Abstract
Autosomal dominant polycystic kidney disease (ADPKD), the most common hereditary disease affecting the kidneys, is caused in 85% of cases by mutations in the PKD1 gene. The protein encoded by this gene, polycystin-1, is a renal epithelial cell membrane mechanoreceptor, sensing morphogenetic cues in the extracellular environment, which regulate the tissue architecture and differentiation. However, how such mutations result in the formation of cyst is still unclear. We performed a precise characterization of mesenchymal differentiation using PAX2, WNT4 and WT1 as a marker, which revealed that impairment of the differentiation process preceded the development of cysts in Pkd1−/− mice. We performed an in vitro organ culture and found that progesterone and a derivative thereof facilitated mesenchymal differentiation, and partially prevented the formation of cysts in Pkd1−/− kidneys. An injection of progesterone or this derivative into the intraperitoneal space of pregnant females also improved the survival of Pkd1−/−embryos. Our findings suggest that compounds which enhance mesenchymal differentiation in the nephrogenesis might be useful for the therapeutic approach to prevent the formation of cysts in ADPKD patients.
Introduction
Polycystic kidney diseases are a leading cause of end-stage renal failure and a common indication for dialysis or renal transplantation. They may arise sporadically as a developmental abnormality or be acquired in adult life, but most forms are hereditary. Autosomal dominant polycystic kidney disease (ADPKD), the most common form of polycystic kidney disease, occurs in 1 in 800 live births[1]. There are two types: type I is caused by mutations in the PKD1 gene and accounts for 85 to 90 percent of cases, and type II is caused by mutations in the PKD2 gene and accounts for 10 to 15 percent of cases[1–2]. PKD1 is transcribed into a 14 kb mRNA and thence translated to a protein, polycystin-1, of 4302 amino acids (aa) with an expected molecular mass of 462 kDa[3], whereas the PKD2 transcript is 5.4 kb and polycystin-2 is a 110 kDa protein of 968 aa[4]. The C-terminus of polycystin-1 interacts, via an α-helical coiled-coil domain, with a region in the C-terminus of polycystin-2[5]. The co-assembled polycystin-1 and polycystin-2 seem to function as a non-selective cation channel[6–7], and to transduce signals to maintain the epithelial architecture of the kidney[8–9]. Recent progress in the understanding of polycystin-1 and polycystin-2 has focused on primary cilia, which act as sensory transducers in renal epithelial cells. New evidence shows that a mechanosensitive signal, cilia bending, activates the polycystin-1- polycystin-2 channel complex. When working properly, this functional complex elicits a transient Ca2+ influx, which is coupled to the release of Ca2+ from intracellular stores[10–12]. Interplay between polycystin-dependent Ca2+ influx and other interacting proteins including E-cadherin/β-catenin[9] and G-proteins may regulate adhesion, differentiation[13–14], and maturation, which are all essential steps of kidney morphogenesis.
To address the molecular mechanism of disease in ADPKD, several groups reported the generation of mouse models by targeted gene disruption of polycystins. Mice heterozygous for inactivating mutations of either locus acquire few cysts during their lifetime, whereas homozygous mutants develop severe cystic disease in utero[15–16]. Genetic analyses revealed that most cysts develop from the clonal expansion of single cells, suggesting that acquired ‘second hits’ give rise to the homozygous inactivation of Pkd1 or Pkd2. Gene disrupted mice carrying an unstable Pkd2 allele prone to inactivation (Pkd2WS25) in combination with a complete null allele (Pkd2−/−) had disease of variable severity that correlated with the degree of somatic inactivation of the unstable allele, which supports these interpretation[15]. Furthermore, Pkd1+/− and Pkd2+/− trans-heterozygous mice had cystic disease that was more severe than that predicted by a simple additive effect in singly heterozygous mice, suggesting that polycystin-1 and polycystin-2 act non-redundantly[17–19]. Polycystin disrupted mice also revealed systemic developmental defects. Pkd1−/− embryos die at later embryonic days from primary cardiovascular defects that include double outflow right ventricle, disorganized myocardium, and abnormal atrio-ventricular septation. Skeletal development is also severely compromised. These abnormalities correlate with the major sites of Pkd1 expression[16, 19–20]. Systemic effects of polycystins on the development suggest that polycystins have an important role in tissue differentiation and organogenesis.
