Abstract
Objectives
Women with a sonographic short cervix in the mid-trimester are at increased risk for preterm delivery. This study was undertaken to determine the ef cacy and safety of using micronized vaginal progesterone gel to reduce the risk of preterm birth and associated neonatal complications in women with a sonographic short cervix.
Methods
This was a multicenter, randomized, double-blind, placebo-controlled trial that enrolled asymptomatic women with a singleton pregnancy and a sonographic short cervix (10–20 mm) at 19 + 0to23 + 6 weeks of gestation. Women were allocated randomly to receive vaginal progesterone gel or placebo daily starting from 20 to 23 + 6 weeks until 36 + 6 weeks, rupture of membranes or delivery, whichever occurred rst. Randomization sequence was strati ed by center and history of a previous preterm birth. The primary endpoint was preterm birth before 33 weeks of gestation. Analysis was by intention to treat.
Results
Of 465 women randomized, seven were lost to follow-up and 458 (vaginal progesterone gel, n = 235; placebo, n = 223) were included in the analysis. Women allocated to receive vaginal progesterone had a lower rate of preterm birth before 33 weeks than did those allocated to placebo (8.9% (n = 21) vs 16.1% (n = 36); relative risk (RR), 0.55; 95% CI, 0.33–0.92; P = 0.02). The effect remained signi cant after adjustment for covariables (adjusted RR, 0.52; 95% CI, 0.31–0.91; P = 0.02). Vaginal progesterone was also associated with a signi cant reduction in the rate of preterm birth before 28 weeks(5.1%vs10.3%; RR, 0.50;95%CI, 0.25–0.97; P = 0.04) and 35 weeks (14.5% vs 23.3%; RR, 0.62; 95% CI, 0.42–0.92; P = 0.02), respiratory distress syndrome (3.0% vs 7.6%; RR, 0.39; 95% CI, 0.17–0.92; P = 0.03), any neonatal morbidity or mortality event (7.7% vs 13.5%; RR, 0.57; 95% CI, 0.33–0.99; P = 0.04) and birth weight < 1500 g (6.4% (15/234) vs 13.6% (30/220); RR, 0.47; 95% CI, 0.26–0.85; P = 0.01). There were no differences in the incidence of treatment-related adverse events between the groups.
Conclusions
The administration of vaginal progesterone gel to women with a sonographic short cervix in the mid-trimester is associated with a 45% reduction in the rate of preterm birth before 33 weeks of gestation and with improved neonatal outcome.
Keywords: pregnancy, preterm delivery, preterm labor, progestins, progestogens, respiratory distress syndrome, transvaginal ultrasound, uterine cervix, vaginal administration
INTRODUCTION
Preterm birth is the leading cause of perinatal morbidity and mortality, and its prevention is an important healthcare priority1. In 2005, 12.9 million births worldwide were preterm2. A sonographic short cervix is a powerful predictor of preterm delivery3–25, yet implementation of a screening program of all pregnant women requires the availability of a clinical intervention able to prevent preterm delivery and improve neonatal outcome26. Strategies that have been considered include progesterone administration27, cervical cerclage28–34 and insertion of a pessary35.
A randomized clinical trial of vaginal progesterone capsules to prevent preterm delivery (<34 weeks of gestation) in women with a short cervix (defined as 15 mm or less) reported a 44% reduction in the rate of preterm delivery (19.2% vs 34.4%; relative risk (RR), 0.56; 95% CI, 0.36–0.86), although this was not associated with a significant improvement in neonatal outcome27. In addition, secondary analyses of a randomized clinical trial36 of vaginal progesterone in patients with a history of preterm birth showed that progesterone administration was associated with delayed cervical shortening37 as pregnancy progressed, a lower rate of preterm birth, a lower frequency of admission to the neonatal intensive care unit (NICU) and a shorter length of NICU stay38. This study was undertaken to determine the efficacy and safety of vaginal progesterone gel in reducing the rate of preterm birth before 33 weeks in asymptomatic women with a mid-trimester sonographic short cervix.
METHODS
Study design and participants
This was a Phase-III, prospective, randomized, placebo-controlled, double-masked, parallel-group, multicenter, international trial. The study was conducted from March 2008 to November 2010 and was approved by the institutional review board of each participating center. Participants provided written informed consent to study coordinators or investigators prior to participation in the trial. Women between 19 + 0 and 23 + 6 weeks of gestation were eligible for screening. During the screening visit, cervical length and gestational age were determined. Women were eligible for the study if they met the following criteria: 1) singleton gestation; 2) gestational age between 19 + 0 and 23 + 6 weeks; 3) transvaginal sonographic cervical length between 10 and 20 mm; and 4) asymptomatic, i.e. without signs or symptoms of preterm labor. Subjects were allocated randomly to receive vaginal progesterone gel or placebo beginning at 20 to 23 + 6 weeks. Gestational age calculation was based on the participant’s reported last menstrual period and fetal biometry39.
Exclusion criteria included: 1) planned cerclage; 2) acute cervical dilation; 3) allergic reaction to progesterone; 4) current or recent progestogen treatment within the previous 4 weeks; 5) chronic medical conditions that would interfere with study participation or evaluation of the treatment (e.g. seizures, psychiatric disorders, uncontrolled chronic hypertension, congestive heart failure, chronic renal failure, uncontrolled diabetes mellitus with end-organ dysfunction, active thrombophlebitis or a thromboembolic disorder, history of hormone-associated thrombophlebitis or thromboembolic disorders, active liver dysfunction or disease, known or suspected malignancy of the breast or genital organs); 6) major fetal anomaly or known chromosomal abnormality; 7) uterine anatomic malformation (e.g. bicornuate uterus, septate uterus); 8) vaginal bleeding; or 9) known or suspected clinical chorioamnionitis.
All sonographers involved in sonographic cervical length measurements were required to participate in a training program and to obtain certification before screening patients for the trial. Moreover, the sonographic images of patients enrolled into the trial were reviewed by a central sonologist for quality assurance. An independent data coordinating center was responsible for randomization and data management. Clinical research monitors (Venn Life Sciences (St. Laurent, Quebec, Canada) and PharmOlam International (Houston, TX, USA)) conducted planned, regular site visits at each center, beginning with a site initiation visit and continuing until study completion, to independently assess compliance with the study protocol, timely collection of data, quality control, data completeness and data accuracy, according to International Conference on Harmonization (ICH) and Food and Drug Administration (FDA) Guidelines for Good Clinical Practice40, 41. The study included 44 centers in 10 countries.
Randomization and masking
The randomization allocation was 1:1 (vaginal progesterone gel: placebo) and was accomplished using a centralized interactive voice response (IVR) system. Randomization was stratified according to: a) center and b) risk strata (previous preterm birth between 20 and 35 weeks or no previous preterm birth) using a permuted blocks strategy with a block size of four (i.e. two placebo and two vaginal progesterone gel). Contact with the IVR system required the input of subject characteristics and center number, after which the IVR system assigned a treatment for the specific subject based on the strata to which the subject belonged and the next assignment within the randomization block.
Allocation concealment was accomplished in three ways. First, subject drug kits at each study site were numbered independently from the treatment assignments in the randomization blocks to avoid identification of dispensing patterns. Second, the IVR system (upon generating a treatment assignment for a new subject) specified which kit number was to be dispensed to the subject. Third, the study drug packaging, applicators and their contents (vaginal progesterone and placebo) were identical in appearance.
Procedures
All of the drug required throughout the treatment interval for a randomized woman was included in drug kits to be assigned to each patient at each study visit in order to prevent dispensing errors. Prior to dispensing the assigned treatment, demographic, medical and obstetric history and physical examination data were collected from each participant. Treatment was to be initiated between 20 + 0 and 23 + 6 weeks’ gestational age. Women selfadministered the study drug once daily in the morning. Study participants were instructed to return to the study center every 2 weeks. During each visit, subjects were interviewed to determine the occurrence of adverse events, use of concomitant medications and compliance with study drug. Women were asked to return unused study drug from the previous 2 weeks, and determination of compliance was based on the amount of study drug not used.
Study drug was continued until 36 + 6 weeks’ gestational age, rupture of membranes or delivery, whichever occurred first. Both the vaginal progesterone gel (Prochieve® 8%, also known as Crinone® 8%) and placebo were supplied by Columbia Laboratories, Inc. (Livingston, NJ, USA) as a soft, white to off-white gel, in a single-use, one-piece, white disposable polyethylene vaginal applicator with a twist-off top. The progesterone and placebo gels were identical in appearance. Each applicator delivered 1.125 g gel containing 90 mg progesterone or placebo, and was wrapped and sealed in unmarked foil over-wrap. Both the active drug and the placebo were supplied in boxes of 14 applicators and were labeled with a unique kit number. Subjects received a 2-week supply at randomization and at each subsequent visit. They also received a 1-week emergency supply kit at the time of randomization and were resupplied during the treatment period if additional applicators were required before attending the next visit.
Patients who developed preterm labor during the study were treated according to the standard practice of the participating institutions, e.g. admission to the hospital, bed rest, intravenous fluids, tocolytic therapy, steroid administration, if clinically indicated. Administration of the study drug was to be continued during treatment for preterm labor, until delivery (in the absence of preterm rupture of membranes). Maternal and neonatal outcome were recorded throughout study participation and after delivery and discharge using a standardized electronic reporting template.
An emergency cerclage was allowed after randomization if the following criteria were met: 1) 21–26 weeks’ gestational age; 2) cervical dilation >2 cm; 3) membranes visible; 4) intact membranes; and 5) absence of uterine contractions, clinical chorioamnionitis and significant vaginal bleeding.
