ABSTRACT
The CsrRS two-component regulatory system of group A Streptococcus (GAS; Streptococcus pyogenes) responds to subinhibitory concentrations of the human antimicrobial peptide LL-37. LL-37 signaling through CsrRS results in upregulation of genes that direct synthesis of virulence factors, including the hyaluronic acid capsule and streptolysin O (SLO). Here, we demonstrate that a consequence of this response is augmented GAS resistance to killing by human oropharyngeal keratinocytes, neutrophils, and macrophages. LL-37-induced upregulation of SLO and hyaluronic acid capsule significantly reduced internalization of GAS by keratinocytes and phagocytic killing by neutrophils and macrophages. Because vitamin D induces LL-37 production by macrophages, we tested its effect on macrophage killing of GAS. In contrast to the reported enhancement of macrophage function in relation to other pathogens, treatment of macrophages with 1α,25-dihydroxy-vitamin D3 paradoxically reduced the ability of macrophages to control GAS infection. These observations demonstrate that LL-37 signals through CsrRS to induce a virulence phenotype in GAS characterized by heightened resistance to ingestion and killing by both epithelial cells and phagocytes. By inducing LL-37 production in macrophages, vitamin D may contribute to this paradoxical exacerbation of GAS infection.
IMPORTANCE
It remains poorly understood why group A Streptococcus (GAS) causes asymptomatic colonization or localized throat inflammation in most individuals but rarely progresses to invasive infection. The human antimicrobial peptide LL-37, which is produced as part of the innate immune response to GAS infection, signals through the GAS CsrRS two-component regulatory system to upregulate expression of multiple virulence factors. This study reports that two CsrRS-regulated GAS virulence factors—streptolysin O and the hyaluronic acid capsule—are critical in LL-37-induced resistance of GAS to killing by human throat epithelial cells and by neutrophils and macrophages. Vitamin D, which increases LL-37 production in macrophages, has the paradoxical effect of increasing GAS resistance to macrophage-mediated killing. In this way, the human innate immune response may promote the transition from GAS colonization to invasive infection.
Introduction
Group A Streptococcus (GAS; Streptococcus pyogenes) causes a wide spectrum of clinical conditions that range from asymptomatic colonization to localized infections (such as pharyngitis and impetigo) to life-threatening invasive disease, including necrotizing fasciitis and streptococcal toxic shock syndrome (1, 2). GAS appears to be uniquely adapted to survive in the human host, as no other significant reservoir of the organism has been identified in animals or the environment. Although GAS can be isolated from other body sites under some circumstances, the oropharynx is its preferred niche and the most common site of local infection. Furthermore, isolates from invasive infections are usually identical to strains associated with pharyngitis or asymptomatic pharyngeal carriage (3, 4). The latter observation has stimulated interest in determining the bacterial and host factors that govern the transition from pharyngeal colonization or localized pharyngitis to invasive infection and systemic dissemination.
The CsrRS (or CovRS) two-component system appears to play an important role in the conversion from asymptomatic carriage or localized infection to invasive and potentially life-threatening disease. Originally identified as a regulator of hyaluronic acid capsule biosynthesis, CsrRS is now known to regulate expression of up to 15% of the GAS genome (5-8). Genes included in the CsrRS regulon vary somewhat in different GAS strains, but they typically include those encoding major virulence determinants such as streptolysin O, NAD-glycohydrolase, streptolysin S, streptokinase, SpyCEP interleukin 8 (IL-8) protease, and the hyaluronic acid capsule-biosynthetic enzymes. Isolates with spontaneous inactivating mutations in csrR or csrS have been associated with clinical invasive infections and with increased virulence in experimental infections in mice (9-11). Because these mutations result in increased expression of most CsrRS-regulated virulence factors, the regulator component CsrR is thought to act predominantly as a transcriptional repressor at regulated promoters. Exposure of GAS to high concentrations (≥10 mM) of extracellular magnesium results in CsrRS-dependent repression of many regulated genes, consistent with a specific signaling role for Mg2+ as an activating ligand for the CsrS sensor kinase (7, 12). The human cathelicidin antimicrobial peptide LL-37 functions in an opposite manner: GAS exposure to concentrations of LL-37 far below those that inhibit bacterial growth results in upregulation of the same genes that are repressed by high levels of Mg2+ in a CsrRS-dependent manner (13, 14).
The finding that LL-37 could stimulate a marked increase in expression of multiple virulence factors in vitro suggested that this innate immune effector might function in vivo as a specific signal to enhance GAS virulence during human infection. LL-37 has been detected in human saliva at concentrations similar to those that signal through CsrRS in vitro (50 to 300 nm), and local concentrations may be increased by secretion from epithelial cells or macrophages or from degranulation of neutrophils (15, 16). The effect of LL-37 signaling through CsrRS is to upregulate expression of several factors predicted to increase GAS resistance to uptake and killing by epithelial cells and professional phagocytes. The goals of this investigation were characterize the effect of LL-37 on interaction of GAS with human cell types implicated in local and systemic control of GAS proliferation and to identify critical virulence determinants in the CsrRS regulon responsible for these effects. We found that LL-37 signaling resulted in a marked increase in GAS resistance to uptake and killing by each of three key cell types—oropharyngeal keratinocytes, neutrophils, and macrophages. Thus, exposure of GAS to this innate immune effector has the paradoxical effect of enabling the organism to overcome local cellular mechanisms to control GAS proliferation. Studies of GAS isogenic mutants deficient in production of specific virulence determinants revealed that the hyaluronic acid capsule and streptolysin O (SLO) were both critical factors in LL-37-induced resistance to killing by human cells. As others have reported, we found that treatment of macrophages with vitamin D induced LL-37 production; however, in striking contrast to effects on macrophage killing of other pathogens, vitamin D treatment of macrophages promoted GAS survival.
RESULTS
LL-37 signals through CsrRS to confer GAS resistance to uptake and killing by oropharyngeal keratinocytes.
During colonization or infection of the throat, GAS must adhere to the pharyngeal epithelium and resist clearance by mechanical forces such as mucous flow as well as internalization and killing by pharyngeal keratinocytes. The GAS hyaluronic acid capsule and streptolysin O have been shown to inhibit internalization of GAS by epithelial cells (17, 18). Since production of both these products is stimulated by LL-37 signaling through CsrRS, we hypothesized that exposure of GAS to LL-37 would make the bacteria more resistant to uptake and killing by oropharyngeal keratinocytes.
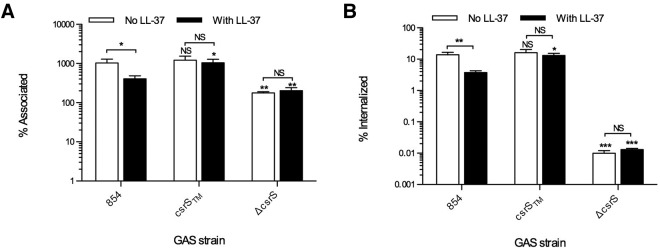
To test this hypothesis, we used antibiotic protection assays to assess uptake of GAS strain 854, an M1T1 clinical isolate, by human oropharyngeal keratinocytes. We found that exposure of strain 854 to 300 nM LL-37 significantly decreased both bacterial attachment and internalization by the keratinocytes (Fig. 1). We used two isogenic mutants to investigate the role of the CsrS sensor kinase in LL-37-induced resistance to internalization. Deletion of CsrS results in constitutive upregulation of several CsrRS-regulated virulence factors and lack of responsiveness to LL-37 (13, 14). Accordingly, such a mutant would be predicted to resist internalization in the presence or absence of LL-37. As expected, the csrS deletion mutant 854ΔcsrS was resistant to internalization in the absence of LL-37, and resistance was not increased further by LL-37 exposure (Fig. 1B). Another mutant, strain 854csrSTM, expresses a CsrS protein that has three point mutations that change a cluster of negatively charged amino acids in the extracellular domain to neutral residues, the effect of which is constitutive repression of CsrRS-regulated genes and relative refractoriness to LL-37-induced regulation of the CsrRS regulon (14). As predicted by the unresponsiveness of this mutant strain to LL-37 signaling, attachment and internalization of strain 854csrSTM were unaffected by exposure to LL-37 (Fig. 1). These results are all consistent with a model in which LL-37 signals through CsrS to induce resistance to GAS internalization by keratinocytes.