Although the PKD genes have been unequivocally shown to be essential for establishing normal tubules, their role in the tissue differentiation and the maintenance of the highly differentiated phenotype is less well established. For example, the earliest stages of ureteric bud branching and induction of mesenchyme appeared to be normal in the Pkd1 and Pkd2 knockout mice. Given that a detailed analysis of the earliest stages of renal development has not been reported, it is possible that subtle changes that are difficult to detect at the gross level have been overlooked. To address this issue, we analyzed the early stages of nephrogenesis using several mesenchymal markers, including PAX2, WNT4, and WT1. Precise characterization revealed subtle, but significant reductions in the expression of PAX2, WNT4, and WT1 in Pkd1−/− mice, which preceded morphological aberrations of the kidney. We further performed an in vitro organ culture to trace the formation of cysts, since Pkd1−/− is embryonic lethal[16, 20]. Interestingly, metanephroi were successfully grown for 4–5 days without cysts developing, suggesting that the culture conditions suppressed the cyst-forming process. We examined each of components used for the organ culture, and found that progesterone effectively prevented cysts from developing and facilitated mesenchymal differentiation. Furthermore, an intraperitoneal injection of progesterone or its derivative prevented cysts from forming and enhanced the survival of the Pkd1−/− fetus. Our observations suggest that progesterone and its derivatives might be applicable for therapeutic approach by influencing the competence of mesodermal induction.
Materials and Methods
Histology and immunohistochemistry
Tissues and embryos for histological analysis were fixed overnight in 4% paraformaldehyde in PBS and stored in 70% ethanol before being embedded in paraffin. Sections of 5 μm thick were cut and stained with hematoxylin and eosin. For immunocytochemistry, the sections were preincubated for 15 minutes in PBS containing 0.1% bovine serum albumin and 10% goat serum, then incubated for 1 hour with either PAX2 (Ztmed), WNT4 (R & D systems), WT1 (Santa cruz) or preimmune serum. Goat anti-rabbit IgG conjugated with Cy5 (Jackson immunoResearch) was used as a secondary antibody. For nuclear counterstaining, slides were also incubated with propidium iodide (0.5 μg/ml; P-4170, Sigma) for 5 minutes. Sections were examined and images were acquired by confocal laser scanning microscopy (Zeiss, LSM510). Examinations were performed using serial sections for precise quantitation (Supplemental table 1). Quantitation was performed by measuring the fluorescence intensity of each 100μm square and summing the values. Skeletal preparations and cartilage staining were carried out as described[21]. Western bloting was performed with the same antibody used for immunohistochemistry.
Metanephric organ culture
Kidney rudiments were dissected from E13.5 wild-type embryos in PBS and placed in 15-ml culture bottles containing 7 ml glucose (10 mg/ml), penicillin G (100 units/ml), streptomycin (100 μg/ml)[22], and given supplements including of culture medum consisting of 10% FCS and 20% centrifuged mouse serum supplemented with 7 X MITO (Becton–Dickinson), 1 μM of progesterone (Sigma) or 3 μM of RU486 (Sigma). The culture bottles were allowed to rotate in an incubator (model no. RKI10–0310, Ikemoto, Tokyo).
Intraperitoneal injection
A solution of 100 μM of progesterone or 33 μM of medroxyprogesterone acetate (Kyowa Hakko) in saline was prepared. Each solution of 300 μl was then injected into the intraperitoneal space of pregnant females of Pkd1−/+ mated with Pkd1−/+ males, followed by sacrifice at a given time. We optimized the injection time and found that an injection at E11.5 was most effective, compared to other points.
