Abstract
Background
Studies have shown that individuals with schizophrenia suffer from memory impairments. In this study, we combined proton magnetic resonance spectroscopy (1H-MRS) and functional magnetic resonance imaging (fMRI) to clarify the neurobiology of memory deficits in schizophrenia.
Methods
We used single-voxel MRS acquired in the left hippocampus and fMRI during performance of a memory task to obtain measures of neurochemistry and functional response in 28 stable, medicated participants with schizophrenia (SZ) and 28 matched healthy controls (HC).
Results
The SZ group had significantly decreased blood oxygen level-dependent (BOLD) signal in left inferior frontal gyrus (IFG) during encoding and in the anterior cingulate cortex (ACC) and superior temporal gyrus (STG) during retrieval. We did not find significant differences in N-acetylaspartate/creatine (NAA/Cr) or glutamate + glutamine (Glx/Cr) levels between the groups, but did find a significant positive correlation between NAA/Cr and Glx/Cr in the HC group that was absent in the SZ group. There were no significant correlations between BOLD and MRS measured in the hippocampus. Further analyses revealed a negative correlation between left IFG BOLD and task performance in the SZ group. Finally, in the HC group, the left IFG BOLD was positively correlated with Glx/Cr.
Conclusions
We replicated findings of reduced BOLD signal in left IFG and of an altered relationship between IFG BOLD response and task performance in the SZ. The absence of correlation between NAA/Cr and Glx/Cr levels in patients might suggest underlying pathologies of the glutamate-glutamine cycle and/or mitochondria.
Keywords: schizophrenia, hippocampus, fMRI, MRS, NAA, glutamate
1 INTRODUCTION
One of the most replicated abnormalities in schizophrenia is impairment in episodic memory (Aleman et al., 1999). This impairment is not related to education level, gender, medication, duration of illness, or severity of illness (Saykin et al., 1994; Seidman et al., 1998) but instead seems to be a core feature of the illness that highlights disruption of neural memory circuitry, which includes the hippocampus. In addition, it is this memory impairment, along with dysfunctions in other cognitive domains, that puts the greatest limitation on the overall level of functioning in individuals with schizophrenia (Goldberg et al., 2007).
Episodic memory impairment in schizophrenia has been explored in some detail using several imaging modalities. Functional Magnetic Resonance Imaging (fMRI) has demonstrated reduced blood oxygen level dependent (BOLD) signal in the medial temporal lobe, including the hippocampus, and prefrontal regions during memory tasks in individuals with schizophrenia (Achim and Lepage, 2005; Ragland et al., 2004; Ragland et al., 2009). Proton magnetic resonance spectroscopy (1H-MRS) studies of the hippocampus in schizophrenia have, in general, documented a reduction in N-acetylaspartate (NAA) levels (Brugger et al., 2011; Steen et al., 2005). Although NAA levels are thought to be an in vivo biomarker of neuronal integrity (Bates et al., 1996; Lentz et al., 2005; Stork and Renshaw, 2005), it is not known whether decreased NAA is related to memory impairment in schizophrenia. On the other hand, MRS studies measuring glutamate in the hippocampus in schizophrenia have been few and inconclusive (Karlsgodt et al., 2008; Olbrich et al., 2008; Tebartz van Elst et al., 2005)
The purpose of this study was to investigate the relationship between 1H-MRS metabolites and fMRI BOLD signal collected from the hippocampus of participants with schizophrenia (SZ) and matched healthy controls (HC). To our knowledge, despite known abnormalities, the relationships between NAA, glutamate, and the BOLD signal in the hippocampus have never been studied in schizophrenia. For the purpose of this study, we acquired single-voxel 1H-MRS measurements exclusively in the left hippocampus, as prior studies have reported NAA reductions there (Bertolino et al., 1998; Maier M, 20002). For the functional study, we chose an episodic memory task known to activate the medial temporal and prefrontal cortices (Otten et al., 2001; Tebartz van Elst et al., 2005).
We hypothesized that we would replicate findings of reduced NAA levels within the left hippocampus, as well as reduced temporofrontal functional engagement during the memory encoding and retrieval tasks in SZ. Since glutamatergic transmission plays a key role in hippocampal memory processes (Bliss and Collingridge, 1993), we hypothesized that glutamate + glutamine (Glx) levels would correlate positively with the BOLD signal measured in the hippocampus during task performance in HC but not in SZ.
2 METHODS AND MATERIALS
2.1 SUBJECTS
Thirty-nine stable, medicated SZ were recruited from the psychiatry clinics at the University of Alabama at Birmingham (UAB). Forty-one HC, matched on age, gender, and parental occupation, were recruited by advertisements in fliers and the University’s newspaper. Exclusion criteria were major medical conditions, substance abuse within 6 months of imaging (Supplement), previous serious head injury, neurological disorders, loss of consciousness, and pregnancy. The study was approved by the UAB IRB, and all participants gave written informed consent.