FIG 1.
LL-37 decreases association and internalization of GAS (group A Streptococcus) by oropharyngeal keratinocytes. OKP7 cells were infected with GAS 854, 854csrSTM, or 854ΔcsrS in the absence or presence of 300 nM LL-37. Numbers of total associated CFU (A) and intracellular CFU (B) are shown as means and SEM from four independent experiments; each experiment assayed each condition in triplicate. Asterisks indicate significant differences from values for strain 854 for the same condition: *, P < 0.05; **, P < 0.01. NS, not significant.
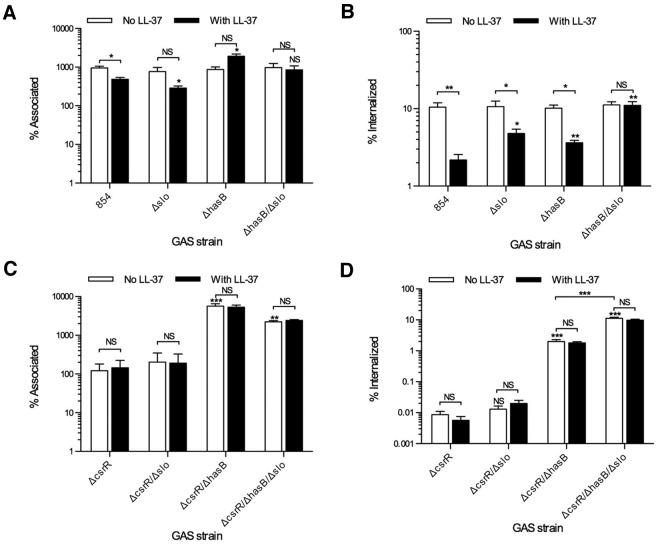
Because both the hyaluronic acid capsule and SLO inhibit GAS internalization by oropharyngeal keratinocytes, we used isogenic mutants deficient in production of these factors to investigate their contribution to the resistance to internalization induced by LL-37. Exposure of the SLO-mutant strain 854Δslo to LL-37 was less effective in inducing resistance to uptake by keratinocytes than was LL-37 treatment of wild-type strain 854 (Fig. 2B). Thus, upregulation of SLO by exposure to LL-37 contributes significantly to GAS resistance to internalization. Similarly, deletion of hyaluronic acid capsule in strain 854ΔhasB resulted in attenuation of LL-37-induced resistance to uptake by keratinocytes. A strain deficient in both SLO and capsule (854Δslo/ΔhasB) was efficiently internalized in either the absence or presence of LL-37. These results indicate that SLO and capsule play critical and nonredundant roles in LL-37-induced GAS resistance to internalization by oropharyngeal keratinocytes.
FIG 2.
SLO and capsule contribute to the phenotype induced by either LL-37 exposure or inactivation of CsrR. OKP7 cells were infected with GAS in the absence or presence of 300 nM LL-37. Total associated (A) and internalized (B) CFU were measured for cells infected with GAS 854, 854Δslo, 854ΔhasB, and 854ΔhasB/Δslo. Similarly, total associated (C) and internalized (D) CFU were measured for cells infected with 854ΔcsrR, 854ΔcsrR/Δslo, 854ΔcsrR/ΔhasB, and 854ΔcsrR/ΔhasB/Δslo. Data are means and SEM from four independent experiments; each experiment assayed each condition in triplicate. Asterisks indicate significant differences from parental strain 854 or 854ΔcsrR for the same condition: *, P < 0.05; **, P < 0.01; ***, P < 0.001. NS, not significant.
Inactivation of CsrR, either by intentional deletion or spontaneous mutation, results in derepression of multiple genes in the CsrRS regulon, including the has operon and slo. This transcriptional profile is similar to that induced in wild-type GAS by exposure to LL-37 (14). Therefore, we anticipated that a CsrR deletion mutant would be resistant to internalization by keratinocytes. Indeed, strain 854ΔcsrR was poorly associated with and highly resistant to internalization by oropharyngeal keratinocytes (Fig. 2C and D). This phenotype was not significantly altered by the deletion of slo (strain 854ΔcsrR/Δslo); however, deletion of hasB (strain 854ΔcsrR/ΔhasB) significantly increased both association and internalization. Deletion of both genes in strain 854ΔcsrR/ΔhasB/Δslo further increased internalization, which approached the rates seen with wild-type 854 in the absence of LL-37 exposure. Of note, the deletion of csrR abolished responsiveness to LL-37 exposure, consistent with the complete derepression of slo and the has operon as a result of csrR deletion (14).
Upregulation of hyaluronic acid capsule by LL-37 makes GAS more resistant to killing by human neutrophils.
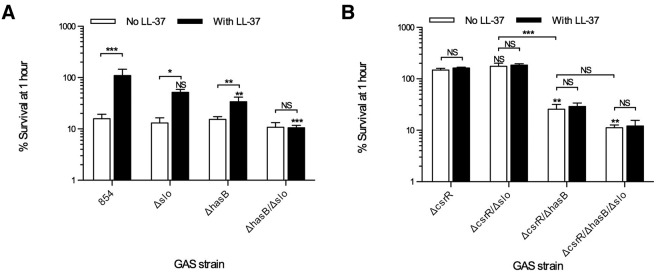
As some of the earliest inflammatory cells at the site of infection, neutrophils play a central role in controlling bacterial invasion, including that by GAS. To study the role of SLO and the hyaluronic acid capsule in resistance to neutrophil killing, isogenic mutants of strain 854 were grown in the absence or presence of LL-37 prior to exposure to human neutrophils and nonimmune serum as a complement source. In the absence of LL-37 exposure, wild-type 854, 854Δslo, 854ΔhasB, and 854ΔhasB/Δslo were all killed equally effectively (Fig. 3A). As demonstrated previously, wild-type strain 854 became more resistant to killing by human neutrophils when the bacteria were exposed to LL-37 (14). However, when capsule synthesis was eliminated by deletion of hasB, pre-exposure to LL-37 had significantly less effect, a result that supports the importance of capsule for LL-37-induced phagocytic resistance (Fig. 3A). In contrast, 854Δslo had survival rates slightly lower than, but not statistically different from, wild-type 854. The double mutant, 854Δslo/ΔhasB, showed no increase in resistance to killing after exposure to LL-37. Thus, capsule appears to be essential for LL-37-induced resistance to neutrophil phagocytic killing, and SLO further enhances the anti-phagocytic phenotype.