Results
Impairment of mesenchymal differentiation precedes the formation of cysts in Pkd−/− mice
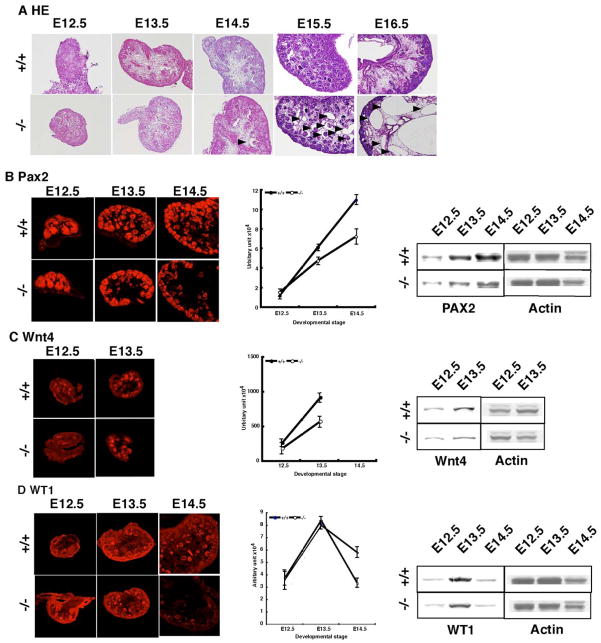
The identification of the human ADPKD genes, PKD1 and PKD2, prompted the characterization and targeted mutagenesis of their mouse orthologs. Although a many of groups have generated mutants of Pkd1 by targeted gene disruption[16, 19–20], the impairment of mesenchymal differentiation has not been precisely characterized. To address this issue, we performed immunohistochemistry with serial sections of the kidneys at several developmental points using three mesenchymal makers, PAX2, WNT4, and WT1 (Supplemental Table 1 and Supplemental Figure 1). This comprehensive analysis allowed us to uncover a subtle but an important developmental defect, which is associated with the disruption of Pkd1. The homozygous mutant was found to develop cysts from embryonic day 15.5 (E15.5) as previously reported (Figure 1A) [16, 19–20]. PAX2 is expressed in the mesonephric duct and its branch, the ureteric bud, which gives rise to the ureteric, renal pelvic, and collecting duct epithelia[23]. The expression of PAX2 in Pkd1−/− mice was normal at E12.5, but gradually decreased from E13.5, which precedes the formation of cysts in the kidney, compared to wild the type controls (Figure 1b). In particular, the reduction was more remarkable in the medulary region, which was also confirmed by Western bloting (Figure 1B). WNT4 is a member of the Wnt family that plays a critical role in genitourinary development.[24] WNT4 is activated in induced metanephric mesenchyme and required for metanephric mesenchymal condensation. It is highly expressed at E13.5, but its expression is rapidly downregulated thereafter, being undetectable at E14.5. In Pkd1−/− mice, the expression of WNT4 is also reduced at E13.5 (Figure 1C). Western bloting supported our observations. Finally, we examined the expression of WT1. WT1 is a recessive oncogene that encodes a putative transcription factor implicated in nephrogenesis[25]. The expression of WT was initially normal in Pkd1−/− mice. In wild type mice, WT1 displayed a peak in expression at E13.5, followed by a gradual decline with the further progression of the nephrogenesis. Although initial upregulation was normal in Pkd1−/− mice, the subsequent down regulation was faster as also confirmed by Western bloting(Figure 1D). These observations suggest that the initial expression of mesenchymal markers is indistinguishable in Pkd1−/− mice compared to wild type mice, however the levels clearly became reduced with the progression of nephrogenesis. In addition, this reduction precedes the cystic dilatation of renal tubes, suggesting that polycystin-1 is essential for the maintenance of mesenchymal differentiation.
Figure 1. Precise characterization of nephrogenesis in Pkd−/− mice.
