Abstract
Solubilizations of the purple membrane from Halobacterium halobium with the detergent Tritain X-100 followed by gel filtration in deoxycholate solution gave bacteriorhodopsin that was more than 99% free from endogenous lipid. The delipidated bacteriorhodopsin was reconstituted with exogenous phospholipids to form vesicles which on illumination efficiently translocated protons. The direction of proton pumping was from the outside to the interior of the vesicles, indicating that the orientation of bacteriorhodopsin in the vesicles was opposite to that in the bacterial membrane. This orientation was confirmed by cleavage of the carboxyl terminus of the protein by proteolysis from the outside of the vesicles.
Full text
PDF
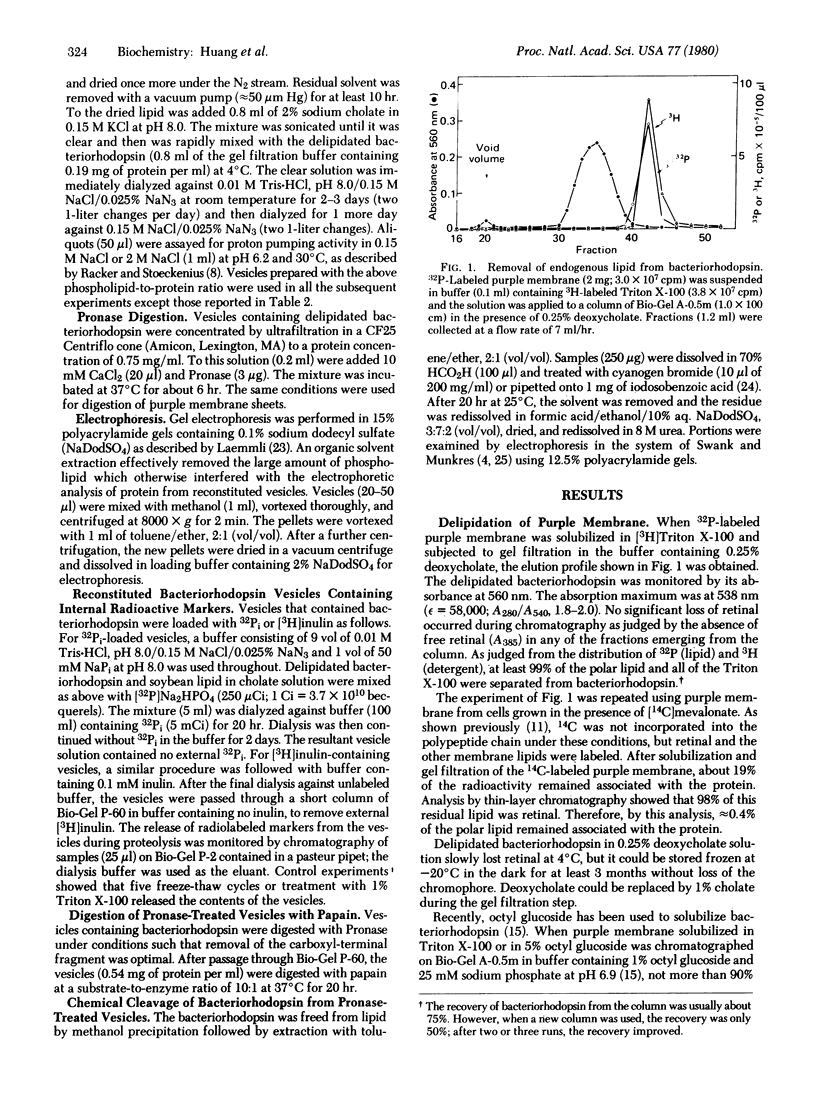
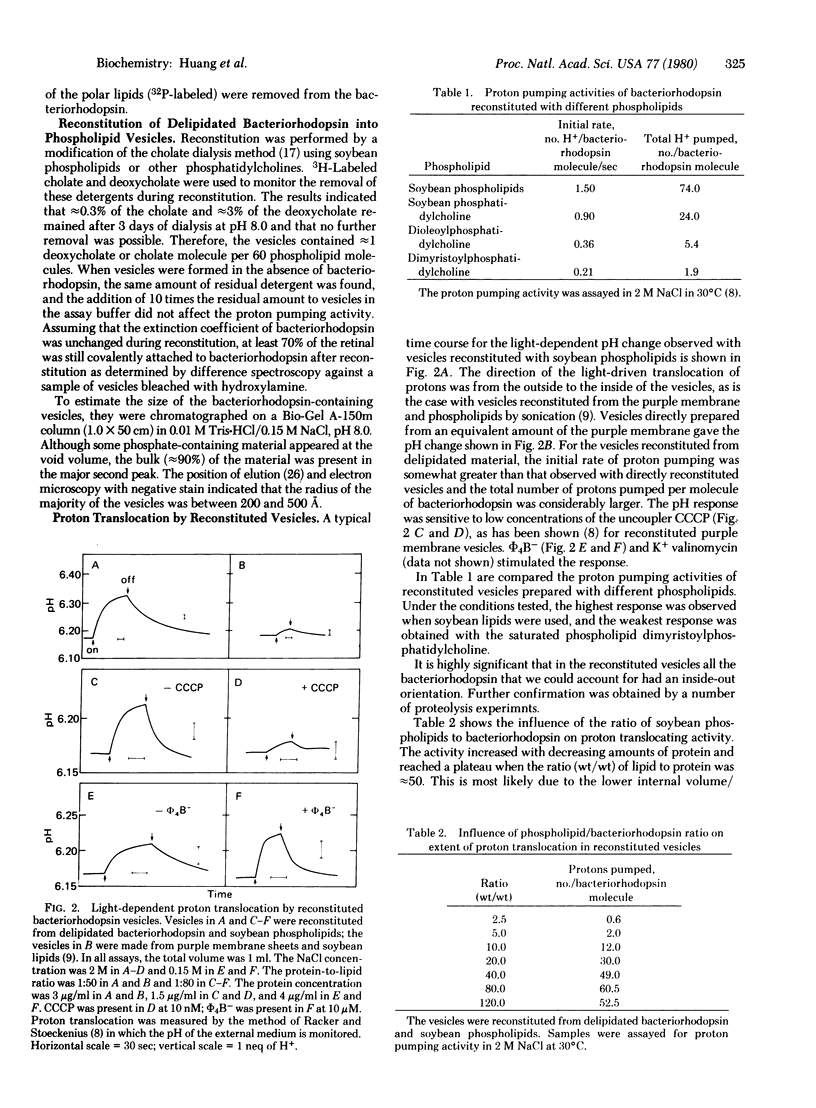


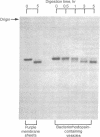
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bakker E. P., Caplan S. R. Phospholipid substitution of the purple membrane. The stoichiometry of light-induced proton release by phospholipid-substituted purple membranes. Biochim Biophys Acta. 1978 Aug 8;503(2):362–379. doi: 10.1016/0005-2728(78)90194-9. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Müller U. Temperature-dependent aggregation of bacteriorhodopsin in dipalmitoyl- and dimyristoylphosphatidylcholine vesicles. J Mol Biol. 1978 May 15;121(2):283–298. doi: 10.1016/s0022-2836(78)80010-2. [DOI] [PubMed] [Google Scholar]
- Dencher N. A., Heyn M. P. Bacteriorhodopsin monomers pump protons. FEBS Lett. 1979 Dec 15;108(2):307–310. doi: 10.1016/0014-5793(79)80552-9. [DOI] [PubMed] [Google Scholar]
- Dencher N. A., Heyn M. P. Formation and properties of bacteriorhodopsin monomers in the non-ionic detergents octyl-beta-D-glucoside and Triton X-100. FEBS Lett. 1978 Dec 15;96(2):322–326. doi: 10.1016/0014-5793(78)80427-x. [DOI] [PubMed] [Google Scholar]