FIG 3.
SLO and capsule contribute to GAS resistance to killing by neutrophils after LL-37 exposure or inactivation of csrR. GAS strains were grown in the absence or presence of 300 nM LL-37 prior to exposure to human neutrophils. CFU were determined by quantitative cultures at the beginning and end of a 1-h incubation and are plotted as percent survival, i.e., number of CFU at 1 h expressed as a percentage of the number present at the beginning of the experiment. (A) Infections with GAS 854, 854Δslo, 854ΔhasB, and 854ΔhasB/Δslo; (B) those with 854ΔcsrR, 854ΔcsrR/Δslo, 854ΔcsrR/ΔhasB, and 854ΔcsrR/ΔhasB/Δslo. Data are means and SEM from four independent experiments; each experiment assayed each condition in duplicate. Asterisks indicate significant differences from parental strain 854 or 854ΔcsrR for the same condition: *, P < 0.05; **, P < 0.01; ***, P < 0.001. NS, not significant.
The CsrR mutant strain 854ΔcsrR was highly resistant to neutrophil phagocytic killing. Growth of 854ΔcsrR in LL-37 resulted in no further increase in resistance, as predicted from the complete derepression of the CsrRS regulon after deletion of csrR (Fig. 3B). There was no statistically significant difference between survival rates of wild-type 854 grown with LL-37 and 854ΔcsrR. Similar to results for wild-type 854 treated with LL-37, when hasB was deleted from 854ΔcsrR (Fig. 3A and B), the survival rate was significantly decreased, whereas deletion of slo had no significant effect (Fig. 3B). Deletion of both slo and hasB from 854ΔcsrR created a strain (854ΔcsrR/ΔhasB/Δslo) that was no more resistant to neutrophil killing than wild-type 854 in the absence of LL-37 stimulation.
LL-37 induces GAS resistance to killing by macrophages.
In addition to keratinocytes and neutrophils, macrophages represent some of the earliest cells GAS encounters during invasion of host tissues. SLO production by GAS is associated with cytotoxic injury to macrophages in vitro, and SLO has been reported to induce macrophage apoptosis, pyroptosis, and oncosis (19-21). Studies of tissue samples from patients with necrotizing fasciitis suggest that GAS can survive within tissue macrophages despite bactericidal antibiotic concentrations in the surrounding milieu (22). Macrophages have been identified as a source of LL-37 in tissue samples from healthy oropharynx and in necrotizing fasciitis caused by GAS (23, 24). Therefore, it is possible that LL-37 produced by macrophages at the site of infection signals through CsrRS to induce expression of factors that enhance GAS resistance to macrophage killing.
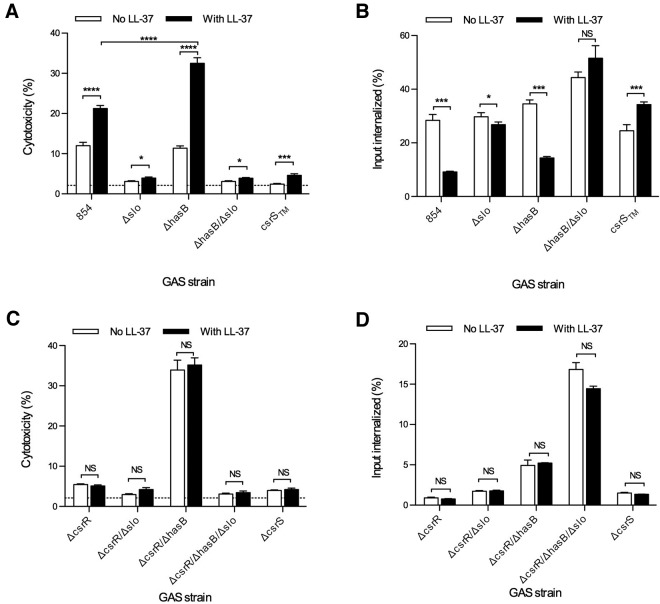
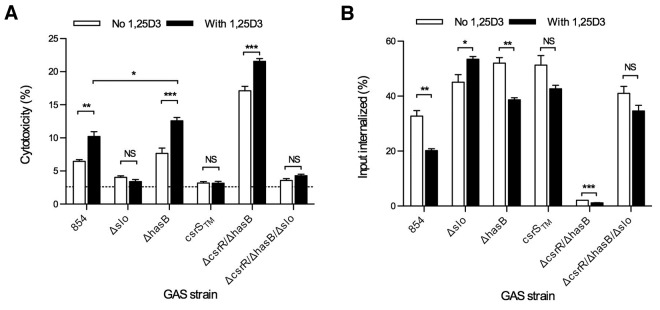
To evaluate the effect of LL-37 on GAS interactions with macrophages, we measured lactate dehydrogenase (LDH) release as a marker of cytotoxicity during exposure of THP-1 macrophages to GAS in the absence or presence of exogenous LL-37. As shown in Fig. 4A, LL-37 significantly increased GAS cytotoxicity for macrophages. The CsrS triple mutant 854csrSTM, in which expression of CsrRS-regulated virulence factors is repressed and which is hyporesponsive to LL-37, was not cytotoxic in the untreated condition and showed only a minimal increase in the presence of LL-37. The SLO mutant strain 854Δslo also produced minimal toxicity under each condition, supporting the importance of SLO for macrophage cytotoxicity. Assays of strain 854ΔhasB revealed that LL-37-induced upregulation of slo in the absence of a concomitant increase in the antiphagocytic capsule led to a marked increase in cytotoxicity. This result suggests that the cytotoxic effect of SLO depends on phagocytic uptake of GAS, which is enhanced by elimination of the capsule. Our observations that capsule overproduction can limit SLO toxicity, as in 854ΔcsrR above, are consistent with this model. To evaluate directly the role of GAS uptake in cytotoxicity, we blocked GAS internalization by macrophages using the actin polymerization inhibitor cytochalasin D. Blocking internalization reduced GAS-induced cytotoxicity nearly to background and abolished any increase in cytotoxicity from exposure to LL-37 (see Fig. S1 in the supplemental material). Thus, GAS-induced cytotoxicity for macrophages requires bacterial internalization, as has been reported previously (21). Consistent with this model, despite expressing approximately five times more SLO than wild-type 854, the csrR mutant strain 854ΔcsrR produced only limited cytotoxicity unless capsule expression was abrogated (strain 854ΔcsrR/ΔhasB) (Fig. 4C). As expected, cytotoxic injury by deletion mutants in csrR or csrS was unaffected by exposure to LL-37.
FIG 4.
LL-37 increases GAS cytotoxicity for THP-1 macrophages. THP-1 cells were infected with GAS in the absence or presence of 300 nM exogenous LL-37. After infection with GAS 854, 854Δslo, 854ΔhasB, 854ΔhasB/Δslo, and 854csrSTM, cytotoxicity was measured as LDH released into the cell culture supernatant (A), and intracellular GAS CFU were counted (B). Infections with 854ΔcsrR, 854ΔcsrR/Δslo, 854ΔcsrR/ΔhasB, 854ΔcsrR/ΔhasB/Δslo, and 854ΔcsrS were also assayed for cytotoxicity (C) and intracellular CFU (D). The background level of LDH release from cells not exposed to GAS is indicated by a broken line and did not vary between conditions. Data represent a single experiment, with five (A and C) or three (B and D) replicates, representative of three independent experiments. *, P < 0.05; ***, P < 0.001; ****, P < 0.0001.
