Abstract
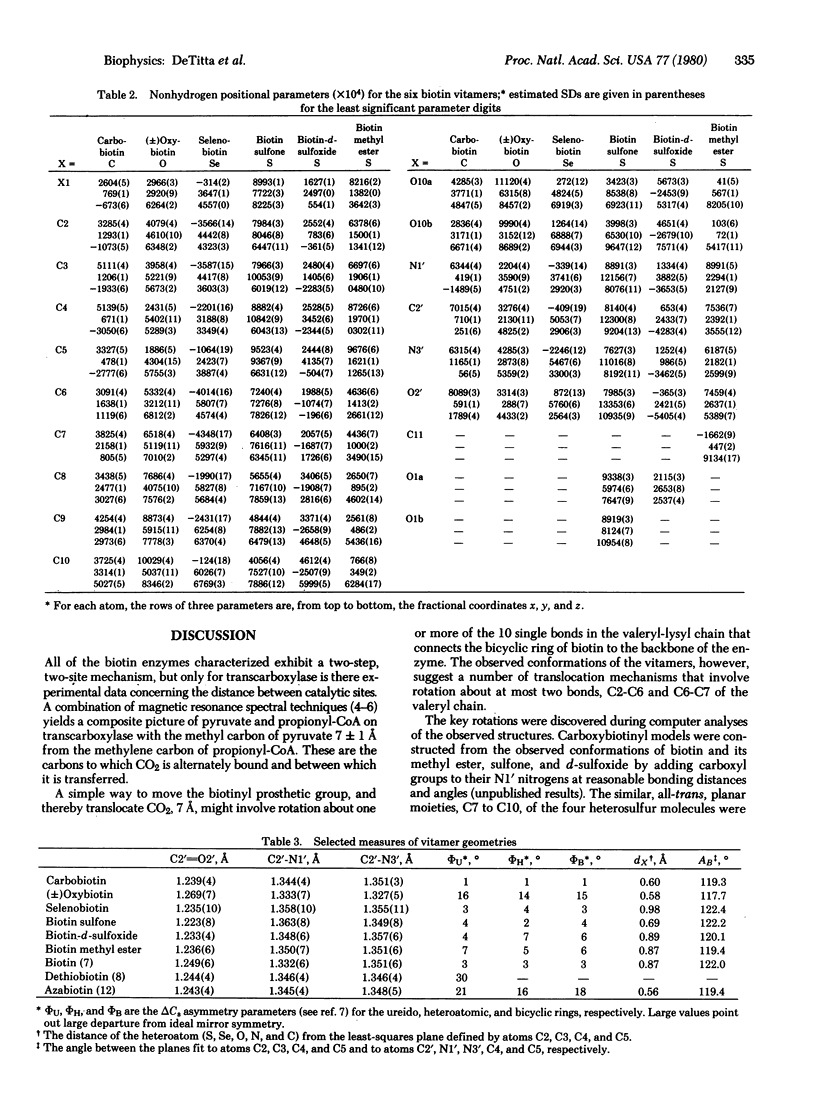
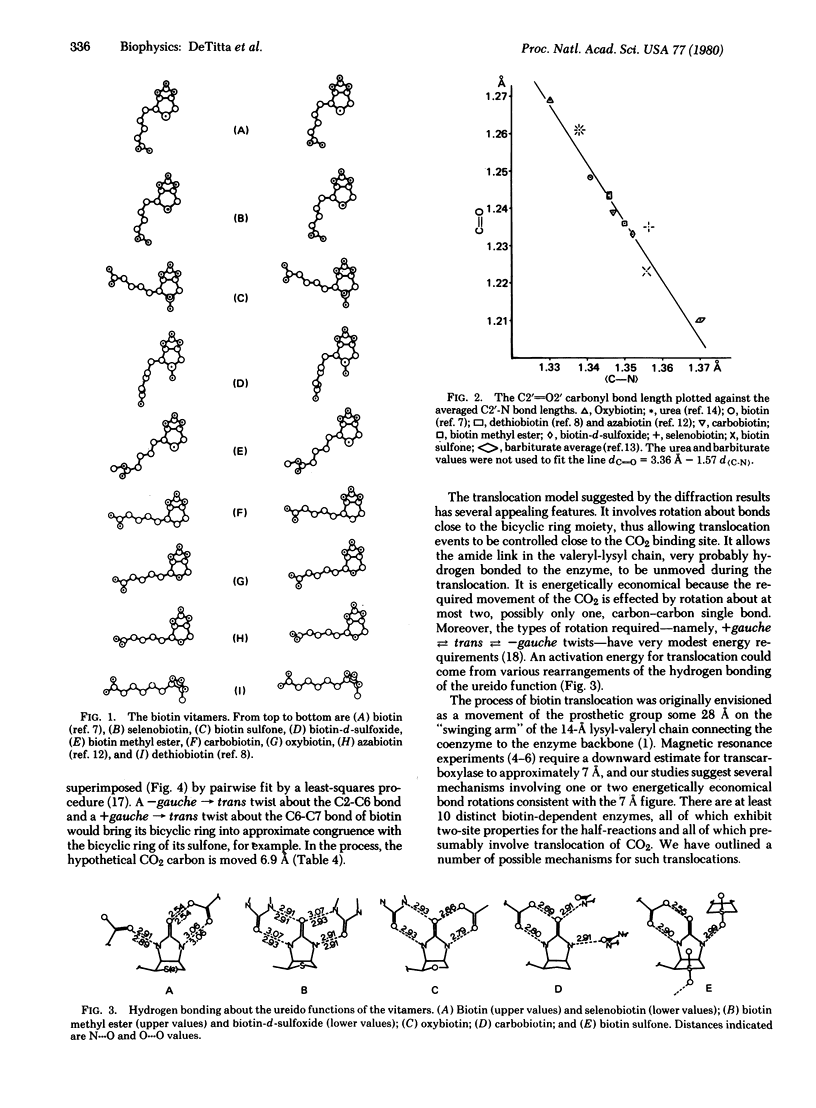
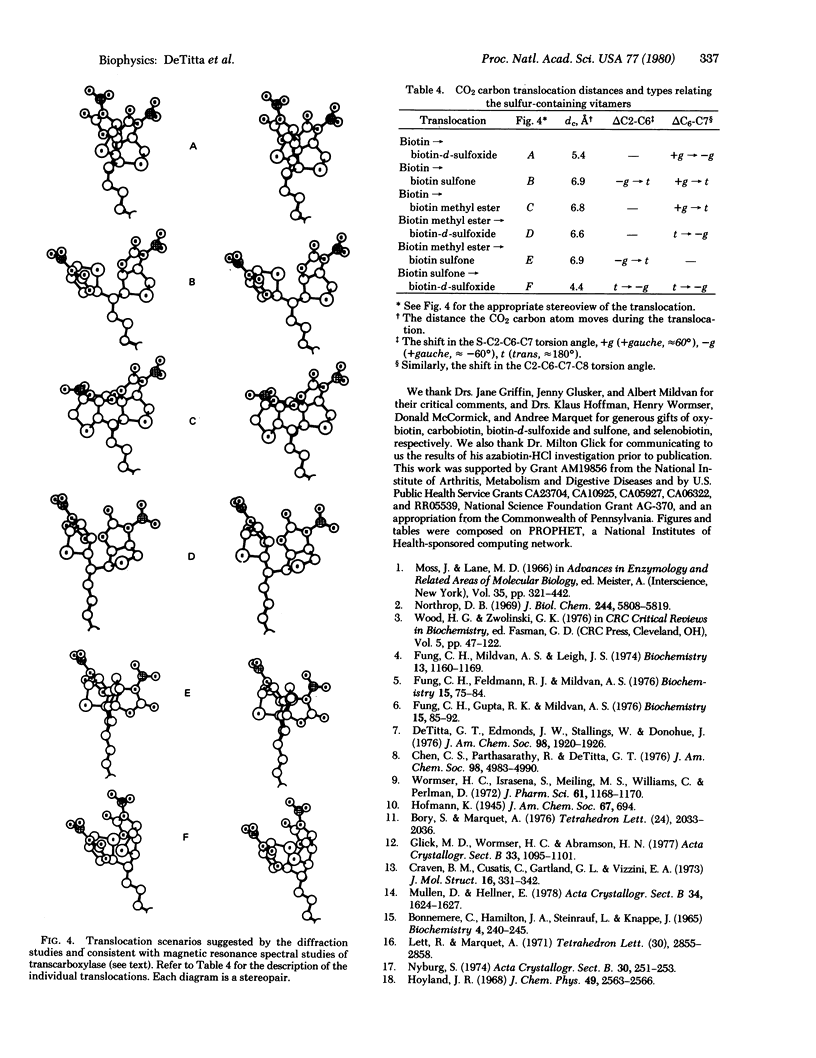
Biotin is a coenzyme that fixes CO2 for transfer in a family of carboxylase, decarboxylase, and transcarboxylase enzymes. Their enzyme reactions involve two basic steps during which a carboxybiotinyl intermediate forms at one site and translocates to a second (distinct) site for CO2 transfer. Our diffraction studies of biotin and its vitamers suggest that translocation involves rotation about one, or at most two, bonds in biotin's valeryl chain. The rotations are energetically economical gauche in equilibrium trans rotations about the two valeryl bonds nearest the biotin bicyclic ring. They move a carbon atom of a CO2 moiety bound at N-1' approximately 7 A, a distance in accord with spectroscopic measurements of one of the biotin enzymes. From our studies we infer that sulfur in biotin imparts to the valeryl chain a conformational variability necessary for bond rotation and, hence, translocation between catalytic sites.