- Gerber G. E., Gray C. P., Wildenauer D., Khorana H. G. Orientation of bacteriorhodopsin in Halobacterium halobium as studied by selective proteolysis. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5426–5430. doi: 10.1073/pnas.74.12.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin S. M., Rhoden V. Reconstitution and "transport specificity fractionation" of the human erythrocyte glucose transport system. A new approach for identification and isolation of membrane transport proteins. J Biol Chem. 1978 Apr 25;253(8):2575–2583. [PubMed] [Google Scholar]
- Happe M., Overath P. Bacteriorhodopsin depleted of purple membrane lipids. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1504–1511. doi: 10.1016/s0006-291x(76)80184-2. [DOI] [PubMed] [Google Scholar]
- Happe M., Teathera R. M., Overath P., Knobling A., Oesterhelt D. Direction of proton translocation in proteoliposomes formed from purple membrane and acidic lipids depends on the pH during reconstitution. Biochim Biophys Acta. 1977 Mar 1;465(2):415–420. doi: 10.1016/0005-2736(77)90092-x. [DOI] [PubMed] [Google Scholar]
- Henderson R. The purple membrane from Halobacterium halobium. Annu Rev Biophys Bioeng. 1977;6:87–109. doi: 10.1146/annurev.bb.06.060177.000511. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Hwang S. B., Stoeckenius W. Purple membrane vesicles: morphology and proton translocation. J Membr Biol. 1977 May 12;33(3-4):325–350. doi: 10.1007/BF01869523. [DOI] [PubMed] [Google Scholar]
- Keana J. F., Roman R. B. Improved synthesis of n-octyl-beta-D-glucoside: a nonionic detergent of considerable potential in membrane biochemistry. Membr Biochem. 1978;1(3-4):323–327. doi: 10.3109/09687687809063854. [DOI] [PubMed] [Google Scholar]
