposito D. Enhanced soluble protein expression using two new fusion tags. Prot Expr Purif. 2006;46:122–129. doi: 10.1016/j.pep.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 24.Narayanan N, Khan M, Chou CP. Enhancing functional expression of heterologous Burkholderia lipase in Escherichia coli. Mol Biotechnol. 2011;47:130–143. doi: 10.1007/s12033-010-9320-3. [DOI] [PubMed] [Google Scholar]
- 25.Pajunen MI, Elizondo MR, Skurnik M, Kieleczawa J, Molineux IJ. Complete nucleotide sequence and likely recombinatorial origin of bacteriophage T3. J Mol Biol. 2002;319:1115–1132. doi: 10.1016/S0022-2836(02)00384-4. [DOI] [PubMed] [Google Scholar]
- 26.Lingohr EJ, Villegas A, She Y-M, Ceyssens P-J, Kropinski AM. The genome and proteome of the Kluyvera bacteriophage KvpI – another member of the T7-like Autographivirinae. Virol J. 2008;5:122. doi: 10.1186/1743-422X-5-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon HJ, Cho SH, Kim T-E, Won YJ, Jeong J, Park SC, Kim J-H, Yoo HS, Park YH, Kim SJ. Characterization of a T7-like lytic bacteriophage (φSG-JL2) of Salmonella enterica serovar Gallinarum biovar Gallinarum. Appl Env Microbiol. 2008;74:6970–6979. doi: 10.1128/AEM.01088-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mass spectrometric analysis of tryptic peptides derived from E. coli IF1 phosphorylated by T7PK in vitro.
Mass spectrometric analysis of tryptic peptides derived from T7PK autophosphorylated in vitro.
Mass spectrometric analysis of tryptic peptides derived from H-pT7PK, directly isolated from E. coli.
Alignment of 0.7 protein sequences of six T7 group phages. The authors thank Dr. Kaye Speicher of the Wistar Institute Proteomics Facility for performing the mass spectrometric analyses of phosphorylated IF1 and autophosphorylated T7PK. The authors also thank Dr. Philip R. Cunningham (Wayne State University) for providing the pET-15b plasmid encoding E. coli IF1 gene, and Dr. Rhonda Nicholson for input on the manuscript. This research was supported in part by the National Institutes of Health (RO1 GM56772).