To examine whether Pkd1−/− mice display an impairment of mesenchymal differentiation, we performed immunohistochemistry using PAX2, WNT4 and WT1 as markers. (A) H-E stain revealed the formation of cysts to be remarkable at E15.5 as previous reports (arrowhead)[16, 20]. A small number of cysts were was detectable from E14.5 (arrowhead). There were no obvious cyst before E13.5. (B) We first analyzed PAX2 expression. At E12.5, the expression was completely normal, but after E13.5 it was significantly reduced compared that in wild type littermates. To quantitate the level precisely, we measured PAX2 expression in serial sections as shown in Supplemental Figure1 and Supplemental Table 1. A Western bloting is shown on the right side. (C) We also examined WNT4 expression. Similarly, the expression was reduced from E13.5. The expression of WNT4 was markedly downregulated thereafter, and was not detectable after E14.5. A Western bloting is shown on the right side. (D) Finally, we examined the expression of WT1. The expression was relatively normal by E13.5, but was more rapidly downregulated in Pkd1−/− mice at E14.5.
Progesterone and its derivative rescued cyst-formation in Pkd−/− mice
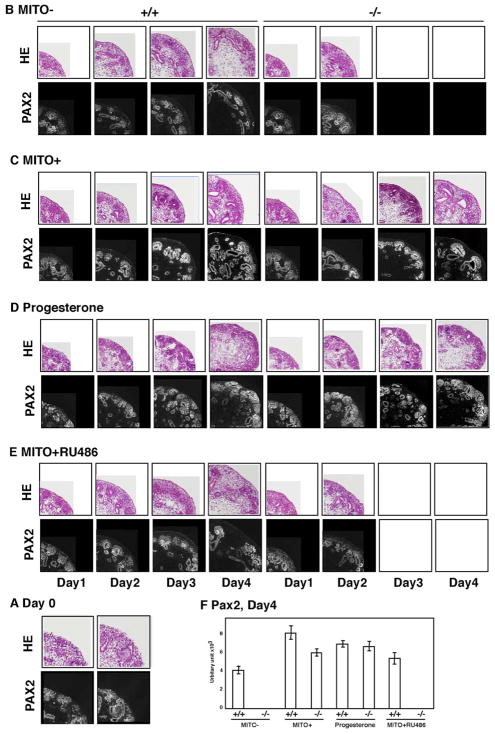
Homozygous mutants develop renal and pancreatic cysts at E15.5, coinciding with the induction of PKD1 expression in normal maturing tubular epithelia. The disease progresses rapidly, with embryonic lethality occurring among most homozygous mutants, making it difficult to trace the process of cyst-formation. To overcome this limitation of murine models of Pkd1 mutants, we employed an in vitro organ culture of the kidney[26]. At E13.5 days, metanephroi were dissected from embryos (Figure 2A) and subjected to organ culture for 4 days, which allowed kidney rudiments to continue to grow in vitro, as assessed by the observation of tubulogenesis and ureteric bud branching (Figure 2B). We also achieved several modifications to recapitulate the cyst forming process in Pkd1−/− mice (Figure 2B). We also examined the differentiation of mesenchyme using PAX2 as a marker. The conditions used for the in vitro organ culture allowed kidney rudiments to continue to grow, as assessed by PAX2 expression. Interestingly, no cysts formed in the presence of a MITO+ serum extender, suggesting that the MITO might suppress the cyst forming process (Figure 2C). To address this issue, we examined the effect of each component of MITO on growth and cystic development, and found that progesterone effectively prevents cysts from forming (Figure 2D). In contrast, RU 486, a synthetic steroid with anti-progesterone and anti-glucocorticoid inhibited the effect of progesterone had on the development of cysts (Figure 2E). The expression of PAX2 was quantified, and correlated with the formation of cysts (Figure 2F). Interestingly, administration of progesterone significantly augmented the expression of PAX2, suggesting that progesterone might enhance mesenchymal differentiation by regulating gene expression. Several lines of evidence indicate that steroids and analogues alter the total number of nephrons formed during nephrogenesis by regulation of gene expression[27–29]. Our observations suggest that progesterone might stimulate the expression of genes located downstream of polycystin-1.
Figure 2. Examination of the influence of progesterone on nephrogenesis in Pkd−/− mice.