Diagnoses were established using participants’ medical records and the Diagnostic Interview for Genetic Studies (DIGS) (Nurnberger et al., 1994). The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph et al., 1998) and the Brief Psychiatric Rating Scale (BPRS) (Overall JE, 1962) were used to characterize general cognitive function and symptom severity, respectively.
Several subjects were excluded from the analyses (Supplement), leaving a combined group of 28 HC and 28 SZ (Table 1).
Table 1.
Group demographics (mean ± stdev)
| Characteristic | HC (n = 28) |
SZ (n = 28) |
|---|---|---|
| Age, years | 35.6 ± 11.1 | 36.7 ± 12.2 |
| Duration of illness, (years) | 15.9 ± 11.9 | |
| Gender, M/F | 17/11 | 20/8 |
| Parental Occupationa | 5.7± 4.3 | 6.0 ± 4.3 |
| RBANSb | ||
| Total index | 101.7 ± 12.3 | 70.4 ± 13.8*** |
| Immediate memory | 102.8 ± 10.2 | 72.9 ± 15.1*** |
| Visuospatial | 96.4 ± 15.9 | 79.6 ± 18.2** |
| Language | 100.4 ± 11.7 | 81.8 ± 15.6*** |
| Attention | 108.8 ± 15.6 | 77.0 ± 20.4*** |
| Delayed memory | 99.1 ± 7.5 | 70.3 ± 19.8*** |
| BPRSc | ||
| Total | 40.5 ± 12.7 | |
| Positive | 8.4 ± 4.2 | |
| Negative | 5.5 ± 2.2 |
HC, healthy control; F, female; M, male; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; SZ, patient with schizophrenia.
Ranks determined from Diagnostic Interview for Genetic Studies (1–18 scale); higher rank (lower numerical value) corresponds to higher socioeconomic status; information not available for three SZ and two HC participants.
Repeatable Battery for the Assessment of Neuropsychological Status
Brief Psychiatric Rating Scale, scored on a 1–7 scale; positive subscale (conceptual disorganization, hallucinatory behavior, and unusual thought content); negative subscale (emotional withdrawal, motor retardation, and blunted affect); data not available for one HC and four SZ participants.
P< .05 significant difference between HC and SZ in independent samples t-test.
P< .01 significant difference between HC and SZ in independent samples t-test.
P < .001 significant difference between HC and SZ in independent samples t-test.
P< .05 significant difference between HC and SZ in the chi-square test.
2.2 IMAGE ACQUISITION: fMRI and MRS
All imaging was performed on a 3T head-only scanner (Siemens Allegra, Erlangen, Germany), equipped with a circularly polarized transmit/receive head coil. fMRI data were acquired using the gradient recalled echo-planar imaging (EPI) sequence (repetition time/echo time [TR/TE] = 2100/30 msec, 70° flip angle, 24 × 24 cm2 field of view, 64 × 64 matrix, 4-mm slice thickness, 1-mm gap, 26 axial slices). A high-resolution structural scan was acquired using the T1-weighted magnetization prepared rapid acquisition gradient-echo (MPRAGE) sequence (TR/TE/inversion time [TI] =2300/3.93/1100 msec, 12° flip angle, 256 × 256 matrix, 1-mm isotropic voxels). An IFIS-SA system (In Vivo Corp., Orlando, Florida) running E-Prime software (version 1.2; Psychology Software Tools, Inc., Pittsburgh, Pennsylvania) controlled stimulus delivery and recorded responses and reaction times.
T1-weighted anatomic scans (gradient-recalled echo sequence, TR/TE = 250/3.48 ms, 70° flip angle, 5-mm slice thickness, 1.5-mm gap, 512 × 512 matrix) were acquired for spectroscopic voxel placement. Slices were aligned to the anatomical midline to control for head tilt. To facilitate voxel placement, the axial images were obtained along the long axis of the hippocampi, as viewed from the sagittal images. The voxel was placed in the left hippocampus such that the amount of gray matter was maximized (Figure 1). Following manual shimming and water suppression, spectra were acquired using the point-resolved spectroscopy sequence (PRESS; TR/TE = 2000/80 msec, 640 averages, 2.7 × 1.5 × 1 cm voxel size, 21 min 20 sec scanning time) to optimize the glutamate signal (Bottomley, 1987).
Figure 1.
(A) Example of magnetic resonance spectroscopy (MRS) voxel placement in the left hippocampus. Image is displayed in radiological convention (right side of image is subject’s left side). (B) Spectra obtained from one healthy control scanned on 5 consecutive days to assess reproducibility of MRS data. Cho, choline; Cr, creatine; Glx, glutamate + glutamine; NAA, N-acetylaspartate; ppm, parts per million.