The primary outcome of this study was preterm birth before 33 weeks of gestation. The key secondary outcomes were neonatal morbidity, including respiratory distress syndrome (RDS), bronchopulmonary dysplasia, Grade III or IV intraventricular hemorrhage, periventricular leukomalacia, proven sepsis, necrotizing enterocolitis and perinatal mortality (fetal death or neonatal death). Four composite outcome scores were also used to assess perinatal mortality and neonatal morbidity (any event, two 0–4 scales and a 0–6 scale). The definitions for individual outcomes and composite scores are provided in the supplementary material online (Appendix S1). The outcome scores (0–4, 0–6) assigned ordinal values based upon the number of morbid events from 0 to 3 or 0 to 5; the highest number, 4 or 6, was assigned to a mortality event. For one of the 0–4 scores, number of NICU days was also used for assignment of the ordinal value. Other pre-specified secondary outcomes included preterm birth before 28, 35 and 37 weeks of gestation, neonatal length, weight and head circumference at birth and incidence of congenital abnormalities. The frequency of adverse events related to treatment was also assessed (see Appendix S2 online for definition of adverse events). All outcomes were determined and the database was locked prior to the unsealing of the randomization code.
Statistical analysis
We estimated that a sample size of 450 women (225 per treatment group) would have >90% power (two-tailed alpha level of 0.05) to detect a 55% reduction in the rate of preterm birth before 33 weeks of gestation, from 22% in the placebo group to 9.9% in the vaginal progesterone group. Analysis of the trial was conducted in three different analysis sets:
Intent-to-treat (ITT) analysis set: all patients randomized to either vaginal progesterone gel or placebo; subjects without a documented delivery date were excluded;
Treated patient analysis set: patients who took at least one dose of either placebo or progesterone gel; women who received placebo and had no documented delivery date were considered as if they had delivered at term (37 weeks of gestation); for women who received vaginal progesterone gel and had no documented delivery date, the date of last contact was used as the delivery date;
Compliant analysis set: patients who used at least 80% of study medication, did not have a cerclage and were not lost to follow-up.
The primary endpoint of the study, preterm birth before 33 weeks, was analyzed using the Cochran–Mantel–Haenszel (CMH) test. The P-value was assessed at the two-sided significance level of 5%. Analysis of the primary efficacy endpoint was also performed using multivariable logistic regression, in which the following variables were included: treatment group, pooled study site, risk strata, gestational age at first dose, maternal age, cervical length, body mass index (BMI) and race. RR with 95% CI was used as the measure of effect. The CMH test was also used for the analysis of the ordinal composite scores described in Appendix S1 online. For this analysis, a modified ranking procedure (modified ridits) was used to calculate the sum of the expected values for each of the ordinal categories for each of the treatment groups. This ranking procedure is equivalent to non-parametric van Elteren scores. The RR for the primary endpoint was calculated unadjusted, partially adjusted (for pooled study site and risk strata) as well as fully adjusted using multivariable logistic regression. We also calculated the number needed to treat42, with 95% CIs for the primary outcome and the most common complication of preterm birth, RDS. All analyses were performed with SAS® 9.2 (SAS Institute Inc., Cary, NC, USA) on a Windows 2003 operating system.
An independent Data and Safety Monitoring Board (DSMB) reviewed unblinded data relevant to safety (not efficacy) after approximately 50% of the subjects had delivered. The observed frequency of adverse events did not exceed that expected or that stated in the informed consent. The DSMB recommended the study continue without modification of the protocol or informed consent. This trial is registered with ClinicalTrials.gov, number NCT00615550.
RESULTS
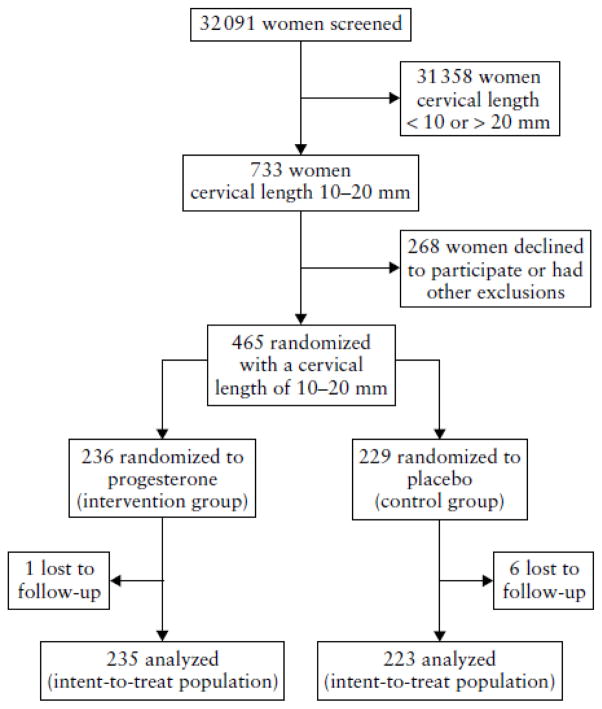
Of the 32 091 women who underwent sonographic measurement of cervical length between 19 +0 and 23 + 6 weeks of gestation, 2.3% (733/32 091) were reported to have a cervical length of 10–20 mm. Four hundred and sixty-five women agreed to participate and were randomized, of whom seven were lost to follow-up (vaginal progesterone gel, n = 1; placebo n = 6). Thus, 458 women were included in the ITT analysis set (vaginal progesterone gel, n = 235; placebo, n = 223). Figure 1 shows the participant flow diagram (see Appendix S3 online for further details regarding patient disposition). The trial ended on the delivery date of the last delivered participant. Of the 458 women, 16% (n = 72) had a history of a previous preterm birth between 20 and 35 weeks of gestation.
Figure 1.
Participant flow diagram
Baseline maternal characteristics were similar between the placebo and the vaginal progesterone groups (Table 1). There were no differences between the two groups in median duration of treatment (14.3 weeks for vaginal progesterone gel and 13.9 weeks for placebo) or mean study drug administration compliance reported by the investigator (93.3% (SD, ±13.1%) for vaginal progesterone gel and 94.0% (SD, ±12.7%) for placebo). A history of cervical surgery was present in 9.4% (22/235) of patients allocated to receive vaginal progesterone gel and in 12.6% (28/223) of those allocated to the placebo group (P = 0.20). Sixteen women (10 in the vaginal progesterone group and six in the placebo group; P = 0.46) underwent an emergency cervical cerclage after randomization.
Table 1.
Baseline and treatment characteristics of 458 asymptomatic women with a singleton pregnancy and sonographic short cervix randomized to receive vaginal progesterone gel or placebo.
| Characteristic | Vaginal progesterone (n = 235) | Placebo (n = 223) |
|---|---|---|
| Age (years) | ||
| Median (range) | 25.3 (18–44) | 25.6 (18–41) |
| Interquartile range | (21.8–30.3) | (21.9–29.4) |
| Mean (SD) | 26.5 (5.8) | 26.2 (5.1) |
| Race (n (%)) | ||
| African-American | 76 (32) | 67 (30) |
| Asian | 76 (32) | 74 (33) |
| Caucasian | 73 (31) | 70 (31) |
| Other | 10 (4) | 12 (5) |
| Body mass index (kg/m2) | ||
| Median (range) | 24.5 (14–47) | 23.6 (14–50) |
| Interquartile range | (20.4–30.0) | (20.5–29.2) |
| Mean (SD) | 25.6 (6.3) | 25.3 (6.8) |
| Obstetric history (n (%)) | ||
| Nulliparous | 125 (53) | 126 (57) |
| No previous PTD* | 204 (87) | 195 (87) |
| ≥ 1 previous PTD* | 31 (13) | 28 (13) |
| Cervical length (mm) | ||
| Median (range) | 18 (10–21) | 18 (10–20) |
| Interquartile range | (16–19) | (15–19) |
| Mean (SD) | 17 (2.5) | 17 (2.8) |
| GA at first dose of progesterone (weeks) | ||
| Median (range) | 21.7 (19–25) | 21.7 (17–25) |
| Interquartile range | (20.7–23.0) | (20.4–22.9) |
| Mean (SD) | 21.9 (1.4) | 21.7 (1.4) |
| Duration of treatment (weeks) | ||
| Median (range) | 14.3 (0–18) | 13.9 (0–18) |
| Interquartile range | (12.6–15.7) | (10.9–15.7) |
| Mean (SD) | 13.0 (4.2) | 12.5 (4.7) |
| † Compliance (%) | ||
| Median (range) | 99.2 (6–100) | 100 (0–100) |
| Interquartile range | (92.7–100) | (93.0–100) |
| Mean (SD) | 93.3 (13.1) | 94.0 (12.7) |
Preterm delivery (PTD) >20 weeks and <32 weeks.
Reported compliance was calculated using the following formula: (Number of vaginal applicators used since last visit/Number of vaginal applicators that should have been used since last visit) × 100. Every 2 weeks, a percentage of compliance was calculated and the compliance for a specific patient was based on the average of all visits. The definition of compliance was based on the formula and percentage indicated above, and a compliant patient was defined as one with an average of >80% compliance. GA, gestational age.