To examine the mechanism by which cysts form in Pkd1−/− mice, metanephroi from E13.5 embryos of wild type or Pkd1−/− mice were cultured in bottles (see methods). (A) Starting materials are shown. (B) Metanephroi from wild type mice developed relatively normally with slower growth speed. Metanephroi from Pkd1−/− mice developed cysts without supplements. Arrowheads indicate cystic dilatation of the duct. (C) Interestingly, 7 X MITO+ serum extender suppressed the formation of cysts and enhanced PAX2 expression. (D) We examined each component of MITO+ serum extender to find which is effective in preventing cysts. We found that progesterone had a rescuing effect in an organ culture. (E) We confirmed the effect of progesterone using RU486, an anti-progesterone reagent. The presence of RU486 inhibited the suppression of cyst-formation by MITO+ serum extender. Arrowheads indicate cystic dilatation of the duct. (F) Quantitation of the amount of PAX2 after 4 days in vitro organ culture. Note the significant augmentation of PAX2 expression in the presence of progesterone.
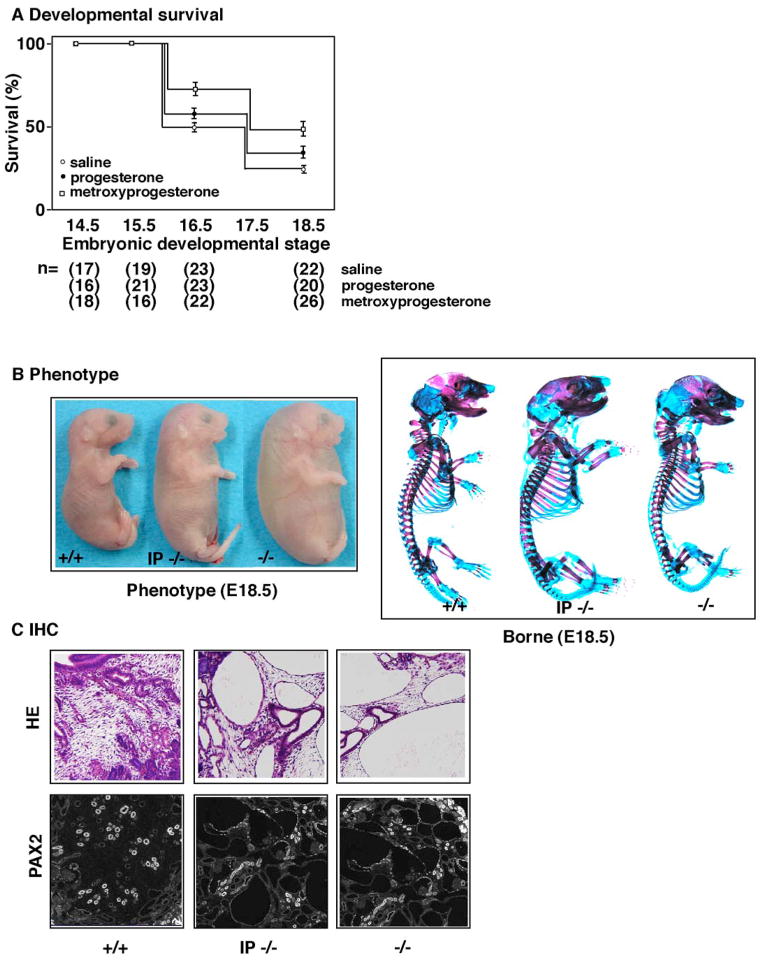
Intraperitoneal injections of progesterone and its derivative improve survival and organogenesis in Pkd1−/− mice
In vitro organ culture experiment suggests that progesterone might improve the phenotypes of Pkd1−/−. We injected progesterone and its derivatives into the intraperitoneal space of pregnant female of Pkd1−/+to examine whether the injection improves the systemic phenotypes of Pkd1−/−. We optimized the time and the dosage of injection as described in the methods. Initially, we traced the survival rate of Pkd1−/−mice (Figure 3A). The majority of Pkd1−/− mice died in the late gestational period, and Pkd1−/− mice were rarely observed at E18.5 in the control, in which saline was injected. In contrast, the injection of progesterone resulted in mild improvement in the rate of survival. Interestingly, medroxyprogesterone acetate[30], a potent derivative of progesterone, achieved an ever more significant improvement of survival. The use of another potent derivative of progesterone, chlormadinone acetate[30], did not result in any improvement, suggesting that the potency of progesterone activity is not correlated with survival (data not shown). Pkd1−/− embryos die from a primary cardiovascular defect, and show the severely compromised skeletal development. We examined these phenotypes at E18.5 (Figure 3B). Pkd1−/− embryos exhibited an edematous appearance compared to the wild type, whereas in the injected groups, this feature was improved. The improvement of edema was also supported by quantitation of fluid volume. We also examined skeletal abnormalities in Pkd1−/− embryos. We found that both the axial skeleton and long bones were abnormal as previously reports[20]. Notably, the long bones were shorter and smaller in diameter, with splaying of the radius and ulna, and histological sectioning revealed the differentiation of cartilage to be delayed (data not shown). These impairments of osteogenesis were ameliorated in the injected groups (Figure 3B). Finally, we examined the development of cysts and mesenchymal differentiation using PAX2 as a marker (Figure 3C). The formation of cysts and expression of PAX2 were partially normalized in the injected group. These observations suggest that progesterone and its derivatives might facilitate the expression of genes downstream of polycystin-1.