2.3 MRS Analyses
MRS data were analyzed in jMRUI (version 3.0) (Naressi et al., 2001) as previously described (Reid et al., 2010). After removing the residual water peak, spectra were quantified in the time domain using the AMARES algorithm (Vanhamme et al., 1997). Prior knowledge derived from in vitro and in vivo metabolite spectra was included in the model (Reid et al., 2010), which consisted of peaks for NAA, choline (Cho), creatine (Cr), and three peaks for Glx. Amplitude, line width, and chemical shift were optimized for each peak. Cramer–Rao lower bounds (CRLB) (Ratiney et al., 2004) were calculated for each peak. Exclusion criteria were 1) line width of water greater than 25 Hz at full width at half maximum (FWHM) during shimming, 2) CRLB greater than 30%, and 3) failure of the fitting algorithm. No data were excluded on the basis of these criteria. One HC was scanned on 5 consecutive days (Figure 1) to assess reproducibility (Supplement). NAA and Glx were quantified with respect to Cr.
We conducted a MANCOVA to assess between-group differences in NAA/Cr and Glx/Cr levels. For all analyses, NAA/Cr and Glx/Cr levels were corrected for age. Because positive correlations between NAA and Glx have been identified in healthy controls (Waddell et al., 2011; Walter et al., 2009), we also assessed the correlations between NAA and Glx in each group. These analyses were tested with an alpha level of .05.
2.4 fMRI TASK
The episodic memory task consisted of an intentional encoding phase, followed by a recognition memory phase after a 15 minute delay. To maximize retrieval performance, a deep encoding paradigm utilizing an animacy decision was used. During the encoding task, participants saw a series of 60 words, presented one at a time for 300 msec followed by a fixation screen. A 2-second prestimulus cue (“Alive?”) indicated that the participant had to answer by button press whether the upcoming word was alive or not alive. Following a 15 minute interval, participants performed the retrieval task, where they saw 60 words, including 30 words previously seen (old words) and 30 new words, presented one at a time for 300 msec. A 2-second warning stimulus (“Ready?”) indicated that the participant had to answer by button press whether the upcoming word was “old” or “new.” For each task, the inter-stimulus interval (fixation screen) was jittered, ranging from 3 to 5 seconds. Behavioral measures were compared across groups using a one-way ANOVA. The alpha level was set at .05.
2.5 fMRI Analysis and Design
Data analyses were implemented in SPM8 running in MATLAB (version R2010b). Preprocessing of the fMRI data included slice timing correction, realignment and reslicing to the mean functional volume, artifact/motion correction (movement > 1mm) using ArtRepair (Mazaika PK, 2005), coregistraton to the structural scan, and normalization to MNI space using DARTEL (Ashburner, 2007) with 4-mm FWHM Gaussian kernel smoothing. Participants were excluded from further analyses if 33% or more of their data were repaired during artifact and motion correction.
The subject-level statistical analysis consisted of an event-related GLM. We included the following regressors: encode correctly and incorrectly retrieved for the encoding condition, and Hits, Misses, Correct Rejects and False Alarms for the retrieval condition (Ragland et al., 2001; Ragland et al., 2004). All events were modeled using a canonical hemodynamic response function, and data were high-pass filtered (cutoff = 256 seconds). Statistical parametric maps were generated for the following contrasts: encode correctly retrieved > encode incorrectly retrieved, and retrieve high (Hits) > retrieve low (Correct Rejects). At the group level, the two contrasts for each individual were entered as conditions in a diagnosis-by-condition factorial design (Achim and Lepage, 2005). Whole-brain results were corrected for multiple comparisons using a cluster-level FDR correction. The cluster size threshold was defined within SPM8, based on Gaussian random field theory, as the number of contiguous voxels with p < .05 (uncorrected) in order to accept the false discovery rate of 0.05 (Chumbley and Friston, 2009).
2.6 MRS, fMRI Correlations
The BOLD signal was extracted as percent signal change using MarsBaR (Brett M, 2002) from a region of interest, defined as the left hippocampus, in WFU pick-atlas’s IBASPM 116 definitions (Maldjian et al., 2003). The relationships between metabolite levels and percent signal change were analyzed by Pearson correlation, which were compared using Fisher’s r to Z transform. The family-wise alpha was held at .05 by using a Holm multiple comparison correction (Holm, 1979).
Since Ragland et al. (2001) found an association between target discriminability and activation in the left IFG similar to the one observed in this study, we conducted exploratory correlations between BOLD response in that region, defined as the LIFG in the pick-atlas, and the index of discriminability, Pr. We also conducted correlations between hippocampal metabolites, LIFG BOLD response, and Pr.