Full text
PDF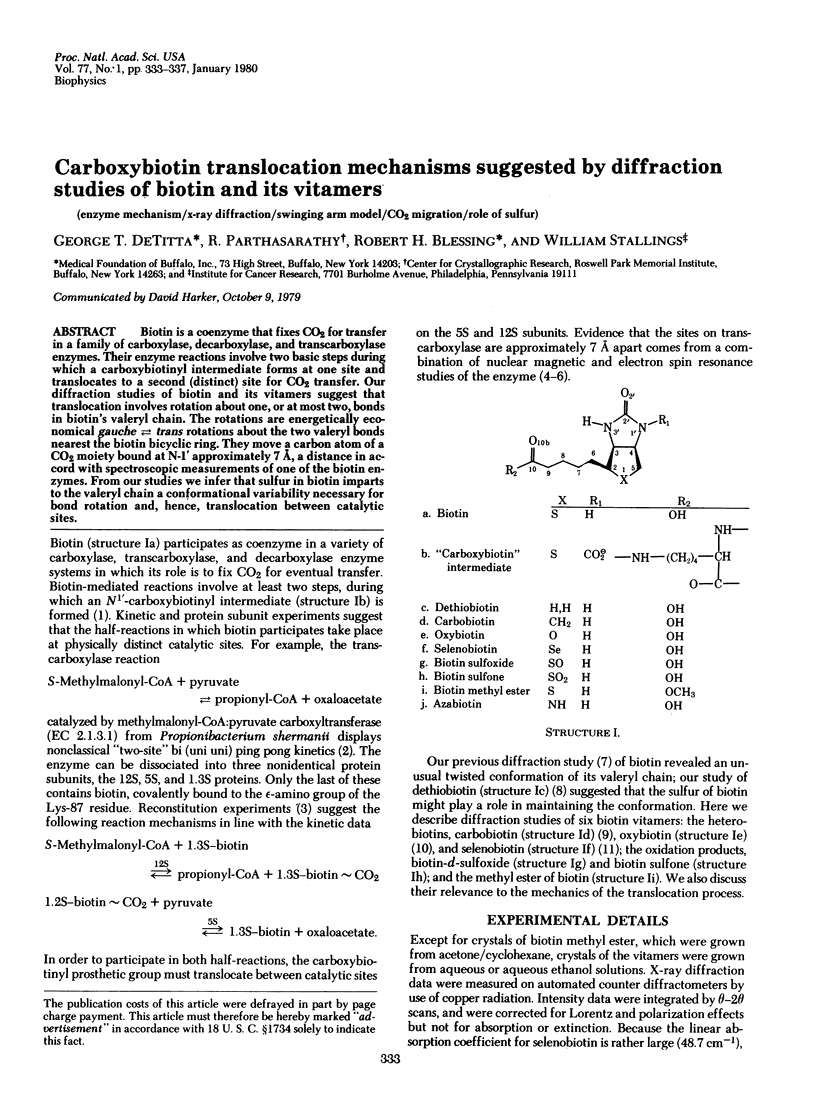

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen C. S., Parthasarathy R., De Titta G. T. Crystal and molecular structure of d, l-dethiobiotin. Role of sulfur in biotin stereochemistry. J Am Chem Soc. 1976 Aug 4;98(16):4983–4990. doi: 10.1021/ja00432a045. [DOI] [PubMed] [Google Scholar]
- DeTitta G. T., Edmonds J. W., Stallings W., Donohue J. Molecular structure of biotin. Results of two independent crystal structure investigations. J Am Chem Soc. 1976 Mar 31;98(7):1920–1926. doi: 10.1021/ja00423a045. [DOI] [PubMed] [Google Scholar]
- Fung C. H., Feldmann R. J., Mildvan A. S. 1H and 31P Fourier transform magnetic resonance studies of the conformation of enzyme-bound propionyl coenzyme A on the transcarboxylase. Biochemistry. 1976 Jan 13;15(1):75–84. doi: 10.1021/bi00646a013. [DOI] [PubMed] [Google Scholar]
- Fung C. H., Gupta R. K., Mildvan A. S. Magnetic resonance studies of the proximity and spatial arrangement of propionyl coenzyme A and pyruvate on a biotin-metalloenzyme, transcarboxylase. Biochemistry. 1976 Jan 13;15(1):85–92. doi: 10.1021/bi00646a014. [DOI] [PubMed] [Google Scholar]
- Fung C. H., Mildvan A. S., Leigh J. S., Jr Electron and nuclear magnetic resonance studies of the interaction of pyruvate with transcarboxylase. Biochemistry. 1974 Mar 12;13(6):1160–1169. doi: 10.1021/bi00703a017. [DOI] [PubMed] [Google Scholar]
- Glick D. The contribution of microchemical methods of histochemistry to the biological sciences. J Histochem Cytochem. 1977 Sep;25(9):1087–1101. doi: 10.1177/25.9.198475. [DOI] [PubMed] [Google Scholar]
- Northrop D. B. Transcarboxylase. VI. Kinetic analysis of the reaction mechanism. J Biol Chem. 1969 Nov 10;244(21):5808–5819. [PubMed] [Google Scholar]
- Wormser H. C., Israsena S., Heiling M. S., Williams C., Perlman D. Synthesis and growth-promoting activity of dl-cis-hexahydro-4-(4-carboxybutyl)-2-cyclopentimidazolone: carbobiotin. J Pharm Sci. 1972 Jul;61(7):1168–1170. doi: 10.1002/jps.2600610731. [DOI] [PubMed] [Google Scholar]