Patients allocated to receive vaginal progesterone gel had a significantly lower rate of preterm birth before 33 weeks of gestation compared with those allocated to placebo (8.9% (n = 21) vs 16.1% (n = 36); RR, 0.55; 95% CI, 0.33–0.92; P = 0.02; adjusted (pooled study site and risk strata) RR, 0.54; 95% CI, 0.33–0.89; P = 0.01). Fourteen women with cervical length between 10 and 20 mm would need to be treated with vaginal progesterone gel to prevent one case of preterm birth before 33 weeks of gestation (95% CI, 8–87). Even after adjustment for pooled study site, risk strata, treatment group, gestational age at first dose, maternal age, cervical length, BMI and race using multivariable logistic regression analysis, the effect of vaginal progesterone gel remained significant for the primary endpoint (adjusted RR, 0.52; 95% CI, 0.31–0.91; P = 0.02). No interaction between treatment and pooled study site was detected (P = 0.2). In women without a history of preterm birth (84% of the population), vaginal progesterone gel administration was associated with a significant reduction in the rate of preterm birth before 33 weeks (7.6% (15/197) vs 15.3% (29/189); RR, 0.50; 95% CI, 0.27–0.90; P = 0.02). However, the reduction in the rate of preterm birth in women with a prior history of preterm birth between 20 and 35 weeks of gestation did not reach statistical significance (15.8% (6/38) vs 20.6% (7/34); RR, 0.77; 95% CI, 0.29–2.06; P = 0.60).
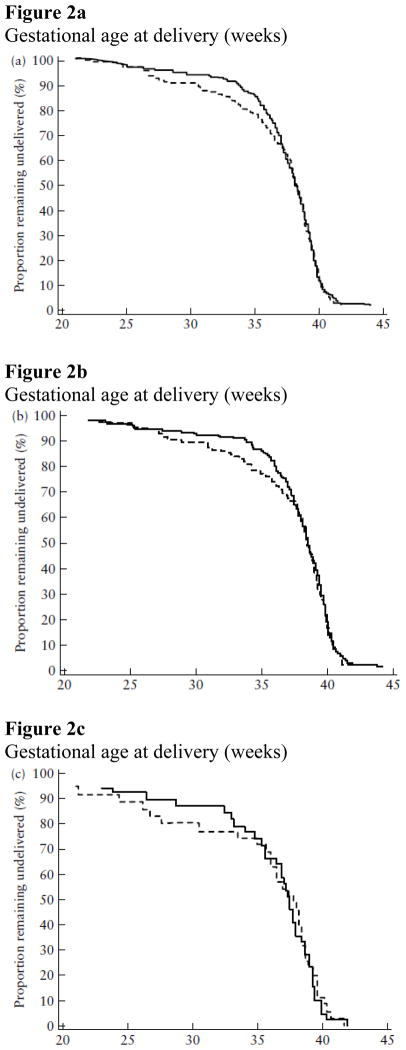
Vaginal progesterone gel was also associated with a significant reduction in the rate of preterm birth before 35 weeks (14.5% (n = 34) vs 23.3% (n = 52); RR, 0.62; 95% CI, 0.42–0.92; P = 0.02) and before 28 weeks of gestation (5.1% (n = 12) vs 10.3% (n = 23); RR, 0.50; 95% CI, 0.25–0.97; P = 0.04). Figure 2 displays the survival analysis for patients in the entire ITT analysis set (Figure 2a), patients with no prior preterm delivery (Figure 2b) and patients with a prior preterm delivery (Figure 2c). The curves demonstrate a separation between patients allocated to receive vaginal progesterone gel and those in the placebo group. However, there was no difference in the proportion of patients who delivered at <37 weeks, because the curves converge and overlap at this point. One interpretation of this is that the administration of vaginal progesterone shifted the proportion of patients who would have delivered very preterm to a later gestational age. In addition, vaginal progesterone was associated with a significant reduction in the rate of neonatal birth weight <1500 g (6.4% (15/234) vs 13.6% (30/220); RR, 0.47; 95% CI, 0.26–0.85; P = 0.01) (Table 2).
Figure 2.
Figure 2a–c Survival analysis of intent-to-treat analysis set showing proportion of patients remaining undelivered according to treatment allocation: vaginal progesterone ( ) vs placebo (- - - -). (a) Entire population (patients with and without a prior history of preterm delivery) (vaginal progesterone n = 235, placebo n = 223); (b) patients without a prior history of preterm delivery (vaginal progesterone n = 197, placebo n = 189); (c) patients with a prior history of preterm delivery (vaginal progesterone n = 38, placebo n = 34). P > 0.05 for all comparisons.
Table 2.
Gestational age at delivery and neonatal outcome in asymptomatic women with a singleton pregnancy and sonographic short cervix allocated to receive vaginal progesterone gel (n = 235) compared with those allocated to receive placebo (n = 223): intent to treat analysis set
| Outcome | Vaginal progesterone (n (%)) | Placebo (n (%)) | Relative risk (95% CI) | P |
|---|---|---|---|---|
| Primary outcome | ||||
| Preterm birth < 33 weeks | 21/235 (8.9) | 36/223 (16.1) | 0.55 (0.33–0.92) | 0.020 |
| Secondary outcomes | ||||
| Preterm birth < 28 weeks | 12/235 (5.1) | 23/223 (10.3) | 0.50 (0.25–0.97) | 0.036 |
| Preterm birth < 35 weeks | 34/235 (14.5) | 52/223 (23.3) | 0.62 (0.42–0.92) | 0.016 |
| Preterm birth < 37 weeks | 71/235 (30.2) | 76/223 (34.1) | 0.89 (0.68–1.16) | 0.376 |
| Respiratory distress syndrome | 7/235 (3.0) | 17/223 (7.6) | 0.39 (0.17–0.92) | 0.026 |
| Bronchopulmonary dysplasia | 4/235 (1.7) | 5/223 (2.2) | 0.76 (0.21–2.79) | 0.678 |
| Proven sepsis | 7/235 (3.0) | 6/223 (2.7) | 1.11 (0.38–3.24) | 0.853 |
| Necrotizing enterocolitis | 5/235 (2.1) | 4/223 (1.8) | 1.19 (0.32–4.36) | 0.797 |
| Intraventricular hemorrhage, Grade III/IV | 0/235 (0.0) | 1/223 (0.5) | 0.32 (0.01–7.73)* | 0.305 |
| Periventricular leukomalacia | 0/235 (0.0) | 0/223 (0.0) | Not estimable | NA |
| Perinatal death | 8/235 (3.4) | 11/223 (4.9) | 0.69 (0.28–1.68) | 0.413 |
| Fetal death | 5/235 (2.1) | 6/223 (2.7) | 0.79 (0.25–2.57) | 0.700 |
| Neonatal death | 3/235 (1.3) | 5/223 (2.2) | 0.57 (0.14–2.35) | 0.431 |
| Composite outcome scores | ||||
| Any morbidity/mortality event | 18/235 (7.7) | 30/223 (13.5) | 0.57 (0.33–0.99) | 0.043 |
| 0–4 without NICU† | 0.048 | |||
| 0–4 with NICU† | 0.068 | |||
| 0–6 without NICU† | 0.048 | |||
| Birth weight < 2500 g | 60/234 (25.6) | 68/220 (30.9) | 0.83 (0.62–1.11) | 0.213 |
| Birth weight < 1500 g | 15/234 (6.4) | 30/220 (13.6) | 0.47 (0.26–0.85) | 0.010 |
Unadjusted relative risk (RR) and 95% CI calculated using the Cochran–Mantel–Haenszel (CMH) test.
Based on Logit estimator with continuity correction.
Frequency of perinatal mortality/neonatal morbidity composite scores are provided in Appendix S4 online. NA, not applicable; NICU, neonatal intensive care unit.
In terms of infant outcome, neonates born to women allocated to receive vaginal progesterone gel had a significantly lower frequency of RDS than did those born to women allocated to receive placebo (3.0% (n = 7) vs 7.6% (n = 17); RR, 0.39; 95% CI, 0.17–0.92; P = 0.03). The number needed to treat for benefit was 22 (95% CI, 12–186). This effect remained significant after adjustment for pooled study site and risk strata (RR, 0.40; 95% CI, 0.17–0.94; P = 0.03). The other neonatal outcomes are listed in Table 2. Pre-specified composite scores to assess perinatal mortality/neonatal morbidity were calculated. The rate of any morbidity or mortality was significantly lower in the neonates of subjects allocated to receive vaginal progesterone gel compared with those allocated to receive placebo (7.7% (n = 18) vs 13.5% (n = 30); RR, 0.57; 95% CI, 0.33–0.99; P = 0.04). The composite scores ‘0–4 scale without NICU’ and ‘0–6 scale without NICU’ were also significantly lower in the progesterone gel group compared with the placebo group (P < 0.05 for both comparisons). After adjustment for pooled study site and risk strata, the effect of vaginal progesterone gel on composite perinatal mortality/neonatal morbidity scores ‘any morbidity/mortality event’, ‘0–4 scale without NICU’ and ‘0–6 scale without NICU’ continued to show trends toward improvement (P = 0.054, 0.065 and 0.065, respectively). The frequency of distributions for the perinatal mortality/neonatal morbidity composite scores can be found in Appendix S4 online.
Adverse events were comparable between patients who received vaginal progesterone gel and those who received placebo. The rate of adverse events related to study treatment was not significantly different in women who received vaginal progesterone gel compared with those who received placebo (12.8% (n = 30) vs 10.8% (n = 24); RR, 1.19; 95% CI, 0.72–1.96; P = 0.51); the most frequently reported adverse events related to study treatment occurred in up to 2% of women and included vaginal pruritus, vaginal discharge, vaginal candidiasis and nausea. Furthermore, no fetal or neonatal safety signal43 was detected for vaginal progesterone gel. Regarding labor and delivery data, there were no meaningful differences in method of delivery. There was one case of a congenital anomaly in the vaginal progesterone group and there were three in the placebo group (RR, 0.32; 95% CI, 0.03–3.02; P = 0.29). Median 1-min and 5-min Apgar scores were comparable between study groups. Women allocated to receive vaginal progesterone gel had a lower rate of neonates born weighing <1500 g compared with those in the placebo group (6.4% (15/234) vs 13.3% (29/218); RR, 0.49; 95% CI, 0.27–0.88; P = 0.01).