Figure 3. Improvement of phenotype in Pkd−/− mice on the intraperitonial injection of progesterone and a derivative.
To examine whether progesterone and its derivatives are effective against the phenotype of Pkd1−/− embryos, we injected them into the intraperitoneal space of pregnant females of Pkd1+/− mice. (A) The rate of survival was examined at a given point in gestation. Most of the Pkd1−/− embryos died before E18.5, whereas in the injected group, over 35% of Pkd1−/− embryos survived. The effect on the rate of survival rate was significant in medroxyprogesterone acetate, but less significant in progesterone. The total number of embryos examined is summarized at the bottom. (B) Surface examination revealed that Pkd1−/− embryos at E17.5 are edematous and pale compared to wild type controls (+/+), whereas Pkd1−/− embryos from injected groups (IP−/−) are improved compared to the uninjected group (−/−) (left panel). Quantitation of fluid volume is summarized at the bottom. Skeletal bone stain revealed an impairment of osteogenesis in Pkd1−/− embryos, whereas Pkd1−/− embryos from injected groups (IP−/−) are partially restored compared to the uninjected group (−/−) (right panel). Wild type (+/+) is shown at the left. (C) We examined H-E staining of Pkd1−/− embryos from injected groups (IP−/−) compared to the uninjected group (−/−), and found a partial effect on the formation of cysts. In these cases, expression of PAX2 was also improved. Wild type (+/+) is shown at the left.
Discussion
ADPKD is a heterogeneous human disease resulting from mutations in either of two genes, PKD1 and PKD2[3–4]. However, PKD1 expression during early nephrogenesis is limited, with weak expression in uninduced and induced mesenchyme and no expression in the ureteric bud and comma and S-shaped bodies[31]. The developmental role of polycystin-1 in ureteric bud growth and branching during kidney development has not been clear, since a precise characterization of nephrogenesis in Pkd1−/− mice at an early stage was not carried out. We performed precise characterization of mesenchymal differentiation using several mesodermal markers. Our observations suggest that an impairment of mesenchymal differentiation precedes cystic dilation. We also performed an in vitro organ culture, and found that progesterone and its derivative prevent cysts from forming. The intraperitoneal injection of a progesterone derivative also improved phenotypes of Pkd−/− mice. Polycystin-1 is a renal epithelial cell membrane mechanoreceptor, sensing morphogenetic cues in the extracellular environment at the basal surface in focal adhesion complexes; at the lateral surface in cell adherens junctions; and in the lumen at the apical primary cilium[10]. Activation via the formation of a multiprotein complex, intracellular signal transduction cascades and the regulation of fetal gene transcription leads to appropriate renal tubule epithelial cell division and differentiation in normal kidneys. Although the molecular mechanism by which cysts form is not clearly understood, trans-differentiation caused by a lack of appropriate maintenance of differentiation is plausible[32]. For example, in the orpk pancreas, cilia numbers are reduced and cilia length is decreased[33]. The expression of polycystin-2, a protein involved in PKD, is mislocalized in orpk mice. Furthermore, the cellular localization of beta-catenin, a protein involved in cell adhesion and Wnt signaling, is altered. Progesterone is a potent regulator of gene expression by binding with intranuclear receptors. The target genes of progesterone might partially overlap with the downstream genes of polycystin-1. The augmentation of PAX2 expression by progesterone supports this possibility. Further characterization of the target genes of polycystin-1 may lead to a therapeutic approach to the prevention of cyst development through the induction of differentiation by steroids and their derivatives.