3 RESULTS
3.1 Behavioral
Behavioral results are summarized in Table 2. The SZ group performed significantly worse compared to HC on all behavioral measures except for the measure of response bias (Br). The percentage of correct responses during encoding and retrieval demonstrated that both groups were attentive and engaged during the task.
Table 2.
Task Performance (mean ± stdev)
| Measure | HC (n= 28) | SZ (n= 28) |
|---|---|---|
| Encode | ||
| Percent Correctb | 0.92 ± 0.06 | 0.79 ± 0.17*** |
| “Alive” Correct Reaction Time (ms) | 1057.9 ± 244.0 | 1315.6 ± 404.0** |
| “Not alive” Correct Reaction Time (ms) | 1093.8 ± 281.0 | 1367.8 ± 384.0** |
| Retrieve | ||
| Hit Rate, HRc | 0.83 ± 0.13 | 0.64 ±0.19*** |
| Percent Correctd | 0.86 ± 0.07 | 0.68 ± 0.12*** |
| False Alarm Rate, FARe | 0.11± 0.10 | 0.27 ± 0.19*** |
| Discrimination Index, Prf | 0.71±0.14 | 0.37 ± 0.23*** |
| Response Bias, Brg | 0.39 ±0.27 | 0.43 ± 0.23 |
| “Old” (Previously Seen) Correct RT (ms) | 1078.21± 235.12 | 1234.01 ± 278.90* |
HC, healthy control; RT, Reaction Time; SZ, schizophrenia.
Mean ± SD unless indicated otherwise.
Encode Percent Correct = correct animacy decision/60
Retrieve Hit Rate, HR = [number of correctly identified old words(Hits)]/30
Retrieve Percentage Correct = (hits + correct rejections)/60
False alarm rate, FAR = number false alarms/30
Index of discriminability, Pr = (HR – FAR)
Response bias, Br = [FAR/(1-Pr)].
P< .05 significant difference between HC and SZ in independent samples t-test.
P< .01 significant difference between HC and SZ in independent samples t-test.
P < .001 significant difference between HC and SZ in independent samples t-test.
3.2 MRS
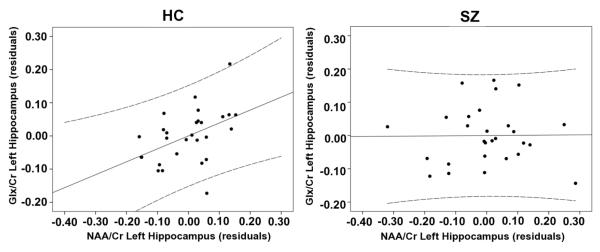
NAA/Cr and Glx/Cr did not significantly differ between the groups (Table 3) (Scatter plots in Supplement). NAA/Cr and Glx/Cr were significantly correlated in the HC group (r = 0.450, p = .019) but not the SZ group (r = 0.010, p = .959). The correlation in the HC group did not survive a multiple comparisons correction (Figure 2).
Table 3.
Results of multiple analysis of covariance, controlling for age, comparing the metabolite ratios in the left hippocampus.
| Metabolite | HC (n=28) Mean ± SD CRLB ± SD (CRLB range) |
SZ (n=28) Mean ± SD CRLB ± SD (CRLB range) |
|---|---|---|
| NAA/Cra, b | 1.27 ± 0.10 3.7% ± 0.5% (2.7%-4.5%) |
1.23 ± 0.15 3.7% ± 0.9% (2.4%-6.8%) |
| Glx/Crb | 0.63 ± 0.08 9.9% ± 1.7% (7.5%-13.9%) |
0.63 ± 0.09 10.8% ± 3.4% (7.2%-19.9%) |
Cr, creatine; CRLB, Cramer-Rao Lower Bounds; Glx, glutamate and glutamine; HC, healthy control participants; HIP, hippocampus, NAA, N-acetylaspartate; SZ, participants with schizophrenia.
There was no significant difference in the raw amplitudes of Cr between groups (t = −.673, p = .78).
There were no differences between the groups for water line width, metabolite peak line width, CRLBs or gray or white matter contents of the left hippocampus (all p > 0.05) (see Supplement for details).
P< .05 significant difference between HC and SZ in independent samples t-test.
Figure 2.
Association between the metabolites NAA/Cr and Glx/Cr both measured in the left hippocampus of healthy controls (HC, n = 28) and participants with schizophrenia (SZ, n = 28). The HC group showed a positive correlation (r = 0.450, p = .019) that was not seen in the SZ group (r = 0.010, p = .959) (Z = 1.68, p = .093). The healthy control group’s positive correlation did not survive multiple comparison testing. Solid lines are linear regressions and dashed lines are 95% confidence intervals.