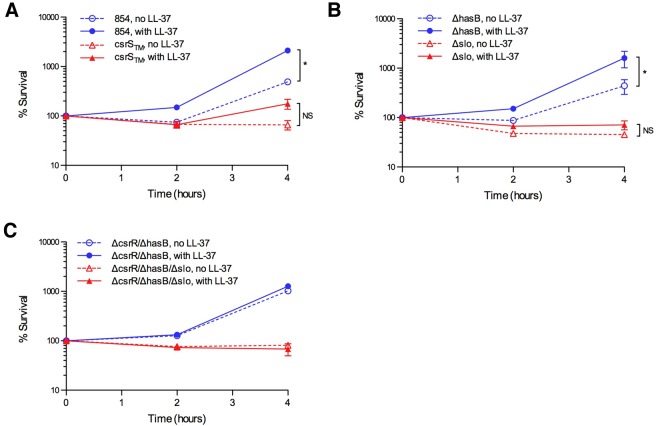
In separate experiments, we assessed the effect of LL-37 signaling on GAS survival in the presence of macrophages. THP-1 macrophages were infected with GAS in the absence or presence of exogenous LL-37 and then were treated with penicillin and gentamicin to kill extracellular bacteria. The cells were then washed and returned to antibiotic-free medium with or without LL-37. GAS that survived intracellularly and those that escaped from the cells were quantified by culturing aliquots of the cell lysate and growth medium 2 or 4 h later. As shown in Fig. 5A, wild-type strain 854 exhibited significantly better survival at 4 h in the presence of LL-37 than in its absence. In contrast, the CsrS triple mutant 854csrSTM survived poorly in macrophages after growth in unsupplemented medium and only slightly better in the presence of LL-37, consistent with the repressed expression of CsrRS-regulated virulence factors in this strain and its blunted response to LL-37. Deletion of slo abrogated the survival benefit conferred by LL-37 exposure, whereas a capsule-deficient strain was not significantly different from wild-type 854 with respect to survival in the absence or presence of LL-37 (Fig. 5B). Together, these results implicate SLO as the critical CsrRS-regulated determinant required for enhanced GAS survival to macrophage killing in response to LL-37. This survival benefit was absent from strains lacking a functional CsrR (ΔcsrR/ΔhasB and ΔcsrR/ΔhasB/Δslo strains), a result that supports signaling through CsrRS as the responsible mechanism and that excludes an inhibitory effect of LL-37 on the macrophages themselves (Fig. 5C).
FIG 5.
LL-37 makes GAS more resistant to killing by macrophages. THP-1 macrophages were infected with GAS in the absence or presence of 300 nM LL-37. GAS 854, 854csrSTM, 854ΔhasB, 854Δslo, 854ΔcsrR, or 854ΔcsrR/ΔhasB were used for infection; data are presented in separate panels for clarity. Viable GAS released into the cell culture medium plus GAS associated with the macrophages were quantified after 90 min of incubation (time zero) and 2 and 4 h later. Data are presented for each strain as a percentage of the GAS at each time point compared to the number at time zero. Data are means and standard errors from at least three separate experiments. *, P < 0.05.
Vitamin D treatment of macrophages augments GAS-induced cytotoxicity and GAS survival.
The experiments described above utilizing exogenously added LL-37 as a signaling ligand raised the possibility that endogenous LL-37 produced by macrophages themselves might cause similar increases in macrophage cytotoxicity and enhanced GAS survival. Others have demonstrated that 1,25-dihydroxy-vitamin D3 (1,25D3) increases expression of LL-37 and its precursor hCAP18 in both human monocyte-derived macrophages and in THP-1 macrophages (25, 26). While 1,25D3-induced upregulation of macrophage cathelicidin expression has been correlated with enhanced killing of intracellular pathogens in vitro, its effect on killing of GAS by human macrophages is less predictable, since the enhancement of macrophage function may be counterbalanced by LL-37-induced upregulation of GAS virulence factor expression via CsrRS signaling (25, 26). To investigate the net effect of 1,25D3 on macrophage interactions with GAS, we compared GAS-induced cytotoxicity of THP-1 macrophages that had and had not been treated with 1,25D3. Upregulation of hCAP18/LL-37 was confirmed by a 38-fold (±3.1-fold) increase in gene expression by quantitative reverse transcription-PCR and by Western blot analysis of macrophage cell lysates (see Fig. S2 in the supplemental material). Macrophages that had been treated with 1,25D3 released significantly more LDH than untreated cells upon infection with GAS 854, consistent with the induction of hCAP18/LL-37 expression in the macrophages and subsequent LL-37 signaling to internalized GAS through CsrRS to increase GAS virulence gene expression (Fig. 6A; also, see Fig. S1 in the supplemental material). The SLO mutant strain 854Δslo and the CsrS triple mutant strain 854csrSTM, which is hyporesponsive to LL-37 stimulation, induced little cytotoxicity in either untreated or 1,25D3-treated macrophages. Despite the absence of a functional CsrRS system, strain 854ΔcsrR/ΔhasB also induced increased cytotoxicity with 1,25D3-pretreated cells, although to a lesser degree than wild-type 854, a result that suggests that other vitamin D-responsive factors contribute to enhanced cytotoxicity in addition to LL-37 signaling through CsrRS. The effects of 1,25D3 on GAS-induced cytotoxicity were mirrored by reciprocal effects on the efficiency of GAS internalization by infected macrophages (Fig. 6B; also, see Fig S1 in the supplemental material).
FIG 6.
Pretreatment of macrophages with 1,25-dihydroxyvitamin D3 (1,25D3) increases GAS cytotoxicity. THP-1 macrophages were pretreated with 20 nM 1,25D3 for 24 h before being infected with GAS 854, 854Δslo, 854ΔhasB, 854csrSTM, 854ΔcsrR/ΔhasB, or 854ΔcsrR/ΔhasB/Δslo. (A) Supernatants were assayed for LDH release as described above; (B) intracellular GAS were measured. GAS strain designations are as in Fig. 2. Results of a single representative experiment with five (A) or three (B) replicate measurements are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001. NS, not significant.
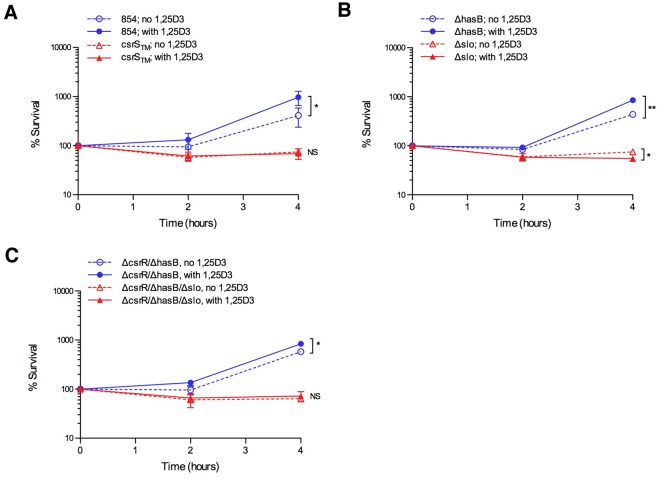
We also tested whether 1,25D3-pretreatment of macrophages affected GAS survival. As shown in Fig. 7, macrophages pretreated with 1,25D3 were significantly impaired in their ability to control proliferation of wild-type GAS 854 compared to untreated macrophages. In contrast, the csrS triple mutant strain 854csrSTM, which is hypo-responsive to LL-37, was controlled effectively under either condition (Fig. 7A). The survival phenotype was dependent on expression of SLO, as the 854Δslo mutant was actually killed more effectively by macrophages pretreated with 1,25D3 than by untreated macrophages (Fig. 7B). The enhanced killing of 854Δslo provides evidence of increased bactericidal effector function in 1,25D3-treated macrophages, consistent with previous reports, and further highlights the paradoxical impairment in killing of wild-type GAS after 1,25D3 treatment. In contrast to SLO, capsule upregulation played little role in increased survival of GAS in macrophages, as 854ΔhasB behaved nearly identically to wild-type 854 (Fig. 7B). Strain 854ΔcsrR/ΔhasB also survived somewhat better in 1,25D3-pretreated macrophages than in untreated cells, which suggests a role for another 1,25D3-responsive factor(s) in addition to LL-37 (Fig. 7C). Together, these experiments demonstrate that 1,25D3 treatment of human macrophages induces increased susceptibility to GAS infection, in striking contrast to the enhanced resistance to other pathogens reported previously (25, 26).