Supplementary Material
Acknowledgments
We thank Takao Kenko, Emi Donoue and Keisuke Inoue for technical support. We also thank to Hiromichi Nishimura and Keiko Fujimoto for mouse breeding. We are grateful to Hirotaka Asakura and Hiroshi Iwao for generous support and encouragement. This work was supported by Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan and by “Ground-based Research Program for Space Utilization” promoted by Japan Space Forum to Shinji Hirotsune. This work was also supported by YASUDA Medical Research Foundation and The Cell Science Research Foundation to Shinji Hirotsune.
References
- 1.Wilson PD. Polycystic kidney disease: new understanding in the pathogenesis. Int J Biochem Cell Biol. 2004;36:1868–1873. doi: 10.1016/j.biocel.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Peters DJ, Breuning MH. Autosomal dominant polycystic kidney disease: modification of disease progression. Lancet. 2001;358:1439–1444. doi: 10.1016/S0140-6736(01)06531-X. [DOI] [PubMed] [Google Scholar]
- 3.Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. The International Polycystic Kidney Disease Consortium. 1995;81:289–298. doi: 10.1016/0092-8674(95)90339-9. [DOI] [PubMed] [Google Scholar]
- 4.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 5.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet. 1997;16:179–183. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- 6.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- 7.Tsiokas L, Arnould T, Zhu C, Kim E, Walz G, Sukhatme VP. Specific association of the gene product of PKD2 with the TRPC1 channel. Proc Natl Acad Sci USA. 1999;96:3934–3939. doi: 10.1073/pnas.96.7.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnould T, Kim E, Tsiokas L, Jochimsen F, Gruning W, Chang JD, Walz G. The polycystic kidney disease 1 gene product mediates protein kinase C alpha-dependent and c-Jun N-terminal kinase-dependent activation of the transcription factor AP-1. J Biol Chem. 1998;273:6013–6018. doi: 10.1074/jbc.273.11.6013. [DOI] [PubMed] [Google Scholar]
- 9.Kim E, Arnould T, Sellin LK, Benzing T, Fan MJ, Gruning W, Sokol SY, Drummond I, Walz G. The polycystic kidney disease 1 gene product modulates Wnt signaling. J Biol Chem. 1999;274:4947–4953. doi: 10.1074/jbc.274.8.4947. [DOI] [PubMed] [Google Scholar]
- 10.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 11.Delmas P. Polycystins: from mechanosensation to gene regulation. Cell. 2004;118:145–148. doi: 10.1016/j.cell.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell. 2006;10:57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Delmas P, Nomura H, Li X, Lakkis M, Luo Y, Segal Y, Fernandez-Fernandez JM, Harris P, Frischauf AM, Brown DA, Zhou J. Constitutive activation of G-proteins by polycystin-1 is antagonized by polycystin-2. J Biol Chem. 2002;277:11276–11283. doi: 10.1074/jbc.M110483200. [DOI] [PubMed] [Google Scholar]
- 14.Kim E, Arnould T, Sellin L, Benzing T, Comella N, Kocher O, Tsiokas L, Sukhatme VP, Walz G. Interaction between RGS7 and polycystin. Proc Natl Acad Sci USA. 1999;96:6371–6376. doi: 10.1073/pnas.96.11.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu G, D’Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, Maeda Y, Le TC, Hou H, Jr, Kucherlapati R, Edelmann W, Somlo S. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell. 1998;93:177–188. doi: 10.1016/s0092-8674(00)81570-6. [DOI] [PubMed] [Google Scholar]