3.3 fMRI
In the between-group comparison of the encode contrast, the SZ group showed significantly reduced BOLD signal in the left IFG compared to HC (Table 4). During the retrieve contrast, the SZ group showed significantly reduced BOLD signal in the anterior cingulate cortex (ACC) and the left superior temporal gyrus (STG) compared to HC (Table 4) (Figure 3). Within-group results can be found in Supplement. Although the encode and retrieve contrasts were based on correct responses, we also analyzed a subset of subjects matched on Pr (HC = 14; SZ = 15). During encode there was a significant group difference in the IFG, but there were no significant group difference during retrieve (Supplement). To evaluate the functional connectivity (FC) between the left IFG and left hippocampus during encode, we conducted a psycho-physiological interaction (PPI) analysis (Friston et al., 1997) within SPM8 (Supplement). In each group, there was a significant FC between these regions and no significant group difference (Supplement).
Table 4.
| Contrast | Hem | Lobe | Region | BA | Peak t value |
Volume (voxels) |
x, y, zd |
|---|---|---|---|---|---|---|---|
| Encode | |||||||
| HC > SZ | |||||||
| L | Frontal | Inferior frontal gyrus, Triangular part |
47 | 4.60 | 675 | −39, 19, −9 | |
| Retrieve | |||||||
| HC > SZ | |||||||
| R | Limbic | Anterior cingulate gyrus | 32 | 4.46 | 323 | 2, 39, 3 | |
| L | Temporal | Superior temporal gyrus | 49 | 3.88 | 360 | −45, −53, 18 |
BA, Brodmann Area, HC, healthy control; Hem, hemisphere; SZ, schizophrenia.
Cluster level False Discovery Rate (FDR) of 0.05 was used to control for multiple comparisons
Encode condition: encode correctly retrieved > encode incorrectly retrieved
Retrieve condition: retrieve Hits > retrieve Correct Rejects
x,y, and z refer to Montreal Neurological Institute coordinates
Figure 3.
Whole-brain between-group difference (healthy control participants > participants with schizophrenia) during the encode task and retrieve task. For encode, the single cluster shown is located at the inferior frontal gyrus (MNI coordinate: x = -39, y = 19, z = -9). For retrieve, the top slices show a cluster located at the anterior cingulate (MNI coordinates: x = 2, y= 39, and z = 3). The bottom slices show a cluster in the left superior temporal gyrus (MNI coordinates: x = -45, y= -53, and z = 18). Multiple comparisons corrected by using a cluster level false discovery rate (FDR) of .05. Activation overlaid on the SPM8 single-subject T1 template. Left, sagittal slices; right axial slices. Numbers below slices indicate x and z coordinates in MNI convention. Color bar indicates t values.
3.4 fMRI/MRS Correlations
MRS and hippocampal BOLD measures were not significantly correlated. However, the retrieve BOLD signal in left IFG and Pr were negatively correlated in SZ (r = -0.386, p = .042) but not in HC (r = 0.163, p = .408), a difference that was statistically significant (z = -2.02, p = .043) (Figure 4). The IFG BOLD was significantly correlated with Glx/Cr in HC (r = 0.489, p = .008) but not in SZ (r = 0.139, p = 0.480) (z = 1.4, p = .162) (Figure 5). There were no significant correlations between metabolites and Pr. Glx/Cr was significantly correlated with the IFG/hippocampus FC beta weights in the HC group (r = 0.428, p = 0.026) but not in the SZ group (r = .078, p = .699) (Supplement).
Figure 4.
Association between blood oxygen level-dependent (BOLD) acquired in the left inferior frontal gyrus and a behavioral index of discriminability, Pr (see methods). BOLD mean percent signal change was acquired during the encode task. A significant negative correlation was found for the SZ group (r = -0.386, p = .042) but not the HC (r = 0.163, p = .408). These two correlations were significantly different (Z = -2.02; p = .043). Solid lines are linear regressions and dashed lines are 95% confidence intervals.
Figure 5.
Association between blood oxygen level-dependent (BOLD) mean percent signal change during the retrieve task and the MRS metabolite Glx/Cr. The BOLD measurement was collected in the left inferior frontal gyrus and the MRS measurement was measured in the left hippocampus. HC showed a significant positive correlation (r = 0.489, p = .008) that was not present in SZ group (r = 0.139, p = .480). These two correlations are not significantly different (Z = 1.4, p = .162). Solid lines are linear regressions and dashed lines are 95% confidence intervals.
4 DISCUSSION
To our knowledge, this is the first study utilizing MRS and fMRI to study hippocampal pathology in a group of stable, medicated SZ patients and matched HC. We observed that patients showed reduced BOLD signal in left IFG during memory encoding and in the ACC and STG during memory retrieval. There were no significant group difference in functional connectivity between IFG and hippocampus. Although NAA/Cr and Glx/Cr did not significantly differ between the groups, we found a positive correlation between NAA/Cr and Glx/Cr in controls that was not present in the patients. We did not find any correlations between hippocampal BOLD and MRS measures. Exploratory analyses revealed a negative correlation between the IFG BOLD response and task performance in patients, but not in controls, a difference that was statistically significant. Finally, in controls, the BOLD signal in IFG was positively correlated with hippocampal Glx/Cr.