FIG 7.
Pretreatment of macrophages with 1,25D3 increases GAS survival through an SLO-dependent mechanism. THP-1 macrophages were pretreated with 100 nM 1,25D3 for 24 h prior to infection with GAS. GAS 854, 854csrSTM, 854ΔhasB, 854Δslo, 854ΔcsrR, and 854ΔcsrR/ΔhasB were used for infection; data are presented in separate panels for clarity. Viable GAS released into the cell culture medium plus GAS associated with the macrophages were quantified after 90 min of incubation (time zero) and 2 and 4 h later. For each strain, the number of GAS at each time point, reported as a percentage of the number at time zero, is shown. Data are means and standard errors from at least three separate experiments. *, P < 0.05; **, P < 0.01. NS, not significant.
DISCUSSION
To successfully colonize and infect its human host, GAS must overcome multiple host defenses. Bacteria encounter a variety of effector molecules of the innate immune system, including antimicrobial peptides such as LL-37, on the pharyngeal mucosa. GAS has evolved a mechanism to sense LL-37 through CsrRS, a signal that is transduced into upregulation of multiple virulence genes and increased resistance to killing by various human cell types.
We found that concentrations of LL-37 comparable to those detected in human saliva significantly alter the interaction of GAS with oropharyngeal keratinocytes in vitro, decreasing association and internalization of GAS by these host cells. Exposure to subinhibitory concentrations of LL-37 also makes GAS more resistant to killing by neutrophils and macrophages. By decreasing internalization and killing by both epithelial cells and phagocytes, LL-37 improves GAS survival, promoting ongoing pharyngeal colonization and the potential for invasive infection.
LL-37 not only is present in saliva but also is produced by various host cells that GAS encounters within the human host. Although oropharyngeal keratinocytes do not appear to express LL-37, cutaneous keratinocytes upregulate LL-37 expression in response to injury and upon exposure to microbial products (27-29). Neutrophil β-granules are a highly concentrated source of LL-37 that can be released during phagocytosis or during neutrophil degranulation (30). Neutrophils are estimated to contain 0.6 µg of hCAP18/LL-37 per 106 cells, in a cellular volume of approximately 300 µm3, yielding an approximate concentration of 100 µM (31, 32). Neutrophil extracellular traps (NETs) are lattices of extruded neutrophil chromatin studded with antimicrobial peptides, including LL-37, that represent another potential site for exposure of GAS to LL-37 in vivo. Concentrations of LL-37 within the neutrophil phagosome may be high enough to inhibit GAS growth and contribute to bacterial killing. However, the concentrations of LL-37 encountered by GAS in the extracellular environment or within cells that produce smaller amounts of the peptide are insufficient for effective antimicrobial activity against GAS. Rather, the subinhibitory amounts of LL-37 encountered by GAS in the latter environments are far more likely to increase virulence factor expression by signaling through CsrRS and to result in enhancement of GAS resistance to internalization and killing by host cells.
Although CsrRS regulates approximately 10 to 15% of the GAS transcriptome, we found particular importance of two virulence factors in the survival phenotype induced by LL-37: SLO and the hyaluronic acid capsule. The upregulation of slo reduced GAS association and internalization by oropharyngeal keratinocytes, enhanced GAS resistance to neutrophil phagocytic killing, was essential to the cytolysis of macrophages, and improved GAS survival of macrophage attack. Increased hyaluronic acid capsule production similarly decreased association and internalization by oropharyngeal keratinocytes and decreased phagocytic killing by neutrophils. LL-37 failed to increase the survival of a strain lacking both slo and hasB, so capsule and SLO appear to be essential to enhanced GAS resistance to killing by host cells. These results do not exclude potential contributions of other CsrRS-regulated virulence factors to improved GAS survival.
The transcriptional changes in GAS induced by LL-37 exposure likely have additional effects on pathogen-host interactions during infection beyond those specifically documented in this study. For example, two elements of the CsrRS regulon upregulated by LL-37 exposure, hyaluronic acid capsule and streptolysin S, have been implicated in the ability of GAS to transmigrate across epithelial layers (14, 33, 34). CsrRS signaling by LL-37 upregulates SpyCEP, which inhibits neutrophil recruitment through degradation of the chemotactic cytokine IL-8 (14, 35, 36). The sda1 gene of M1T1 strains, which encodes a DNase active against NETs, is upregulated by LL-37 exposure, thereby contributing to GAS escape from extracellular killing by neutrophil antimicrobial peptides (14, 37, 38). In addition to the effects on keratinocytes, neutrophils, and macrophages demonstrated here, SLO inhibits maturation of dendritic cells, thereby impairing development of an adaptive immune response (39). Capsule limits binding of specific antibodies to the GAS surface protein GRAB, so upregulation of capsule by LL-37 may reduce the effectiveness of antibody-mediated opsonization (40). Taken together, these observations suggest that GAS responses to LL-37 signaling modify host-pathogen interactions at multiple stages of colonization, infection, and immune response.
Studies in mice have shown a protective role for the murine cathelicidin CRAMP in experimental GAS soft tissue infection (41, 42). However, despite its similar alpha-helical structure and antimicrobial activity, murine cathelicidin does not signal through CsrRS (13). Therefore, the mouse model does not reflect the human situation, in which the predominant effect of LL-37 is likely to be enhanced resistance to phagocytosis and increased invasiveness from upregulation of CsrRS-regulated virulence factors, rather than inhibition of GAS growth by LL-37’s antimicrobial activity. Similar limitations apply to previous studies of the effect of vitamin D on resistance to GAS infection. Treatment with 1,25D3 has been shown to increase cathelicidin gene expression in human macrophages and to promote macrophage killing of Mycobacterium tuberculosis (25, 26). In mice, deficiency of 1,25D3 inhibited the induction of cathelicidin gene expression in skin keratinocytes and was associated with increased lesion size in experimental GAS skin infection (43). These studies support a causal relationship between vitamin D and cathelicidin expression, but the net effect on GAS virulence in human infection cannot be extrapolated from the murine model, since mouse cathelicidin lacks CsrRS signaling activity. The studies presented here demonstrate a novel instance in which effects of vitamin D on the innate immune system appear to be detrimental to the host by inducing pathogen resistance to macrophage killing.