Compliant analysis set
A pre-specified analysis was conducted in a subgroup (84%, 387/459; vaginal progesterone gel, n = 194; placebo, n = 193) of the treated patient analysis set, excluding those who had <80% treatment compliance (n = 53), those who did not have a documented delivery date (n = 4), or who had a cerclage (n = 17). One subject had <80% compliance and a cerclage and one subject had no delivery date and a cerclage.
This compliant analysis set showed for unadjusted analyses that patients allocated to vaginal progesterone gel had a significantly lower frequency of preterm birth than did those allocated to placebo for delivery <28 weeks of gestation (3.1% (6/194) vs 7.8% (15/193); RR, 0.40; 95% CI, 0.16–1.00; P = 0.04), delivery <33 weeks of gestation (5.7% (11/194) vs 13.0% (25/193); RR, 0.44; 95% CI, 0.22–0.86; P = 0.01) and delivery <35 weeks of gestation (10.3% (20/194) vs 20.2% (39/193); RR, 0.51; 95% CI, 0.31–0.84; P < 0.01). There was no significant difference in the rate of preterm delivery before 37 weeks of gestation (26.8% (52/194) vs 30.6% (59/193); RR, 0.88; 95% CI, 0.64–1.20; P = 0.41). Table 4 displays results of primary outcome and secondary outcomes, RDS and any morbidity/mortality event.
Table 4.
Gestational age at delivery and neonatal outcome in asymptomatic women with a singleton pregnancy and sonographic short cervix allocated to receive vaginal progesterone gel (n = 194) compared with those allocated to receive placebo (n = 193): compliant analysis set
| Outcome | Vaginal progesterone (n (%)) | Placebo (n (%)) | Unadjusted RR (95% CI)* |
|---|---|---|---|
| Primary outcome | |||
| Preterm birth < 33 weeks | 21 (8.9) | 34 (15.2) | 0.59 (0.35–0.98) |
| Secondary outcomes | |||
| Preterm birth < 28 weeks | 12 (5.1) | 21 (9.4) | 0.54 (0.27–1.08) |
| Preterm birth < 35 weeks | 34 (14.5) | 50 (22.3) | 0.65 (0.44–0.96) |
| Preterm birth < 37 weeks | 71 (30.2) | 74 (33.0) | 0.91 (0.70–1.20) |
| RDS | 7 (3.0) | 16 (7.1) | 0.42 (0.17–0.99) |
| BPD | 4 (1.7) | 5 (2.2) | 0.77 (0.21–2.80) |
| Proven sepsis | 7 (3.0) | 5 (2.2) | 1.33 (0.43–4.14) |
| NEC | 5 (2.1) | 4 (1.8) | 1.19 (0.32–4.38) |
| IVH Grade III/IV | 0 | 1 (0.5) | 0.32 (0.01–7.76)‡ |
| PVL | 0 | 0 | Not estimable |
| Perinatal death | 8 (3.4) | 10 (4.5) | 0.76 (0.31–1.90) |
| Neonatal death | 3 (1.3) | 5 (2.2) | 0.57 (0.14–2.37) |
| Any morbidity/mortality event | 18 (7.7) | 28 (12.5) | 0.61 (0.35–1.08) |
| Birth weight < 2500 g | 60/234 (25.6) | 67/218 (30.7) | 0.83 (0.62–1.12) |
Unadjusted relative risk (RR) and 95% CI calculated using the Cochran Mantel Haenszel (CMH) method; P-value based on CMH test.
RR and 95% CI calculated using the CMH method adjusted for pooled study site and risk strata; P-value based on CMH test adjusted for pooled study site and risk strata.
Based on Logit estimator with continuity correction. BPD, bronchopulmonary dysplasia; GA, gestational age; IVH, intraventricular hemorrhage; NA, not applicable; NEC, necrotizing enterocolitis; PVL, periventricular leukomalacia; RDS, respiratory distress syndrome.
After adjustment for study site and risk strata, the effect of vaginal progesterone gel remained significant for the reduction in the primary endpoint–the rate of preterm birth before 33 weeks of gestation (RR, 0.42; 95% CI, 0.22–0.82; P < 0.01) and preterm birth before 35 weeks of gestation (RR, 0.50; 95% CI, 0.31–0.82; P < 0.01). Pre-specified composite scores to assess perinatal mortality/neonatal morbidity (0–4 scale without NICU, 0–4 scale with NICU and 0–6 scale without NICU) showed trends towards significance (P = 0.058, 0.049 and 0.058, respectively).
In summary, there was no evidence of a safety signal, and the evidence for the efficacy of vaginal progesterone gel was demonstrated in a similar manner for both of these additional analysis sets to that demonstrated for the intent-to-treat analysis set.
DISCUSSION
Principal findings of the study
Administration of vaginal progesterone gel to women with a short cervix (10–20 mm) was associated with: 1) a substantial reduction in the rate of preterm delivery <33 weeks (primary endpoint), <35 weeks and <28 weeks of gestation; 2) a significant decrease in the rate of RDS; 3) a similar rate of treatment-related adverse events in patients allocated to progesterone or placebo gel; and 4) no evidence of a ‘safety signal’.
Clinical implications of the study
The prevention of preterm birth is a major healthcare priority. The ultimate purpose of interventions designed to reduce preterm birth is improvement in infant outcome. To date, no intervention in an asymptomatic patient with a risk factor has demonstrated both a reduction in preterm birth and an improvement in infant outcome, without a safety signal44. The results of this trial indicate that a combined approach, in which transvaginal sonographic cervical length is used to identify patients at risk for preterm delivery, followed by the administration of vaginal progesterone gel from the mid-trimester of pregnancy until term, reduces the rate of both preterm birth before 33 weeks of gestation and RDS, the most common complication of preterm neonates. In addition to the primary and secondary endpoints related to gestational age, administration of vaginal progesterone gel was associated with a significant reduction in the proportion of infants with any morbidity/mortality event, and a significant improvement in neonatal outcome was demonstrated through two additional composite scores as well as a significant reduction in birth weight <1500 g. Of note, vaginal progesterone gel was well-tolerated and compliance was substantial (>90%).
Results in the context of other studies
The primary result of this trial is similar to that reported by Fonseca et al.27, who found that vaginal progesterone (200 mg vaginal capsules) administered to women with a cervical length ≤15 mm at a median gestational age of 23 weeks reduced the rate of spontaneous preterm (<34 weeks) delivery by 44%. In our trial, there was a 45% reduction in the rate of preterm delivery before 33 weeks. This finding is robust because it was supported by a significant 38% reduction in the rate of preterm birth <35 weeks, a 50% reduction at <28 weeks, and a 53% reduction in the rate of birth weight <1500 g. In addition, the reduction in preterm birth observed in this trial translated into the improvement of clinically important neonatal outcomes such as RDS and three composite perinatal mortality/neonatal morbidity scores.
Both the study by Fonseca et al.27 and the current trial used a similar approach to identify the patients at risk, namely, screening with transvaginal sonography to diagnose a short cervix. Differences between the trials are that: 1) our study excluded twin gestations, which have not been shown to benefit from the prophylactic administration of progesterone45 or 17 alpha-hydroxyprogesterone caproate46, 47; 2) the cervical length for entry into our study was 10–20 mm. Patients with a cervical length of 10 mm or less have a higher rate of intra-amniotic infection/inflammation48 and are less likely to benefit from progesterone administration than are patients with a longer cervix. We extended the upper limit of cervical length to 20 mm to explore whether vaginal progesterone gel would have a beneficial effect beyond 15 mm and therefore expand its therapeutic range; 3) the treatment protocol in our study called for initiation of vaginal progesterone as early as 20 weeks of gestation, continuing until 36 + 6 weeks, while Fonseca et al.27 began at 24 weeks and stopped at 34 weeks (it is possible that earlier treatment may confer more beneficial effects); and 4) the formulation of vaginal progesterone was different. Fonseca et al.27 used oil capsules containing 200 mg progesterone, while we employed a bioadhesive gel with 90 mg progesterone. The vaginal gel preparation has been shown to be biologically active in supporting pregnancies in the first trimester undergoing assisted reproductive technology and, despite the lower dose of progesterone, our current trial results indicate that the dose was sufficient to reduce the rate of preterm delivery. We postulate that this is attributable to the bioadhesive nature of the preparation, which may enhance bioavailability.
Strengths and limitations of the study
The strengths of this study are that it was a multicenter, placebo-controlled, double-masked, randomized trial with rigorous standards for the allocation of treatment and concealment of the identity of the treatment. The placebo and vaginal progesterone gel preparations were identical in appearance and procedures were in place to reduce the risk of other biases. We also performed an additional sensitivity analysis in the ITT analysis set to provide a ‘worst-case’ scenario, in which women lost to follow-up who received vaginal progesterone were considered as if they had a preterm birth before 33 weeks of gestation whereas women lost to follow-up who received placebo were considered as if they had a term delivery (≥37 weeks of gestation). Even in this worst-case scenario of the ITT analysis set, the beneficial effect of vaginal progesterone on the rate of preterm birth before 33 weeks of gestation remained significant (9.3% (22/236) vs 15.7% (36/229); RR, 0.59; 95% CI, 0.36–0.98; P = 0.04).
Another strength of this study is its apparent external validity, supported by the following: 1) our primary results were consistent with those of a similar trial27 that tested the effects of vaginal progesterone capsules in women with a short cervix and reported a similar effect size; 2) the preterm delivery rate in the placebo arm was similar to that reported in studies in the literature12, 17, 49; 3) there was no treatment by site interaction albeit with the necessity to pool sites for this test; and 4) the multinational nature of the trial, in which there was substantial representation (approximately 30%) for each of the following ethnic groups: African-American, Asian and Caucasian.