Our fMRI results are consistent with two meta-analyses (Achim and Lepage, 2005; Ragland et al., 2009) that found evidence of reduced left IFG activation during encoding and reduced ACC and temporal lobe activation during retrieval in schizophrenia. These reductions are thought to reflect a dysfunction of cognitive processes engaged during a memory task (Ragland et al., 2009). We did not find significantly reduced hippocampal activation in the schizophrenia group, a finding consistent with Ragland’s (2009) but not with Achim and Lepage’s (2005) meta-analysis.
We did not find a significant decrease in NAA/Cr between the groups; however, the direction was that of a reduction of about 3% in our patients. A meta-analysis of 64 MRS studies in schizophrenia found consistent evidence of reductions of NAA in hippocampus of approximately 5% (Steen et al., 2005); thus, our sample sizes may have prevented us from detecting the small drop in NAA/Cr.
We reliably quantified Glx/Cr in a group of medicated patients and found no significant differences compared to controls. Only a few studies in schizophrenia have reported glutamate measurements in hippocampus. One study (van Elst et al, 2005) reported higher glutamate levels in a group of acutely exacerbated medicated patients, while another (Lutkenhoff et al, 2010) failed to identify such differences in stable medicated participants. In addition, Olbrich (Olbrich et al, 2008) did not find a glutamate difference in a small group of medicated, first-episode patients. Clearly, these results need to be replicated with larger, well-characterized groups of participants.
We identified a positive correlation between NAA/Cr and Glx/Cr in controls but not in patients. Such correlations have been previously identified in healthy controls (Waddell et al., 2011; Walter et al., 2009). NAA and Glx are inherently linked through a series of biochemical reactions, including the tricarboxylic acid cycle (TCA) in the mitochondria and the glutamate-glutamine cycle between neurons and glia (Moffett et al., 2007). The altered correlation could be driven by a variety of underlying pathologies. Postmortem studies in schizophrenia have revealed abnormalities in mitochondrial morphology, function, and gene expression (Clay et al., 2011; Rosenfeld et al., 2011), including in the hippocampus (Altar et al, 2005); abnormal expression of the enzymes regulating the glutamate-glutamine cycle (Bauer et al., 2008; Bruneau et al., 2005; Steffek et al., 2008), and alterations of the glutamatergic signaling pathway (Harrison, 2004; Tamminga et al., 2010). These findings support the notion that the regulation between NAA and Glx could be altered in schizophrenia.
This study points towards significant functional deficit in left IFG during memory processing in schizophrenia. First, the IFG BOLD response was decreased in patients, even when the groups were matched on a behavioral index of discriminability. Second, replicating prior results (Ragland et al., 2001), we found the relationship between IFG BOLD response and task performance to be altered in patients. While HC showed a small positive correlation between IFG BOLD and Pr, these were negatively correlated in patients. Interestingly, a large number of patients had a decreased BOLD response in IFG during task, and those were the ones with high Pr (Figure 4). Speculatively, these SZ might have relied on compensatory neural network to perform the task. Although functional connectivity between the prefrontal cortex and hippocampus has been found impaired in schizophrenia (Benetti et al., 2009; Meyer-Lindenberg et al., 2005), we did not find significant differences in functional connectivity between the groups. This discrepancy could be related to differences in seed region or task type.
In addition, we observed a robust correlation between hippocampal Glx and left IFG activation in controls and found this correlation contained more variation in patients. A previous study using MRS and electroencephalography found correlations between hippocampal glutamate levels and frontally-recorded theta activity during task performance, which suggests a coupling between these regions (Gallinat et al., 2006). Interestingly, in our study, Glx levels were correlated with the beta weight of the IFG/hippocampus functional connectivity. Hippocampal Glx levels might provide an index of the coupling between these two regions during a memory task.
A major limitation of this study was that all patients were treated with antipsychotic drugs, which complicates the interpretation of the fMRI and MRS data (Bustillo et al., 2008; Choe et al., 1996; Fannon et al., 2003).
In conclusion, we replicated findings of reduced BOLD signal in left IFG during memory encoding and in ACC and STG during memory retrieval, as well as altered relationship between left IFG BOLD response and task performance in patients. We found that the positive correlation between NAA and Glx levels in healthy controls was absent in patients, perhaps suggesting underlying pathologies of the glutamate-glutamine cycle and/or mitochondria. Finally, we observed an altered relationship between hippocampal Glx and IFG BOLD response, suggesting an uncoupling between hippocampal biochemistry and IFG function in schizophrenia. These data further our knowledge of hippocampal pathology in schizophrenia.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a National Institute of Mental Health grant R01 MH081014 (ACL). We want to thank all the volunteers with schizophrenia who so graciously took part in this project, as well as the staff of the Community Psychiatry Program at The University of Alabama at Birmingham. In addition, we thank Dr. Rosalyn Weller for editorial help and Dr. Richard Kennedy for statistical assistance. N.H. would like to thank the Howard Hughes Medical Institute’s Medical to Graduate Initiative for providing him with first year funding and ongoing training in translational research.
