Abstract
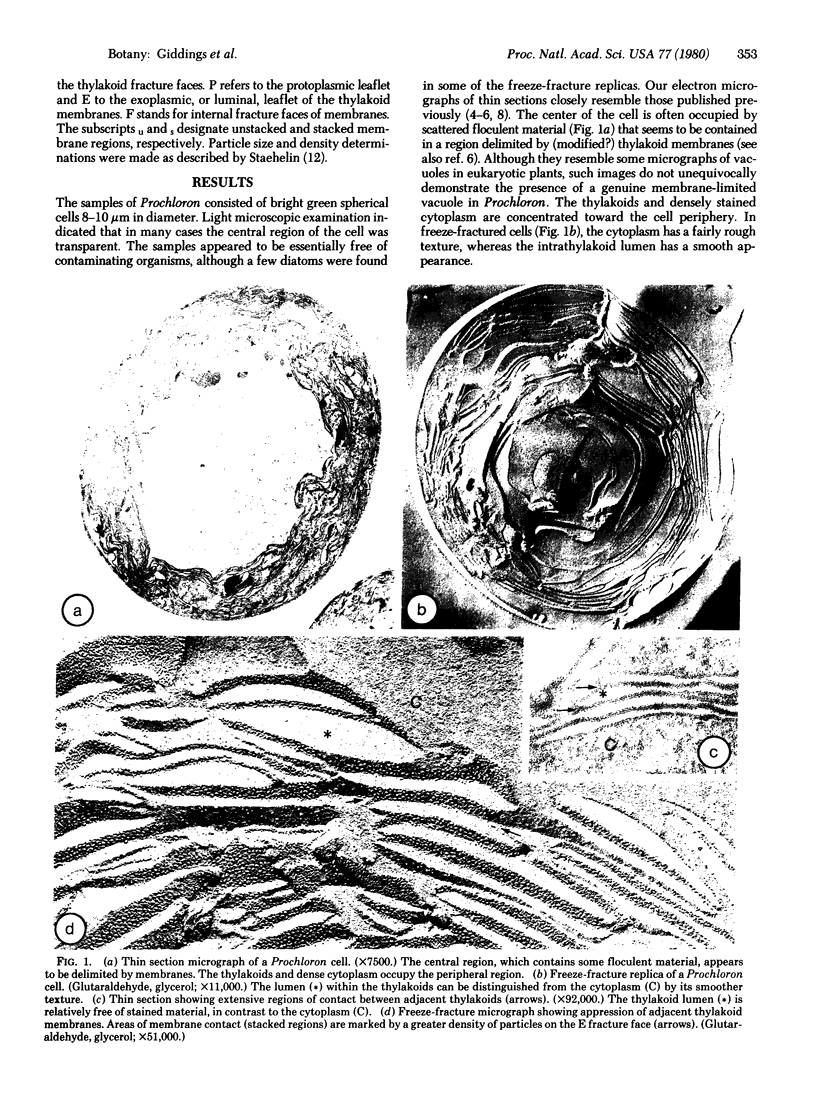
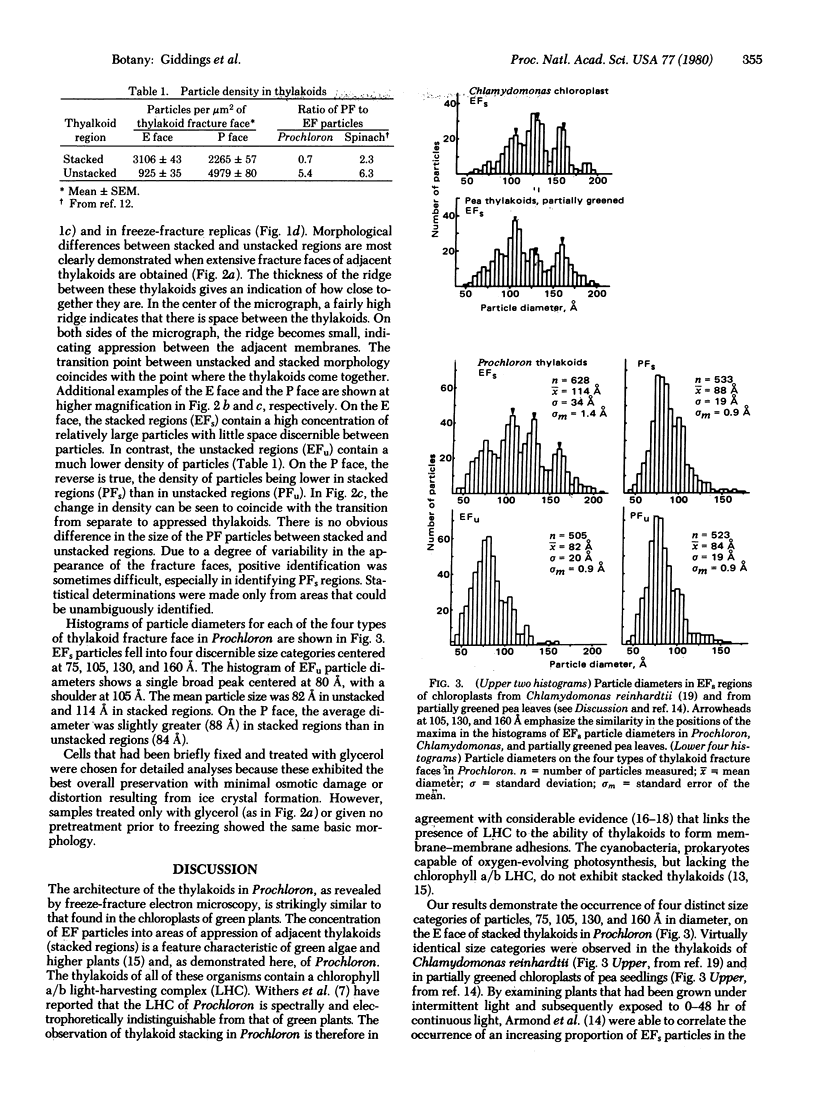
Freeze-fracture replicas of the photosynthetic prokaryote Prochloron sp., collected at Coconut Island, Hawaii, show that the thylakoids are differentiated into stacked and unstacked regions much like the thylakoids of green algae and higher plants. On the exoplasmic (E) fracture face, the particle density is greater in stacked regions (≈3100 particles/μm2) than in unstacked regions (≈925 particles/μm2). On the complementary protoplasmic (P) fracture face, the particle density is lower in stacked regions (≈2265 particles/μm2 than in unstacked regions (≈4980 particles/μm2). The histogram of the diameters of E face particles in unstacked regions shows a single broad peak centered at 80 Å. In stacked regions, four distinct peaks, at 75, 105, 130, and 160 Å, are observed. These size classes are virtually identical to those found on E faces of thylakoids of the green alga Chlamydomonas and of greening pea chloroplasts. In the latter systems, the different size classes of E face particles are believed to represent photosystem II units surrounded by variable amounts of chlorophyll a/b light-harvesting complex. We propose that the same interpretation applies to the thylakoids of Prochloron, which contain a similar chlorophyll a/b complex. Our results add to the evidence supporting the view of the chlorophyll a/b complex as both a light-harvesting complex and a membrane adhesion factor. The similarity of the architecture of the thylakoids of Prochloron to that of green algal and plant chloroplasts also provides additional evidence of an evolutionary relationship between Prochloron and the chloroplasts of green plants.