A limitation of the study is that the primary endpoint is a surrogate for infant outcome. The use of surrogate endpoints is common in clinical trials because of the pragmatic challenges in the execution of trials when infant outcome is the primary outcome of interest. Our study was not powered to detect differences in the outcome according to risk strata (presence or absence of a previous preterm birth).
Sonographic cervical length to identify the patient at risk for preterm delivery
It is now well-established that the shorter the sonographic cervical length in the mid-trimester, the higher the risk of preterm delivery12, 14–23, 25. Indeed, it is possible to assign an individualized risk50 for preterm delivery using sonographic cervical length and other maternal risk factors, such as maternal age, ethnic group, BMI and previous cervical surgery. Among these factors, sonographic cervical length is the most powerful predictor for preterm birth in the index pregnancy, and is more informative than is a history of previous preterm birth14, 17. Selecting patients for prophylactic administration of progestogens based only on a history of a previous preterm birth36, 51–53 would have an effect (albeit limited) on the prevention of preterm delivery worldwide, because most women who deliver preterm neonates do not have this history. Moreover, such strategy cannot be implemented in nulliparous women; therefore, universal risk assessment (primigravidae and parous women) is possible with transvaginal cervical ultrasound. A pharmacoeconomic study is in progress to address the issue of cost-effectiveness, based on the observations of this study.
The effect of progesterone on the uterine cervix
Although the original focus of the effect of progesterone in pregnancy maintenance was on the myometrium54–63, it is now clear that this hormone exerts biological effects on the chorioamniotic membranes64–67 and the uterine cervix68–96. Indeed, progesterone is considered key in the control of cervical ripening70–78, 80–84, 86, 87, 89, 91, 92, 94–96. The precise mechanism by which progesterone prevents preterm delivery in women with a short cervix has not been established. A local effect is likely, given the high concentrations of circulating progesterone in pregnant women97, 98.
Differences among progestogens
The term ‘progestogen’, like ‘progestin’, includes both natural progesterone and synthetic compounds with progesterone-like actions. The compound used in this study is identical to natural progesterone, as was the case in the study by Fonseca et al.27. Progesterone is currently approved to support pregnancies in the first trimester in patients undergoing assisted reproductive technologies in the United States99, Europe and other countries. The safety profile of the preparation used in this study is well-established. In contrast, there are no data to date to support the use of 17-alpha hydroxyprogesterone caproate, a synthetic progestogen, to prevent preterm birth in women with a sonographic short cervix.
Future studies
Additional studies are necessary to determine if treatment of women with a short cervix in the early second trimester may further reduce the rate of preterm delivery100. Moreover, it is important to determine if women with twin gestations who have a short cervix may also benefit from vaginal progesterone. The previous negative results of a randomized clinical trial in twin gestations could be attributed to the inclusion of patients with a long cervix who thus may not have benefited from vaginal progesterone. The optimal treatment of patients with a cervical length <10 mm remains a challenge. Similarly, whether vaginal progesterone may modify the effect of vaginal cerclage remains to be determined.
Importance of the findings
The potential impact of this intervention in clinical practice can be surmised from the estimate that 14 patients need to be treated to prevent one preterm birth before 33 weeks of gestation. Moreover, 22 patients need to be treated to prevent one episode of RDS. These figures compare well with those of two interventions used widely in obstetrics; 100 patients with pre-eclampsia need to be treated with magnesium sulfate to prevent one case of eclampsia101 and 13 women at high risk of preterm birth need to receive antenatal corticosteroids to prevent one case of RDS102.
Implications for clinical practice
The main implication of this study for clinical practice is that universal screening of women with transvaginal sonography to measure cervical length in the midtrimester to identify patients at risk can now be coupled with an intervention–the administration of vaginal progesterone gel–to reduce the frequency of preterm birth and improve neonatal outcome.
Supplementary Material
Table 3.
Gestational age at delivery and neonatal outcome in asymptomatic womenwith a singleton pregnancy and sonographic short cervix allocated to receive vaginal progesterone gel (n = 235) compared with those allocated to receive placebo (n = 224): treated patient analysis set
| Outcome | Vaginal progesterone (n (%)) | Placebo (n (%)) | Unadjusted RR (95% CI)* | P* |
|---|---|---|---|---|
| Primary outcome | ||||
| Preterm birth < 33 weeks | 21 (8.9) | 34 (15.2) | 0.59 (0.35–0.98) | 0.040 |
| Secondary outcomes | ||||
| Preterm birth < 28 weeks | 12 (5.1) | 21 (9.4) | 0.54 (0.27–1.08) | 0.077 |
| Preterm birth < 35 weeks | 34 (14.5) | 50 (22.3) | 0.65 (0.44–0.96) | 0.030 |
| Preterm birth < 37 weeks | 71 (30.2) | 74 (33.0) | 0.91 (0.70–1.20) | 0.516 |
| RDS | 7 (3.0) | 16 (7.1) | 0.42 (0.17–0.99) | 0.041 |
| BPD | 4 (1.7) | 5 (2.2) | 0.77 (0.21–2.80) | 0.683 |
| Proven sepsis | 7 (3.0) | 5 (2.2) | 1.33 (0.43–4.14) | 0.617 |
| NEC | 5 (2.1) | 4 (1.8) | 1.19 (0.32–4.38) | 0.792 |
| IVH Grade III/IV | 0 | 1 (0.5) | 0.32 (0.01–7.76)‡ | 0.306 |
| PVL | 0 | 0 | Not estimable | NA |
| Perinatal death | 8 (3.4) | 10 (4.5) | 0.76 (0.31–1.90) | 0.559 |
| Neonatal death | 3 (1.3) | 5 (2.2) | 0.57 (0.14–2.37) | 0.435 |
| Any morbidity/mortality event | 18 (7.7) | 28 (12.5) | 0.61 (0.35–1.08) | 0.085 |
| Birth weight < 2500 g | 60/234 (25.6) | 67/218 (30.7) | 0.83 (0.62–1.12) | 0.229 |
| Birth weight < 1500 g | 15/234 (6.4) | 29/218 (13.3) | 0.48 (0.27–0.87) | 0.014 |
Unadjusted relative risk (RR) and 95% CI calculated using the Cochran–Mantel–Haenszel (CMH) method; P-value based on CMH test.
RR and 95% CI calculated using the CMH method adjusted for pooled study site and risk strata; P value based on CMH test adjusted for pooled study site and risk strata.
Based on Logit estimator with continuity correction. BPD, bronchopulmonary dysplasia; GA, gestational age; IVH, intraventricular hemorrhage; NA, not applicable; NEC, necrotizing enterocolitis; PVL, periventricular leukomalacia; RDS, respiratory distress syndrome.
Acknowledgments
Role of the funding source
The study was funded in part by the Intramural Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health and Columbia Laboratories, Inc.
The authors were responsible for the study design, data collection and interpretation of the results of the data analysis. The Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)/National Institutes of Health (NIH) was responsible for the writing of the report and the decision to submit the paper for publication. The funding sources (NICHD/NIH and Columbia Laboratories, Inc.) were not involved in writing the report or the decision to submit the paper for publication.
This clinical trial was conducted pursuant to a Clinical Trials Agreement between the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health (NIH), Department of Health and Human Services (DHHS) of the United States, and Columbia Laboratories, Inc., Livingston, New Jersey, USA. We would like to thank the patients who participated in this trial, as well as the coordinators, physicians, nurses, sonographers and administrative staff who assisted in the execution of this study.
Footnotes
Investigators participating in the study
The PREGNANT Trial investigators included: L. Mazheika, S. Zanko (Belarus, Republic of); J. Ortiz Castro, E. Oyarzun (Chile); P. Calda (Czech Republic); S. Fusey, P. Sambarey, Y. Trivedi, D. Vidyadhari, J. Vijayaraghavan (India); A. Bashiri, Y. Hazan, I. Hendler (Israel); M.T. Gervasi (Italy); A. Mikhailov (Russia); P. Soma-Pillay (South Africa); V. Astakhov, D. Manchulenko, V. Potapov, A. Senchuk, O. Yuzko (Ukraine); R. Artal, J. Balducci, J.K. Baxter, M. Beall, L. Bracero, B. Dattel, A. Dayal, S.S. Hassan, B. Howard, J. Hwang, G. Kazzi, M. Khandelwal, W. Kinzler, J. Kipikasa, J. O’Brien, A. Odibo, K. Porter, R. Quintero, H. Sehdev, A. Sheikh, C. Weiner, D. Wing, Y.C. Yang (United States). The investigatorswould like to thank the following central coordinators of the trial: J. Bieda, S. Krafft, J.R. Parella, K. Zubovskiy and E. Richardson. The authors would like to acknowledge the contributions of the following individuals: G. Bega, V. Berghella and K. Dukes.
Contributors
S.S.H., R.R., D.V., S.F., J.K.B., M.K., J.V., Y.T., P.S.P., P.S., A.D., V.P., J.O., V.A., O.Y., W.K., B.D., H.S., L.M., D.M., M.T.G. and G.W.C. contributed to the conception, design, management and interpretation of data, drafting and critically revising the manuscript for important intellectual content, and approving the final version to be published. J.A.P., L.S. and A.C.A. contributed to data analysis and interpretation, as well as drafting and critically revising the manuscript for important intellectual content, and approving the final version to be published. L.S. and A.C.A. were funded exclusively by NICHD/NIH and not Columbia Laboratories, Inc.