Over the past two years, ACL has received research funds from the National Institute of Health (R01 MH081014), and an investigator-initiated grant from Pfizer. RCK works as a neurology subspecialist in epilepsy (35% effort), performs neuroimaging in his clinical practice (10% effort), has received honoraria from UCB Pharma, is funded by NIH grants R01 NS053998 and U01 NS058634, and is conducting clinical trial research for Eisai Pharmaceuticals, protocols E2007-G000-304 and 307.
Role of the funding source Funding for this study was provided by NIH Grant MH081014 to ACL. The NIH had no further role in the study design, subject recruitment, data collection or data analysis. The NIH was also not involved in writing of the report or the decision to submit this report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES All other authors reported no biomedical financial interests or potential conflicts of interest.
Conflicts of interest All authors declare that they have no conflicts of interest.
Contributors Nathan Hutcheson analyzed and interpreted the study data and prepared the written paper. Meredith Reid helped create the model for the acquisition of the MRS data and processed the resulting MRS spectra. David White was involved in subject recruitment, data collection, and data interpretation. Nina Kraguljac helped process the MRS data. Kathy Avsar designed the memory task word list. Mark Bolding helped with data collection and management. Robert Knowlton was responsible for reviewing all MRI images for incidental findings. Jan den Hollander helped design the imaging acquisition parameters used to collect the MRS and also the algorithms used to quantify the spectra. As the P.I. on this project, Adrienne Lahti designed the study. She supervised subject recruitment, data collection and analyses, and interpretation of the data.
References
- Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: meta-analysis. Br J Psychiatry. 2005;187:500–509. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport. 1996;7:1397–1400. [PubMed] [Google Scholar]
- Bauer D, Gupta D, Harotunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophrenia Research. 2008;104:108–120. doi: 10.1016/j.schres.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetti S, Mechelli A, Picchioni M, Broome M, Williams S, McGuire P. Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain. 2009;132:2426–2436. doi: 10.1093/brain/awp098. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Callicott JH, Elman I, Mattay VS, Tedeschi G, Frank JA, Breier A, Weinberger DR. Regionally specific neuronal pathology in untreated patients with schizophrenia: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry. 1998;43:641–648. doi: 10.1016/s0006-3223(97)00555-6. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- Brett M, A.J., Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16:497. [Google Scholar]
- Brugger S, Davis JM, Leucht S, Stone JM. Proton magnetic resonance spectroscopy and illness stage in schizophrenia--a systematic review and meta-analysis. Biol Psychiatry. 2011;69:495–503. doi: 10.1016/j.biopsych.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Bruneau EG, McCullumsmith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Increased expression of glutaminase and glutamine synthetase mRNA in the thalamus in schizophrenia. Schizophrenia Research. 2005;75:27–34. doi: 10.1016/j.schres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Rowland LM, Jung R, Brooks WM, Qualls C, Hammond R, Hart B, Lauriello J. Proton magnetic resonance spectroscopy during initial treatment with antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2008;33:2456–2466. doi: 10.1038/sj.npp.1301631. [DOI] [PubMed] [Google Scholar]
- Choe BY, Suh TS, Shinn KS, Lee CW, Lee C, Paik IH. Observation of metabolic changes in chronic schizophrenia after neuroleptic treatment by in vivo hydrogen magnetic resonance spectroscopy. Invest Radiol. 1996;31:345–352. doi: 10.1097/00004424-199606000-00006. [DOI] [PubMed] [Google Scholar]
- Chumbley JR, Friston KJ. False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage. 2009;44:62–70. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Clay HB, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. International Journal of Developmental Neuroscience. 2011;29:311–324. doi: 10.1016/j.ijdevneu.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannon D, Simmons A, Tennakoon L, O’Ceallaigh S, Sumich A, Doku V, Shew C, Sharma T. Selective deficit of hippocampal N-acetylaspartate in antipsychotic-naive patients with schizophrenia. Biol Psychiatry. 2003;54:587–598. doi: 10.1016/s0006-3223(03)00185-9. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and Modulatory Interactions in Neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Kunz D, Senkowski D, Kienast T, Seifert F, Schubert F, Heinz A. Hippocampal glutamate concentration predicts cerebral theta oscillations during cognitive processing. Psychopharmacology (Berl) 2006;187:103–111. doi: 10.1007/s00213-006-0397-0. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, Woerner MG, Schooler NR, Kane JM, Robinson DG. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Karlsgodt KH, Sun D, Jimenez AM, Lutkenhoff ES, Willhite R, van Erp TGM, Cannon TD. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Development and Psychopathology. 2008;20:1297–1327. doi: 10.1017/S095457940800062X. [DOI] [PubMed] [Google Scholar]