MATERIALS AND METHODS
Ethics statement.
The aspects of this study relating to human subjects were approved by the institutional review board of Boston Children’s Hospital. Written informed consent was provided by study participants.
Bacterial strains and cell lines.
GAS strains used in this study are listed in Table 1. GAS 854 is an M-type 1 strain originally isolated from a patient with a retroperitoneal abscess (13). GAS strains were grown at 37°C in Todd-Hewitt broth (Difco) supplemented with 0.5% yeast extract (THY) or on THY agar or Trypticase-soy agar (BD Biosciences) supplemented with 5% defibrinated sheep blood. Escherichia coli strain DH5α (Zymogen) was used for cloning and was grown in Luria broth (LB). Antibiotics were used when necessary at the following concentrations: for GAS, erythromycin at 1 µg/ml; for E. coli, erythromycin at 200 µg/ml and carbenicillin at 100 µg/ml.
TABLE 1 .
Bacterial strains used in this study
| Strain | Reference | Genotype |
|---|---|---|
| 854 (M-type 1) | 13 | Wild type |
| 854csrSTM | 14 | csrS(D148N/E151Q/D152N) |
| 854ΔcsrR | 14 | ΔcsrR |
| 854ΔcsrS | This study | ΔcsrS |
| 854ΔhasB | This study | ΔhasB |
| 854Δslo | This study | Δslo |
| 854ΔhasB/Δslo | This study | ΔhasB Δslo |
| 854ΔcsrR/ΔhasB | This study | ΔcsrR ΔhasB |
| 854ΔcsrR/Δslo | This study | ΔcsrR Δslo |
| 854ΔcsrR/ΔhasB/Δslo | This study | ΔcsrR ΔhasB Δslo |
Human cell culture.
The human oropharyngeal keratinocyte cell line OKP7/bmi1/TERT (referred to here as OKP7) was described previously (44, 45). These cells were a gift of James Rheinwald and were provided through the Harvard Skin Disease Research Center. Keratinocytes were propagated in keratinocyte serum-free medium (KSFM; Invitrogen).
THP-1 cells were purchased from the American Type Culture Collection (ATCC) and maintained in RPMI medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen).
Chemicals and supplements.
Phorbol 12-myristate 13-acetate (PMA) was purchased from Sigma-Aldrich, dissolved in dimethyl sulfoxide (DMSO), and stored in aliquots at −20°C. 1α,25-Dihydroxyvitamin D3 (1,25D3) was purchased from Sigma-Aldrich, dissolved in ethanol, and stored as aliquots in light-impermeable tubes at −80°C. The human cathelicidin LL-37 was purchased from AnaSpec, dissolved in 0.01% acetic acid to a 1-mg/ml stock, and stored in aliquots at −20°C.
GAS mutagenesis.
Deletion constructs for hasB and csrS were generated by overlap PCR using Easy-A high-fidelity DNA polymerase (Stratagene), using sets of primers listed in Table 2, as described in previous work (14). The final PCR fragments were ligated into pGEM-T (Promega) and sequenced, before being excised by digestion with SalI/BamHI and cloned into the temperature-sensitive shuttle vector pJRS233 (46). The plasmid pJSLO (47) was used to delete slo.
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequence |
|---|---|
| delhasB1 | GGATCCGATGTGATACTGATGTAC |
| delhasB2 | GCATTTTCATAAAATTACTCCTTC |
| delhasB3 | GTAATTTATGAAAATAGCAAGAGACTAGCCATGTCCTTCATC |
| delhasB4 | GTCGACGCCCTCTTGGGTGGCTTTGAC |
| delcsrS1 | CGAGGATCCGTTTGATTTCCTCCTGCTTGAC |
| delcsrS2 | CTGATTTTCCATATGACTTATTTC |
| delcsrS3 | TAAGTCATATGGAAAATCAGCAGTCTAAAGAGAGTTAGAGTAGC |
| delcsrS4 | GAATTCGTCGACCCAATAGCAGTACTTGACACTTC |
Generation of deletion mutants via allelic exchange was achieved using the recombinant shuttle plasmid pJRS233, as previously described (48). Mutant genotypes were confirmed by PCR amplification of target loci from chromosomal DNA and subsequent DNA sequencing (Genewiz). The absence of SLO in culture supernatants from Δslo strains was confirmed in hemolysis assays and Western blots; NADase activity was preserved in these culture supernatants, a result that excludes a polar effect on expression of nga, which encodes NADase and is expressed from the same operon (see Fig. S3 in the supplemental material). The absence of hyaluronic acid capsule from ΔhasB strains was confirmed as described previously (18) (data not shown).
The CsrS triple mutant 854csrSTM and the csrR deletion mutant 854ΔcsrR were described previously (14).
Opsonophagocytic killing assays with human neutrophils.
GAS resistance to phagocytic killing was evaluated in vitro as previously described (13, 14). GAS strains were grown to early exponential phase in the absence or presence of 300 nM LL-37 in THY. At a multiplicity of infection (MOI) of 2 to 3, GAS were mixed with freshly isolated human peripheral blood leukocytes in the presence of 10% nonimmune human serum as a complement source. Aliquots were removed for quantitative culture immediately upon mixing and after 1 h of incubation at 37°C with end-over-end rotation. Results were expressed as the percentage of the inoculum surviving after 1 h. Donors of nonimmune serum were identified by the ability of their peripheral blood to support growth of GAS 854 in vitro (49).
Infections of oropharyngeal keratinocytes.
The human oropharyngeal keratinocyte cell line OKP7 was used for antibiotic protection assays as described previously (17, 45). Cells were seeded in 24-well plates at 105 cells/ml and grown for 3 to 4 days to confluence at 37°C in 5% CO2. GAS were grown to early exponential phase in THY in the absence of LL-37, pelleted, washed, resuspended in KSFM, and diluted. OKP7 cells were washed once with phosphate-buffered saline (PBS). GAS were added to cells at a MOI of 5, followed by addition of KSFM with or without 300 nM LL-37. Infections proceeded for 2 h and 15 min at 37°C in 5% CO2 before addition of penicillin (20 µg/ml) and gentamicin (200 µg/ml) to half of the wells to kill extracellular GAS. At 3 h postinfection, cells were washed with PBS, treated with trypsin (0.25%), and lysed in water for 15 min before being serially diluted for quantitative culture on sheep blood agar. After growth for 24 h at 37°C in 5% CO2, colonies were counted; numbers of total cell-associated GAS were determined from wells which had not received antibiotics, while numbers of internalized GAS were determined from wells treated with antibiotics.
Infection of THP-1 macrophages.
THP-1 cells were seeded in 24-well plates at 5 × 105 cells/ml in RPMI with 10% FBS in the presence of 25 nM PMA. After incubation for 24 h at 37°C in 5% CO2, cells were washed three times with PBS before the medium was replaced with RPMI with 10% FBS with or without 20 nM 1,25D3, after which the cells were incubated for an additional 24 h under identical conditions. GAS were grown in THY to early exponential phase in the absence of LL-37, pelleted, washed, resuspended in RPMI with 10% FBS, and diluted. THP-1 macrophages were washed once with PBS before GAS were added at MOI of 1, followed by addition of RPMI with 10% FBS, with or without 300 nM LL-37. Infections proceeded for 90 min, before penicillin (20 µg/ml) and gentamicin (200 µg/ml) were added to all wells. Thirty minutes later, supernatants were removed for measurement of LDH using a cytotoxicity measurement kit (Roche). Uninfected THP-1 cells were lysed with Triton per the manufacturer’s instructions, as calibration for complete cell lysis. To measure intracellular GAS, cells were washed with PBS, treated with 0.25% trypsin, and lysed in water for 15 min before being serially diluted for quantitative culture on sheep blood agar. When needed, cytochalasin D was added 30 min prior to infection at 5 µg/ml, and the medium, during infection, was supplemented to the same concentration.