CONFLICTS OF INTEREST
S.S.H., R.R., M.T.G., A.C.A., W.K. and L.S. have no financial interest. Author-investigators D.V., S.F., J.B., M.K., J.V., Y.T., P.S.-P., P.S., A.D., V.P., J.O.’B., V.A., O.Y., B.D., H.S., L.M. and D.M. conducted this study with the support of grants awarded by Columbia Laboratories, Inc. for the specific purpose of conducting this trial. The terms and conditions for the awarding of the grants were consistent with those which are customary for this type of industry-sponsored trial and all payments were independent of the outcome of the trial. In addition, J.K.B. and J.O.’B. have also received consulting fees and travel expenses related to Preterm Birth Advisory Committee meetings related to the project. J.O.’B. is an inventor on a patent for the use of progesterone in the prevention of preterm birth. J.A.P. received remuneration as a statistical consultant to Columbia Laboratories, Inc. G.W.C. is an employee of Columbia Laboratories, Inc.
References
- 1.Behrman RE, Butler AS, editors. Preterm Birth Causes, Consequences, and Prevention. Institute of Medicine of the National Academies. The National Academies Press; Washington D.C: 2007. Committee on Understanding Premature Birth and Assuring Healthy Outcomes, Board on Health Sciences Policy. [PubMed] [Google Scholar]
- 2.Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PF. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstine RL, Lee SH, Crawford WL, Shimek MP. Sonographic evaluation of the incompetent cervix. J Clin Ultrasound. 1981;9:417–420. doi: 10.1002/jcu.1870090804. [DOI] [PubMed] [Google Scholar]
- 4.Feingold M, Brook I, Zakut H. Detection of cervical incompetence by ultrasound. Acta Obstet Gynecol Scand. 1984;63:407–410. doi: 10.3109/00016348409156693. [DOI] [PubMed] [Google Scholar]
- 5.Michaels WH, Montgomery C, Karo J, Temple J, Ager J, Olson J. Ultrasound differentiation of the competent from the incompetent cervix: prevention of preterm delivery. Am J Obstet Gynecol. 1986;154:537–546. doi: 10.1016/0002-9378(86)90598-3. [DOI] [PubMed] [Google Scholar]
- 6.Ayers JW, DeGrood RM, Compton AA, Barclay M, Ansbacher R. Sonographic evaluation of cervical length in pregnancy: diagnosis and management of preterm cervical effacement in patients at risk for premature delivery. Obstet Gynecol. 1988;71 (6 Pt 1):939–944. [PubMed] [Google Scholar]
- 7.Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol. 1990;163:859–867. doi: 10.1016/0002-9378(90)91084-p. [DOI] [PubMed] [Google Scholar]
- 8.Kushnir O, Vigil DA, Izquierdo L, Schiff M, Curet LB. Vaginal ultrasonographic assessment of cervical length changes during normal pregnancy. Am J Obstet Gynecol. 1990;162:991–993. doi: 10.1016/0002-9378(90)91302-s. [DOI] [PubMed] [Google Scholar]
- 9.Okitsu O, Mimura T, Nakayama T, Aono T. Early prediction of preterm delivery by transvaginal ultrasonography. Ultrasound Obstet Gynecol. 1992;2:402–409. doi: 10.1046/j.1469-0705.1992.02060402.x. [DOI] [PubMed] [Google Scholar]
- 10.Tongsong T, Kamprapanth P, Srisomboon J, Wanapirak C, Piyamongkol W, Sirichotiyakul S. Single transvaginal sonographic measurement of cervical length early in the third trimester as a predictor of preterm delivery. Obstet Gynecol. 1995;86:184–187. doi: 10.1016/0029-7844(95)00152-h. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa I, Tanaka K, Takahashi K, Tanaka T, Aoki K, Torii Y, Okai T, Saji F, Takahashi T, Sato K, Fujimura M, Ogawa Y. Transvaginal ultrasonographic cervical assessment for the prediction of preterm delivery. J Matern Fetal Med. 1996;5:305–309. doi: 10.1002/(SICI)1520-6661(199611/12)5:6<305::AID-MFM2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 12.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, Thom E, McNellis D, Copper RL, Johnson F, Roberts JM. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567–572. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 13.Berghella V, Tolosa JE, Kuhlman K, Weiner S, Bolognese RJ, Wapner RJ. Cervical ultrasonography compared with manual examination as a predictor of preterm delivery. Am J Obstet Gynecol. 1997;177:723–730. doi: 10.1016/s0002-9378(97)70259-x. [DOI] [PubMed] [Google Scholar]
- 14.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312–317. doi: 10.1046/j.1469-0705.1998.12050312.x. [DOI] [PubMed] [Google Scholar]
- 15.Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18–22 weeks’ gestation and the risk of preterm delivery. Obstet Gynecol. 1998;92:902–907. doi: 10.1016/s0029-7844(98)00346-9. [DOI] [PubMed] [Google Scholar]
- 16.Watson WJ, Stevens D, Welter S, Day D. Observations on the sonographic measurement of cervical length and the risk of premature birth. J Matern Fetal Med. 1999;8:17–19. doi: 10.1002/(SICI)1520-6661(199901/02)8:1<17::AID-MFM4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, Wolfe HM. Patients with an ultrasonographic cervical length< or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–1467. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 18.Heath VC, Daskalakis G, Zagaliki A, Carvalho M, Nicolaides KH. Cervicovaginal fibronectin and cervical length at 23 weeks of gestation: relative risk of early preterm delivery. BJOG. 2000;107:1276–1281. doi: 10.1111/j.1471-0528.2000.tb11620.x. [DOI] [PubMed] [Google Scholar]
- 19.Hibbard JU, Tart M, Moawad AH. Cervical length at 16–22 weeks’ gestation and risk for preterm delivery. Obstet Gynecol. 2000;96:972–978. doi: 10.1016/s0029-7844(00)01074-7. [DOI] [PubMed] [Google Scholar]
- 20.Owen J, Yost N, Berghella V, Thom E, Swain M, Dildy GA, 3rd, Miodovnik M, Langer O, Sibai B, McNellis D. Midtrimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340–1348. doi: 10.1001/jama.286.11.1340. [DOI] [PubMed] [Google Scholar]
- 21.To MS, Skentou C, Liao AW, Cacho A, Nicolaides KH. Cervical length and funneling at 23 weeks of gestation in the prediction of spontaneous early preterm delivery. Ultrasound Obstet Gynecol. 2001;18:200–203. doi: 10.1046/j.1469-0705.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 22.Guzman ER, Walters C, Ananth CV, O’Reilly-Green C, Benito CW, Palermo A, Vintzileos AM. A comparison of sonographic cervical parameters in predicting spontaneous preterm birth in high-risk singleton gestations. Ultrasound Obstet Gynecol. 2001;18:204–210. doi: 10.1046/j.0960-7692.2001.00526.x. [DOI] [PubMed] [Google Scholar]
- 23.Matijevic R, Grgic O, Vasilj O. Is sonographic assessment of cervical length better than digital examination in screening for preterm delivery in a low-risk population? Acta Obstet Gynecol Scand. 2006;85:1342–1347. doi: 10.1080/00016340600935722. [DOI] [PubMed] [Google Scholar]
- 24.Crane JM, Hutchens D. Transvaginal sonographic measurement of cervical length to predict preterm birth in asymptomatic women at increased risk: a systematic review. Ultrasound Obstet Gynecol. 2008;31:579–587. doi: 10.1002/uog.5323. [DOI] [PubMed] [Google Scholar]
- 25.Vaisbuch E, Romero R, Erez O, Kusanovic JP, Mazaki-Tovi S, Gotsch F, Romero V, Ward C, Chaiworapongsa T, Mittal P, Sorokin Y, Hassan SS. Clinical significance of early (<20 weeks) vs. late (20–24 weeks) detection of sonographic short cervix in asymptomatic women in the mid-trimester. Ultrasound Obstet Gynecol. 2010;36:471–481. doi: 10.1002/uog.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero R. Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment. Ultrasound Obstet Gynecol. 2007;30:675–686. doi: 10.1002/uog.5174. [DOI] [PubMed] [Google Scholar]
- 27.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl JMed. 2007;357:462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 28.Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185:1106–1112. doi: 10.1067/mob.2001.118655. [DOI] [PubMed] [Google Scholar]
- 29.Rust OA, Atlas RO, Reed J, van Gaalen J, Balducci J. Revisiting the short cervix detected by transvaginal ultrasound in the second trimester: why cerclage therapy may not help. Am J Obstet Gynecol. 2001;185:1098–1105. doi: 10.1067/mob.2001.118163. [DOI] [PubMed] [Google Scholar]
- 30.To MS, Alfirevic Z, Heath VC, Cicero S, Cacho AM, Williamson PR, Nicolaides KH. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363:1849–1853. doi: 10.1016/S0140-6736(04)16351-4. [DOI] [PubMed] [Google Scholar]
- 31.Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191:1311–1317. doi: 10.1016/j.ajog.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 32.Alfirevic Z. Cerclage: we all know how to do it but can’t agree when to do it. Obstet Gynecol. 2006;107 (2 Pt 1):219–220. doi: 10.1097/01.AOG.0000194479.93493.2c. [DOI] [PubMed] [Google Scholar]
- 33.Owen J, Hankins G, Iams JD, Berghella V, Sheffield JS, Perez- Delboy A, Egerman RS, Wing DA, Tomlinson M, Silver R, Ramin SM, Guzman ER, Gordon M, How HY, Knudtson EJ, Szychowski JM, Cliver S, Hauth JC. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201:375.e1–8. doi: 10.1016/j.ajog.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simcox R, Seed PT, Bennett P, Teoh TG, Poston L, Shennan AH. A randomized controlled trial of cervical scanning vs history to determine cerclage in women at high risk of preterm birth (CIRCLE trial) Am J Obstet Gynecol. 2009;200:623.e1–6. doi: 10.1016/j.ajog.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Arabin B, Halbesma JR, Vork F, Hubener M, van Eyck J. Is treatment with vaginal pessaries an option in patients with a sonographically detected short cervix? J Perinat Med. 2003;31:122–133. doi: 10.1515/JPM.2003.017. [DOI] [PubMed] [Google Scholar]
- 36.O’Brien JM, Adair CD, Lewis DF, Hall DR, Defranco EA, Fusey S, Soma-Pillay P, Porter K, How H, Schackis R, Eller D, Trivedi Y, Vanburen G, Khandelwal M, Trofatter K, Vidyadhari D, Vijayaraghavan J, Weeks J, Dattel B, Newton E, Chazotte C, Valenzuela G, Calda P, Bsharat M, Creasy GW. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:687–696. doi: 10.1002/uog.5158. [DOI] [PubMed] [Google Scholar]
- 37.O’Brien JM, Defranco EA, Adair CD, Lewis DF, Hall DR, How H, Bsharat M, Creasy GW. Effect of progesterone on cervical shortening in women at risk for preterm birth: secondary analysis from a multinational, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2009;34:653–659. doi: 10.1002/uog.7338. [DOI] [PubMed] [Google Scholar]
- 38.DeFranco EA, O’Brien JM, Adair CD, Lewis DF, Hall DR, Fusey S, Soma-Pillay P, Porter K, How H, Schakis R, Eller D, Trivedi Y, Vanburen G, Khandelwal M, Trofatter K, Vidyadhari D, Vijayaraghavan J, Weeks J, Dattel B, Newton E, Chazotte C, Valenzuela G, Calda P, Bsharat M, Creasy GW. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:697–705. doi: 10.1002/uog.5159. [DOI] [PubMed] [Google Scholar]
- 39.ACOG Committee on Practice Bulletins – Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetricians-gynecologists. Number 55, September 2004 (replaces practice pattern number 6, October 1997). Management of Postterm Pregnancy. Obstet Gynecol. 2004;104:639–646. doi: 10.1097/00006250-200409000-00052. [DOI] [PubMed] [Google Scholar]
- 40.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) adopts Consolidated Guideline on Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use. Int Dig Health Legis. 1997;48:231–234. [PubMed] [Google Scholar]
- 41.Dixon JR., Jr The International Conference on Harmonization Good Clinical Practice Guideline. Qual Assur. 1998;6:65–74. doi: 10.1080/105294199277860. [DOI] [PubMed] [Google Scholar]
- 42.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317:1309–1312. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.United States Department of Health and Human Services Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER) [Accessed 25 March 2011];Guidance for Industry: Good Pharmacovigilance Practices and Pharmacoepidemiologic Assessment. 2005 [cited; Available from: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM126834.pdf.