- Lentz MR, Kim JP, Westmoreland SV, Greco JB, Fuller RA, Ratai EM, He J, Sehgal PK, Halpern EF, Lackner AA, Masliah E, Gonzalez RG. Quantitative neuropathologic correlates of changes in ratio of N-acetylaspartate to creatine in macaque brain. Radiology. 2005;235:461–468. doi: 10.1148/radiol.2352040003. [DOI] [PubMed] [Google Scholar]
- Maier M, M.J., Toone BK, Trimble M, Ron MA. Schizophrnia, temporal lobe epilepsy and psychosis: an in vivo magnetic resonance spectroscopy and imaging study of the hippocampus/amygdala complex. Psychol Med. 30:571–581. doi: 10.1017/s0033291799001993. 20002. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mazaika PK, W.-G.S., Reiss A, Glover G. Artifact repair of fMRI data from high motion clinical subjects (with new results from 3-D large motion correction). 11th Annual Meeting of the Organization for Human Brain Mapping Tronto Ontario; Canada. 2005. [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF. Regionally Specific Disturbance of Dorsolateral Prefrontal–Hippocampal Functional Connectivity in Schizophrenia. Archives of General Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12:141–152. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr., Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863-844. [DOI] [PubMed] [Google Scholar]
- Olbrich HM, Valerius G, Rüsch N, Büchert M, Thiel T, Hennig J, Ebert D, Tebartz Van Elst L. Frontolimbic glutamate alterations in first episode schizophrenia: Evidence from a magnetic resonance spectroscopy study. World Journal of Biological Psychiatry. 2008;9:59–63. doi: 10.1080/15622970701227811. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain. 2001;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Overall JE, G.D. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Ragland JD, Gur RC, Raz J, Schroeder L, Kohler CG, Smith RJ, Alavi A, Gur RE. Effect of schizophrenia on frontotemporal activity during word encoding and recognition: a PET cerebral blood flow study. Am J Psychiatry. 2001;158:1114–1125. doi: 10.1176/appi.ajp.158.7.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, Siegel S, Kanes S, Gur RE. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. Am J Psychiatry. 2004;161:1004–1015. doi: 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry. 2009;166:863–874. doi: 10.1176/appi.ajp.2009.08091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Ratiney H, Coenradie Y, Cavassila S, van Ormondt D, Graveron-Demilly D. Time-domain quantitation of 1H short echo-time signals: background accommodation. MAGMA. 2004;16:284–296. doi: 10.1007/s10334-004-0037-9. [DOI] [PubMed] [Google Scholar]
- Reid MA, Stoeckel LE, White DM, Avsar KB, Bolding MS, Akella NS, Knowlton RC, den Hollander JA, Lahti AC. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol Psychiatry. 2010;68:625–633. doi: 10.1016/j.biopsych.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M, Brenner-Lavie H, Ari SG-B, Kavushansky A, Ben-Shachar D. Perturbation in Mitochondrial Network Dynamics and in Complex I Dependent Cellular Respiration in Schizophrenia. Biological Psychiatry. 2011;69:980–988. doi: 10.1016/j.biopsych.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Stone WS, Jones R, Harrison RH, Mirsky AF. Comparative effects of schizophrenia and temporal lobe epilepsy on memory. J Int Neuropsychol Soc. 1998;4:342–352. [PubMed] [Google Scholar]
- Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30:1949–1962. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- Steffek AE, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Cortical expression of glial fibrillary acidic protein and glutamine synthetase is decreased in schizophrenia. Schizophr Res. 2008;103:71–82. doi: 10.1016/j.schres.2008.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Tebartz van Elst L, Valerius G, B¸chert M, Thiel T, R¸sch N, Bubl E, Hennig J.r., Ebert D, Olbrich HM. Increased Prefrontal and Hippocampal Glutamate Concentration in Schizophrenia: Evidence from a Magnetic Resonance Spectroscopy Study. Biological Psychiatry. 2005;58:724–730. doi: 10.1016/j.biopsych.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129:35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- Waddell KW, Zanjanipour P, Pradhan S, Xu L, Welch EB, Joers JM, Martin PR, Avison MJ, Gore JC. Anterior cingulate and cerebellar GABA and Glu correlations measured by (1)H J-difference spectroscopy. Magn Reson Imaging. 2011;29:19–24. doi: 10.1016/j.mri.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, Schnepf B, Boeker H, Boesiger P, Northoff G. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66:478–486. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.