- Khorana H. G., Gerber G. E., Herlihy W. C., Gray C. P., Anderegg R. J., Nihei K., Biemann K. Amino acid sequence of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5046–5050. doi: 10.1073/pnas.76.10.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Martin W. G. Characterization and composition of the purple and red membrane from Halobacterium cutirubrum;. Can J Biochem. 1975 Mar;53(3):284–292. doi: 10.1139/o75-040. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahoney W. C., Hermodson M. A. High-yield cleavage of tryptophanyl peptide bonds by o-iodosobenzoic acid. Biochemistry. 1979 Aug 21;18(17):3810–3814. doi: 10.1021/bi00584a026. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Abdulaev N. G., Feigina M. Y., Kiselev A. V., Lobanov N. A. The structural basis of the functioning of bacteriorhodopsin: an overview. FEBS Lett. 1979 Apr 15;100(2):219–224. doi: 10.1016/0014-5793(79)80338-5. [DOI] [PubMed] [Google Scholar]
- Racker E. A new procedure for the reconstitution of biologically active phospholipid vesicles. Biochem Biophys Res Commun. 1973 Nov 1;55(1):224–230. doi: 10.1016/s0006-291x(73)80083-x. [DOI] [PubMed] [Google Scholar]
- Racker E., Stoeckenius W. Reconstitution of purple membrane vesicles catalyzing light-driven proton uptake and adenosine triphosphate formation. J Biol Chem. 1974 Jan 25;249(2):662–663. [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Warren G. B., Toon P. A., Birdsall N. J., Lee A. G., Metcalfe J. C. Reconstitution of a calcium pump using defined membrane components. Proc Natl Acad Sci U S A. 1974 Mar;71(3):622–626. doi: 10.1073/pnas.71.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenauer D., Khorana H. G. The preparation of lipid-depleted bacteriorhodopsin. Biochim Biophys Acta. 1977 Apr 18;466(2):315–324. doi: 10.1016/0005-2736(77)90227-9. [DOI] [PubMed] [Google Scholar]
- Zingsheim H. P., Neugebauer D. C., Henderson R. Properties of the two sides of the purple membrane correlated. J Mol Biol. 1978 Aug 5;123(2):275–278. doi: 10.1016/0022-2836(78)90326-1. [DOI] [PubMed] [Google Scholar]