- 44.Food and Drug Administration. [Accessed 25 March 2011];Review by the Division of Reproductive and Urologic Products. 2006 Aug 2;:iii. [cited; Available from: ( http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4227B1-02-01-FDA-Background.pdf.
- 45.Norman JE, Mackenzie F, Owen P, Mactier H, Hanretty K, Cooper S, Calder A, Mires G, Danielian P, Sturgiss S, MacLennan G, Tydeman G, Thornton S, Martin B, Thornton JG, Neilson JP, Norrie J. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373:2034–2040. doi: 10.1016/S0140-6736(09)60947-8. [DOI] [PubMed] [Google Scholar]
- 46.Rouse DJ, Caritis SN, Peaceman AM, Sciscione A, Thom EA, Spong CY, Varner M, Malone F, Iams JD, Mercer BM, Thorp J, Sorokin Y, Carpenter M, Lo J, Ramin S, Harper M, Anderson G. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357:454–461. doi: 10.1056/NEJMoa070641. [DOI] [PubMed] [Google Scholar]
- 47.Combs CA, Garite T, Maurel K, Das A, Porto M. 17- hydroxyprogesterone caproate for twin pregnancy: a doubleblind, randomized clinical trial. Am J Obstet Gynecol. 2011;204:221.e1–8. doi: 10.1016/j.ajog.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 48.Vaisbuch E, Hassan SS, Mazaki-Tovi S, Nhan-Chang CL, Kusanovic JP, Chaiworapongsa T, Dong Z, Yeo L, Mittal P, Yoon BH, Romero R. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202:433.e1–8. doi: 10.1016/j.ajog.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berghella V, Roman A, Daskalakis C, Ness A, Baxter JK. Gestational age at cervical length measurement and incidence of preterm birth. Obstet Gynecol. 2007;110 (2 Pt 1):311–317. doi: 10.1097/01.AOG.0000270112.05025.1d. [DOI] [PubMed] [Google Scholar]
- 50.Celik E, To M, Gajewska K, Smith GC, Nicolaides KH. Cervical length and obstetric history predict spontaneous preterm birth: development and validation of a model to provide individualized risk assessment. Ultrasound Obstet Gynecol. 2008;31:549–554. doi: 10.1002/uog.5333. [DOI] [PubMed] [Google Scholar]
- 51.da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–424. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- 52.Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, Spong CY, Hauth JC, Miodovnik M, Varner MW, Leveno KJ, Caritis SN, Iams JD, Wapner RJ, Conway D, O’Sullivan MJ, Carpenter M, Mercer B, Ramin SM, Thorp JM, Peaceman AM, Gabbe S. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl JMed. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 53.Cetingoz E, Cam C, Sakalli M, Karateke A, Celik C, Sancak A. Progesterone effects on preterm birth in high-risk pregnancies: a randomized placebo-controlled trial. Arch Gynecol Obstet. 2011;283:423–429. doi: 10.1007/s00404-009-1351-2. [DOI] [PubMed] [Google Scholar]
- 54.Word RA, Cornwell TL. Regulation of cGMP-induced relaxation and cGMP-dependent protein kinase in rat myometrium during pregnancy. Am J Physiol. 1998;274 (3 Pt 1):C748–756. doi: 10.1152/ajpcell.1998.274.3.C748. [DOI] [PubMed] [Google Scholar]
- 55.Fomin VP, Cox BE, Word RA. Effect of progesterone on intracellular Ca2+ homeostasis in human myometrial smooth muscle cells. Am J Physiol. 1999;276 (2 Pt 1):C379–385. doi: 10.1152/ajpcell.1999.276.2.C379. [DOI] [PubMed] [Google Scholar]
- 56.Pieber D, Allport VC, Hills F, Johnson M, Bennett PR. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol Hum Reprod. 2001;7:875–879. doi: 10.1093/molehr/7.9.875. [DOI] [PubMed] [Google Scholar]
- 57.Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002;87:2924–2930. doi: 10.1210/jcem.87.6.8609. [DOI] [PubMed] [Google Scholar]
- 58.Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci USA. 2003;100:9518–9523. doi: 10.1073/pnas.1633616100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madsen G, Zakar T, Ku CY, Sanborn BM, Smith R, Mesiano S. Prostaglandins differentially modulate progesterone receptor-A and -B expression in human myometrial cells: evidence for prostaglandin-induced functional progesterone withdrawal. J Clin Endocrinol Metab. 2004;89:1010–1013. doi: 10.1210/jc.2003-031037. [DOI] [PubMed] [Google Scholar]
- 60.Condon JC, Hardy DB, Kovaric K, Mendelson CR. Upregulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20:764–775. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- 61.Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol. 2006;20:2724–2733. doi: 10.1210/me.2006-0112. [DOI] [PubMed] [Google Scholar]
- 62.Merlino AA, Welsh TN, Tan H, Yi LJ, Cannon V, Mercer BM, Mesiano S. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92:1927–1933. doi: 10.1210/jc.2007-0077. [DOI] [PubMed] [Google Scholar]
- 63.Renthal NE, Chen CC, Williams KC, Gerard RD, Prange- Kiel J, Mendelson CR. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci USA. 2010;107:20828–20833. doi: 10.1073/pnas.1008301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pieber D, Allport VC, Bennett PR. Progesterone receptor isoform A inhibits isoform B-mediated transactivation in human amnion. Eur J Pharmacol. 2001;427:7–11. doi: 10.1016/s0014-2999(01)01189-x. [DOI] [PubMed] [Google Scholar]
- 65.Oh SY, Kim CJ, Park I, Romero R, Sohn YK, Moon KC, Yoon BH. Progesterone receptor isoform (A/B) ratio of human fetal membranes increases during term parturition. Am J Obstet Gynecol. 2005;193 (3 Pt 2):1156–1160. doi: 10.1016/j.ajog.2005.05.071. [DOI] [PubMed] [Google Scholar]
- 66.Lee RH, Stanczyk FZ, Stolz A, Ji Q, Yang G, Goodwin TM. AKR1C1 and SRD5A1 messenger RNA expression at term in the human myometrium and chorioamniotic membranes. Am J Perinatol. 2008;25:577–582. doi: 10.1055/s-0028-1085626. [DOI] [PubMed] [Google Scholar]
- 67.Merlino A, Welsh T, Erdonmez T, Madsen G, Zakar T, Smith R, Mercer B, Mesiano S. Nuclear progesterone receptor expression in the human fetal membranes and decidua at term before and after labor. Reprod Sci. 2009;16:357–363. doi: 10.1177/1933719108328616. [DOI] [PubMed] [Google Scholar]
- 68.Naftolin F, Stubblefield P. Dilatation of the Uterine Cervix: Connective Tissue Biology and Clinical Management. Raven Press Books; New York, New York: 1980. [Google Scholar]
- 69.Liggins G. Cervical ripening as an inflammatory reaction. In: Ellwood D, Anderson A, editors. The Cervix in Pregnancy and Labour: Clinical and Biochemical Investigations. Churchill Livingstone; Edinburgh: 1981. pp. 1–9. [Google Scholar]
- 70.Saito Y, Takahashi S, Maki M. Effects of some drugs on ripening of uterine cervix in nonpregnant castrated and pregnant rats. Tohoku J Exp Med. 1981;133:205–220. doi: 10.1620/tjem.133.205. [DOI] [PubMed] [Google Scholar]
- 71.Zuidema LJ, Khan-Dawood F, Dawood MY, Work BA., Jr Hormones and cervical ripening: dehydroepiandrosterone sulfate, estradiol, estriol, and progesterone. Am J Obstet Gynecol. 1986;155:1252–1254. doi: 10.1016/0002-9378(86)90154-7. [DOI] [PubMed] [Google Scholar]
- 72.Hegele-Hartung C, Chwalisz K, Beier HM, Elger W. Ripening of the uterine cervix of the guinea-pig after treatment with the progesterone antagonist onapristone (ZK 98. 299): an electron microscopic study. Hum Reprod. 1989;4:369–377. doi: 10.1093/oxfordjournals.humrep.a136909. [DOI] [PubMed] [Google Scholar]
- 73.Stiemer B, Elger W. Cervical ripening of the rat in dependence on endocrine milieu; effects of antigestagens. J Perinat Med. 1990;18:419–429. doi: 10.1515/jpme.1990.18.6.419. [DOI] [PubMed] [Google Scholar]