For bacterial survival assays, THP-1 cells were treated and infected as described above, through antibiotic treatments. After 30 min of incubation with penicillin and gentamicin, wells were washed three times with PBS. For time zero measurements, cells were immediately treated with 0.25% trypsin and lysed in water for 15 min before being serially diluted for quantitative culture on sheep blood agar. Replicate wells had 0.5 ml of RPMI with 10% FBS (without antibiotics and with or without LL-37) added and were further incubated at 37°C for 2 or 4 h. At these time points, the culture medium was removed and set aside, while adherent well contents were trypsinized and lysed in water, as described above; the culture medium was returned to the cell lysate prior to diluting for quantitative culture.
Statistical analysis.
All graphs were generated in Prism version 5.0d (GraphPad Software, Inc.), showing the mean ± standard error of the mean (SEM). The significance of the differences between groups was calculated using paired t tests for all experiments except LDH assays, for which nonpaired t tests were used.
SUPPLEMENTAL MATERIAL
Internalization of GAS is required for SLO-mediated cytotoxicity. THP-1 macrophages were pretreated with the actin polymerization inhibitor cytochalasin D before and during infection with wild-type GAS 854. Infections were performed in the absence (white) or presence (gray) of exogenous LL-37; where indicated, cells were pretreated with 1,25D3 (black). (A) Supernatants were assayed for LDH release as described above; (B) numbers of intracellular GAS were determined. Data represent a single experiment, with five (A) or three (B) independent replicates, representative of three separate experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Download Figure S1, TIF file, 0.8 MB.
Pretreatment of THP-1 macrophages with 1,25D3 increases expression of hCAP18/LL-37. After differentiation, THP-1 macrophages were treated with 20 nM 1,25D3 for 24 h and compared to untreated cells. (A) qRT-PCR demonstrates upregulation of hCAP18/LL-37 mRNA (mean from three independent experiments). (B) Western blot using antiserum for LL-37 is shown; lanes are (1) synthesized LL-37, (2) extract of human peripheral blood granulocytes, (3) extract of untreated THP-1 macrophages, and (4) extract of THP-1 macrophages after 24-h treatment with 20 nM 1,25D3. Note that the Western blot demonstrates both the unprocessed hCAP18 (upper band) and the processed LL-37 (lower band). **, P < 0.01. Download Figure S2, TIF file, 0.7 MB.
Deletion of slo eliminates hemolytic activity while leaving NADase activity unaffected. Supernatants of cultures of GAS 854, 854Δslo, and 854ΔhasB were assayed for hemolytic activity as a measure of SLO activity (A) and subjected to Western blot using anti-SLO antibody (B). NADase activity was also measured (C). Quantitative data are presented as percentages of wild-type GAS 854, representing the mean of three independent experiments. NS, nonsignificant; ****, P < 0.0001. Download Figure S3, TIF file, 0.8 MB.
ACKNOWLEDGMENTS
We thank Maghnus O’Seaghdha and Ioannis Gryllos for valuable discussions.
This work was supported in part by grants AI029952 and AI070926 from the National Institutes of Health. J.F.L. was supported in part by training grant T32 AI07061 and individual NRSA F32 AI085965, both from the National Institutes of Health.
Footnotes
Citation Love JF, Tran-Winkler HJ, and Wessels MR. 2012. Vitamin D and the human antimicrobial peptide LL-37 enhance group A Streptococcus resistance to killing by human cells. mBio 3(5):e00394-12. doi:10.1128/mBio.00394-12.
REFERENCES
- 1. Cunningham MW. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stevens DL. 2000. Group A beta-hemolytic streptococci: virulence factors, pathogenesis, and spectrum of clinical infections, p 19–36 In Stevens DL, Kaplan EL, Streptococcal infections. Clinical aspects, microbiology, and molecular pathogenesis. Oxford University Press, New York, NY. [Google Scholar]
- 3. Cockerill FR, III, et al. 1997. An outbreak of invasive group A streptococcal disease associated with high carriage rates of the invasive clone among school-aged children. JAMA 277:38–43 [PubMed] [Google Scholar]
- 4. Weiss K, et al. 1999. Group A streptococcus carriage among close contacts of patients with invasive infections. Am. J. Epidemiol. 149:863–868 [DOI] [PubMed] [Google Scholar]
- 5. Dalton TL, Collins JT, Barnett TC, Scott JR. 2006. RscA, a member of the MDR1 family of transporters, is repressed by CovR and required for growth of Streptococcus pyogenes under heat stress. J. Bacteriol. 188:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Graham MR, et al. 2002. Virulence control in group A streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc. Natl. Acad. Sci. U. S. A. 99:13855–13860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gryllos I, et al. 2007. Mg(2+) signalling defines the group A streptococcal CsrRS (CovRS) regulon. Mol. Microbiol. 65:671–683 [DOI] [PubMed] [Google Scholar]
- 8. Levin JC, Wessels MR. 1998. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol. Microbiol. 30:209–219 [DOI] [PubMed] [Google Scholar]
- 9. Engleberg NC, Heath A, Miller A, Rivera C, DiRita VJ. 2001. Spontaneous mutations in the CsrRS two-component regulatory system of Streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. J. Infect. Dis. 183:1043–1054 [DOI] [PubMed] [Google Scholar]
- 10. Ikebe T, et al. 2010. Highly frequent mutations in negative regulators of multiple virulence genes in group A streptococcal toxic shock syndrome isolates. PLoS Pathog. 6:e1000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. 2006. Genome-wide analysis of group a streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2:e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gryllos I, Levin JC, Wessels MR. 2003. The CsrR/CsrS two-component system of group A streptococcus responds to environmental Mg2+. Proc. Natl. Acad. Sci. U. S. A. 100:4227–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gryllos I, et al. 2008. Induction of group A streptococcus virulence by a human antimicrobial peptide. Proc. Natl. Acad. Sci. U. S. A. 105:16755–16760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tran-Winkler HJ, Love JF, Gryllos I, Wessels MR. 2011. Signal transduction through CsrRS confers an invasive phenotype in group A streptococcus. PLoS Pathog. 7:e1002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dale BA, Fredericks LP. 2005. Antimicrobial peptides in the oral environment: expression and function in health and disease. Curr. Issues Mol. Biol. 7:119–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davidopoulou S, Diza E, Menexes G, Kalfas S. 2012. Salivary concentration of the antimicrobial peptide LL-37 in children. Arch. Oral Biol. 57:865–869 [DOI] [PubMed] [Google Scholar]
- 17. Håkansson A, Bentley CC, Shakhnovic EA, Wessels MR. 2005. Cytolysin-dependent evasion of lysosomal killing. Proc. Natl. Acad. Sci. U. S. A. 102:5192–5197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schrager HM, Rheinwald JG, Wessels MR. 1996. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J. Clin. Invest. 98:1954–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldmann O, Sastalla I, Wos-Oxley M, Rohde M, Medina E. 2009. Streptococcus pyogenes induces oncosis in macrophages through the activation of an inflammatory programmed cell death pathway. Cell. Microbiol. 11:138–155 [DOI] [PubMed] [Google Scholar]