- 16.Lu W, Peissel B, Babakhanlou H, Pavlova A, Geng L, Fan X, Larson C, Brent G, Zhou J. Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat Genet. 1997;17:179–181. doi: 10.1038/ng1097-179. [DOI] [PubMed] [Google Scholar]
- 17.Koptides M, Mean R, Demetriou K, Pierides A, Deltas CC. Genetic evidence for a trans-heterozygous model for cystogenesis in autosomal dominant polycystic kidney disease. Hum Mol Genet. 2000;9:447–452. doi: 10.1093/hmg/9.3.447. [DOI] [PubMed] [Google Scholar]
- 18.Watnick T, He N, Wang K, Liang Y, Parfrey P, Hefferton D, St George-Hyslop P, Germino G, Pei Y. Mutations of PKD1 in ADPKD2 cysts suggest a pathogenic effect of trans-heterozygous mutations. Nat Genet. 2000;25:143–144. doi: 10.1038/75981. [DOI] [PubMed] [Google Scholar]
- 19.Wu G, Tian X, Nishimura S, Markowitz GS, D’Agati V, Park JH, Yao L, Li L, Geng L, Zhao H, Edelmann W, Somlo S. Trans-heterozygous Pkd1 and Pkd2 mutations modify expression of polycystic kidney disease. Hum Mol Genet. 2002;11:1845–1854. doi: 10.1093/hmg/11.16.1845. [DOI] [PubMed] [Google Scholar]
- 20.Boulter C, Mulroy S, Webb S, Fleming S, Brindle K, Sandford R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci USA. 2001;98:12174–12179. doi: 10.1073/pnas.211191098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogan BBR, Costantini F, Lacy E. A laboratory manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. Manipulating the mouse embryo. [Google Scholar]
- 22.Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest. 2000;105:863–873. doi: 10.1172/JCI8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dressler GR, Douglass EC. Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proc Natl Acad Sci USA. 1992;89:1179–1183. doi: 10.1073/pnas.89.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 25.Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- 26.Avner ED, Villee DB, Schneeberger EE, Grupe WE. An organ culture model for the study of metanephric development. J Urol. 1983;129:660–664. doi: 10.1016/s0022-5347(17)52273-9. [DOI] [PubMed] [Google Scholar]
- 27.Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Mullerian) epithelial differentiation. Dev Biol. 2001;240:194–211. doi: 10.1006/dbio.2001.0458. [DOI] [PubMed] [Google Scholar]
- 28.Briere N, Bertrand L, Ferrari J. Mouse fetal kidneys in serum-free organ culture: effects of epidermal growth factor and hydrocortisone. Comp Biochem Physiol. 1991;98:421–430. doi: 10.1016/0300-9629(91)90426-d. [DOI] [PubMed] [Google Scholar]
- 29.Rowley DR. Glucocorticoid regulation of transforming growth factor-beta activation in urogenital sinus mesenchymal cells. Endocrinology. 1992;131:471–478. doi: 10.1210/endo.131.1.1612028. [DOI] [PubMed] [Google Scholar]
- 30.Westhoff C. Bone mineral density and DMPA. J Reprod Med. 2002;47:795–799. [PubMed] [Google Scholar]
- 31.Griffin MD, O’Sullivan DA, Torres VE, Grande JP, Kanwar YS, Kumar R. Expression of polycystin in mouse metanephros and extra-metanephric tissues. Kidney Int. 1997;52:1196–1205. doi: 10.1038/ki.1997.444. [DOI] [PubMed] [Google Scholar]
- 32.Valkova N, Yunis R, Mak SK, Kang K, Kultz D. Nek8 mutation causes overexpression of galectin-1, sorcin, and vimentin and accumulation of the major urinary protein in renal cysts of jck mice. Mol Cell Proteomics. 2005;4:1009–1018. doi: 10.1074/mcp.M500091-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Cano DA, Murcia NS, Pazour GJ, Hebrok M. Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development. 2004;131:3457–3467. doi: 10.1242/dev.01189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.