- 74.Uldbjerg N, Ulmsten U. The physiology of cervical ripening and cervical dilatation and the effect of abortifacient drugs. Baillieres Clin Obstet Gynaecol. 1990;4:263–282. doi: 10.1016/s0950-3552(05)80226-3. [DOI] [PubMed] [Google Scholar]
- 75.Cabrol D, Carbonne B, Bienkiewicz A, Dallot E, Alj AE, Cedard L. Induction of labor and cervical maturation using mifepristone (RU 486) in the late pregnant rat. Influence of a cyclooxygenase inhibitor (Diclofenac) Prostaglandins. 1991;42:71–79. doi: 10.1016/0090-6980(91)90095-w. [DOI] [PubMed] [Google Scholar]
- 76.Norman J. Antiprogesterones. Br J Hosp Med. 1991;45:372–375. [PubMed] [Google Scholar]
- 77.Ito A, Imada K, Sato T, Kubo T, Matsushima K, Mori Y. Suppression of interleukin 8 production by progesterone in rabbit uterine cervix. Biochem J. 1994;301 (Pt 1):183–186. doi: 10.1042/bj3010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carbonne B, Brennand JE, Maria B, Cabrol D, Calder AA. Effects of gemeprost and mifepristone on the mechanical properties of the cervix prior to first trimester termination of pregnancy. Br J Obstet Gynaecol. 1995;102:553–558. doi: 10.1111/j.1471-0528.1995.tb11360.x. [DOI] [PubMed] [Google Scholar]
- 79.Mahendroo MS, Cala KM, Russell DW. 5 alpha-reduced androgens play a key role in murine parturition. Mol Endocrinol. 1996;10:380–392. doi: 10.1210/mend.10.4.8721983. [DOI] [PubMed] [Google Scholar]
- 80.Chwalisz K, Garfield RE. Regulation of the uterus and cervix during pregnancy and labor. Role of progesterone and nitric oxide. Ann N Y Acad Sci. 1997;828:238–253. doi: 10.1111/j.1749-6632.1997.tb48545.x. [DOI] [PubMed] [Google Scholar]
- 81.Elliott CL, Brennand JE, Calder AA. The effects of mifepristone on cervical ripening and labor induction in primigravidae. Obstet Gynecol. 1998;92:804–809. doi: 10.1016/s0029-7844(98)00284-1. [DOI] [PubMed] [Google Scholar]
- 82.Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol. 1999;13:981–992. doi: 10.1210/mend.13.6.0307. [DOI] [PubMed] [Google Scholar]
- 83.tenlund PM, Ekman G, Aedo AR, Bygdeman M. Induction of labor with mifepristone–a randomized, double-blind study versus placebo. Acta Obstet Gynecol Scand. 1999;78:793–798. [PubMed] [Google Scholar]
- 84.Carbonne B, Dallot E, Haddad B, Ferré F, Cabrol D. Effect of progesterone on prostaglandin E(2)-induced changes in glycosaminoglycan synthesis by human cervical fibroblasts in culture. Mol Hum Reprod. 2000;6:661–664. doi: 10.1093/molehr/6.7.661. [DOI] [PubMed] [Google Scholar]
- 85.Bennett P, Allport V, Loudon J, Elliott C. Prostaglandins, the fetal membranes and the cervix. Front Horm Res. 2001;27:147–164. doi: 10.1159/000061024. [DOI] [PubMed] [Google Scholar]
- 86.Ekman-Ordeberg G, Stjernholm Y, Wang H, Stygar D, Sahlin L. Endocrine regulation of cervical ripening in humans – potential roles for gonadal steroids and insulin-like growth factor-I. Steroids. 2003;68:837–847. doi: 10.1016/j.steroids.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 87.Stjernholm-Vladic Y, Wang H, Stygar D, Ekman G, Sahlin L. Differential regulation of the progesterone receptor A and B in the human uterine cervix at parturition. Gynecol Endocrinol. 2004;18:41–46. doi: 10.1080/09513590310001651777. [DOI] [PubMed] [Google Scholar]
- 88.Tornblom SA, Patel FA, Bystrom B, Giannoulias D, Malmstrom A, Sennstrom M, Lye SJ, Challis JR, Ekman G. 15-hydroxyprostaglandin dehydrogenase and cyclooxygenase 2 messenger ribonucleic acid expression and immunohistochemical localization in human cervical tissue during term and preterm labor. J Clin Endocrinol Metab. 2004;89:2909–2915. doi: 10.1210/jc.2003-031149. [DOI] [PubMed] [Google Scholar]
- 89.Marx SG, Wentz MJ, Mackay LB, Schlembach D, Maul H, Fittkow C, Given R, Vedernikov Y, Saade GR, Garfield RE. Effects of progesterone on iNOS, COX-2, and collagen expression in the cervix. J Histochem Cytochem. 2006;54:623–639. doi: 10.1369/jhc.5A6759.2006. [DOI] [PubMed] [Google Scholar]
- 90.Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 91.Andersson S, Minjarez D, Yost NP, Word RA. Estrogen and progesterone metabolism in the cervix during pregnancy and parturition. J Clin Endocrinol Metab. 2008;93:2366–2374. doi: 10.1210/jc.2007-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elovitz MA, Gonzalez J. Medroxyprogesterone acetate modulates the immune response in the uterus, cervix and placenta in a mouse model of preterm birth. J Matern Fetal Neonatal Med. 2008;21:223–230. doi: 10.1080/14767050801923680. [DOI] [PubMed] [Google Scholar]
- 93.Xu H, Gonzalez JM, Ofori E, Elovitz MA. Preventing cervical ripening: the primary mechanism by which progestational agents prevent preterm birth? Am J Obstet Gynecol. 2008;198:314.e1–8. doi: 10.1016/j.ajog.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 94.Yellon SM, Ebner CA, Elovitz MA. Medroxyprogesterone acetate modulates remodeling, immune cell census, and nerve fibers in the cervix of a mouse model for inflammation-induced preterm birth. Reprod Sci. 2009;16:257–264. doi: 10.1177/1933719108325757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vladic-Stjernholm Y, Vladic T, Blesson CS, Ekman- Ordeberg G, Sahlin L. Prostaglandin treatment is associated with a withdrawal of progesterone and androgen at the receptor level in the uterine cervix. Reprod Biol Endocrinol. 2009;7:116. doi: 10.1186/1477-7827-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hassan SS, Romero R, Tarca AL, Nhan-Chang CL, Mittal P, Vaisbuch E, Gonzalez JM, Chaiworapongsa T, Ali-Fehmi R, Dong Z, Than NG, Kim CJ. The molecular basis for sonographic cervical shortening at term: identification of differentially expressed genes and the epithelial-mesenchymal transition as a function of cervical length. Am J Obstet Gynecol. 2010;203:472.e1–14. doi: 10.1016/j.ajog.2010.06.076. [DOI] [PubMed] [Google Scholar]
- 97.Csapo AI, Knobil E, van der Molen HJ, Wiest WG. Peripheral plasma progesterone levels during human pregnancy and labor. Am J Obstet Gynecol. 1971;110:630–632. doi: 10.1016/0002-9378(71)90242-0. [DOI] [PubMed] [Google Scholar]
- 98.Csapo AI, Puri CP, Tarro S. Relationship between timing of ovariectomy and maintenance of pregnancy in the guinea-pig. Prostaglandins. 1981;22:131–140. doi: 10.1016/0090-6980(81)90060-5. [DOI] [PubMed] [Google Scholar]
- 99.United States Food and Drug Administration. [Accessed 25 March 2011];Drugs@FDA; FDA Approved Drug Products: Crinone. cited; Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Overview&DrugName=CRINONE&CFID=57107255&CFTOKEN=4044debbda6c922-92747269-0699-E743-C01D338314370C9F.
- 100.Greco E, Lange A, Ushakov F, Calvo JR, Nicolaides KH. Prediction of spontaneous preterm delivery from endocervical length at 11 to 13 weeks. Prenat Diagn. 2011;31:84–89. doi: 10.1002/pd.2640. [DOI] [PubMed] [Google Scholar]
- 101.Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, Smith D. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877–1890. doi: 10.1016/s0140-6736(02)08778-0. [DOI] [PubMed] [Google Scholar]
- 102.Sinclair JC. Meta-analysis of randomized controlled trials of antenatal corticosteroid for the prevention of respiratory distress syndrome: discussion. Am J Obstet Gynecol. 1995;173:335–344. doi: 10.1016/0002-9378(95)90223-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.