- 20. Harder J, et al. 2009. Activation of the Nlrp3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-kappa B activation but proceeds independently of TLR signaling and P2X7 receptor. J. Immunol. 183:5823–5829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Timmer AM, et al. 2009. Streptolysin O promotes group A streptococcus immune evasion by accelerated macrophage apoptosis. J. Biol. Chem. 284:862–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thulin P, et al. 2006. Viable group A streptococci in macrophages during acute soft tissue infection. PLoS Med. 3:e53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johansson L, et al. 2008. LL-37 in severe Streptococcus pyogenes soft tissue infections. Infect. Immun. 183:3399–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sigurdardottir SL, et al. 2012. The anti-microbial peptide LL-37 modulates immune responses in the palatine tonsils where it is exclusively expressed by neutrophils and a subset of dendritic cells. Clin. Immunol. 142:139–149 [DOI] [PubMed] [Google Scholar]
- 25. Liu PT, et al. 2006. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773 [DOI] [PubMed] [Google Scholar]
- 26. Yuk JM, et al. 2009. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 6:231–243 [DOI] [PubMed] [Google Scholar]
- 27. Dorschner RA, et al. 2001. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A streptococcus. J. Invest. Dermatol. 117:91–97 [DOI] [PubMed] [Google Scholar]
- 28. Frohm M, et al. 1997. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272:15258–15263 [DOI] [PubMed] [Google Scholar]
- 29. Kim JE, et al. 2005. Expression and modulation of LL-37 in normal human keratinocytes, HaCaT cells, and inflammatory skin diseases. J. Korean Med. Sci. 20:649–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cowland JB, Johnsen AH, Borregaard N. 1995. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 368:173–176 [DOI] [PubMed] [Google Scholar]
- 31. Sørensen O, Cowland JB, Askaa J, Borregaard N. 1997. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J. Immunol. Methods 206:53–59 [DOI] [PubMed] [Google Scholar]
- 32. Ting-Beall HP, Needham D, Hochmuth RM. 1993. Volume and osmotic properties of human neutrophils. Blood 81:2774–2780 [PubMed] [Google Scholar]
- 33. Cywes C, Wessels MR. 2001. Group A streptococcus tissue invasion by CD44-mediated cell signalling. Nature 414:648–652 [DOI] [PubMed] [Google Scholar]
- 34. Sumitomo T, et al. 2011. Streptolysin S contributes to group A streptococcal translocation across an epithelial barrier. J. Biol. Chem. 286:2750–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Edwards RJ, et al. 2005. Specific C-terminal cleavage and inactivation of interleukin-8 by invasive disease isolates of Streptococcus pyogenes. J. Infect. Dis. 192:783–790 [DOI] [PubMed] [Google Scholar]
- 36. Sumby P, et al. 2008. A chemokine-degrading extracellular protease made by group A streptococcus alters pathogenesis by enhancing evasion of the innate immune response. Infect. Immun. 76:978–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buchanan JT, et al. 2006. DNase expression allows the pathogen group A streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 16:396–400 [DOI] [PubMed] [Google Scholar]
- 38. Sumby P, et al. 2005. Extracellular deoxyribonuclease made by group A streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc. Natl. Acad. Sci. U. S. A. 102:1679–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cortés G, Wessels MR. 2009. Inhibition of dendritic cell maturation by group A streptococcus. J. Infect. Dis. 200:1152–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dinkla K, et al. 2007. Upregulation of capsule enables Streptococcus pyogenes to evade immune recognition by antigen-specific antibodies directed to the G-related alpha2-macroglobulin-binding protein GRAB located on the bacterial surface. Microbes Infect. 9:922–931 [DOI] [PubMed] [Google Scholar]
- 41. Lee PH, et al. 2005. Expression of an additional cathelicidin antimicrobial peptide protects against bacterial skin infection. Proc. Natl. Acad. Sci. U. S. A. 102:3750–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nizet V, et al. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454–457 [DOI] [PubMed] [Google Scholar]
- 43. Muehleisen B, et al. 2012. PTH/PTHrP and vitamin D control antimicrobial peptide expression and susceptibility to bacterial skin infection. Sci. Transl. Med. 4:135ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dickson MA, et al. 2000. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 20:1436–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Logsdon LK, Håkansson AP, Cortés G, Wessels MR. 2011. Streptolysin O inhibits clathrin-dependent internalization of group A streptococcus. mBio 2:e00332-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Perez-Casal J, Price JA, Maguin E, Scott JR. 1993. An M protein with a single C repeat prevents phagocytosis of Streptococcus pyogenes: use of a temperature-sensitive shuttle vector to deliver homologous sequences to the chromosome of S. pyogenes. Mol. Microbiol. 8:809–819 [DOI] [PubMed] [Google Scholar]
- 47. Sierig G, Cywes C, Wessels MR, Ashbaugh CD. 2003. Cytotoxic effects of streptolysin O and streptolysin S enhance the virulence of poorly encapsulated group A streptococci. Infect. Immun. 71:446–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ashbaugh CD, Albertí S, Wessels MR. 1998. Molecular analysis of the capsule gene region of group A Streptococcus: the hasAB genes are sufficient for capsule expression. J. Bacteriol. 180:4955–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lancefield RC. 1962. Current knowledge of the type-specific M antigens of group A streptococci. J. Immunol. 89:307–313 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Internalization of GAS is required for SLO-mediated cytotoxicity. THP-1 macrophages were pretreated with the actin polymerization inhibitor cytochalasin D before and during infection with wild-type GAS 854. Infections were performed in the absence (white) or presence (gray) of exogenous LL-37; where indicated, cells were pretreated with 1,25D3 (black). (A) Supernatants were assayed for LDH release as described above; (B) numbers of intracellular GAS were determined. Data represent a single experiment, with five (A) or three (B) independent replicates, representative of three separate experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Download Figure S1, TIF file, 0.8 MB.
Pretreatment of THP-1 macrophages with 1,25D3 increases expression of hCAP18/LL-37. After differentiation, THP-1 macrophages were treated with 20 nM 1,25D3 for 24 h and compared to untreated cells. (A) qRT-PCR demonstrates upregulation of hCAP18/LL-37 mRNA (mean from three independent experiments). (B) Western blot using antiserum for LL-37 is shown; lanes are (1) synthesized LL-37, (2) extract of human peripheral blood granulocytes, (3) extract of untreated THP-1 macrophages, and (4) extract of THP-1 macrophages after 24-h treatment with 20 nM 1,25D3. Note that the Western blot demonstrates both the unprocessed hCAP18 (upper band) and the processed LL-37 (lower band). **, P < 0.01. Download Figure S2, TIF file, 0.7 MB.
Deletion of slo eliminates hemolytic activity while leaving NADase activity unaffected. Supernatants of cultures of GAS 854, 854Δslo, and 854ΔhasB were assayed for hemolytic activity as a measure of SLO activity (A) and subjected to Western blot using anti-SLO antibody (B). NADase activity was also measured (C). Quantitative data are presented as percentages of wild-type GAS 854, representing the mean of three independent experiments. NS, nonsignificant; ****, P < 0.0001. Download Figure S3, TIF file, 0.8 MB.