Keywords: photosynthesis, thylakoids, chlorophyll a/b protein, freeze-fracture
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. The molecular organization of chloroplast thylakoids. Biochim Biophys Acta. 1975 Aug 15;416(2):191–235. doi: 10.1016/0304-4173(75)90007-5. [DOI] [PubMed] [Google Scholar]
- Armond P. A., Staehelin L. A., Arntzen C. J. Spatial relationship of photosystem I, photosystem II, and the light-harvesting complex in chloroplast membranes. J Cell Biol. 1977 May;73(2):400–418. doi: 10.1083/jcb.73.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Giddings T. H., Jr, Staehelin L. A. Changes in thylakoid structure associated with the differentiation of heterocysts in the cyanobacterium, Anabaena cylindrica. Biochim Biophys Acta. 1979 Jun 5;546(3):373–382. doi: 10.1016/0005-2728(79)90074-4. [DOI] [PubMed] [Google Scholar]
- Lewin R. A. Prochlorophyta as a proposed new division of algae. Nature. 1976 Jun 24;261(5562):697–698. doi: 10.1038/261697b0. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A. Reversible particle movements associated with unstacking and restacking of chloroplast membranes in vitro. J Cell Biol. 1976 Oct;71(1):136–158. doi: 10.1083/jcb.71.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Cohen-Bazire G. Phototrophic prokaryotes: the cyanobacteria. Annu Rev Microbiol. 1977;31:225–274. doi: 10.1146/annurev.mi.31.100177.001301. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Alberte R. S., Hunter F. A., Shiozawa J. A., Kan K. S. The organization of chlorophyll in the plant photosynthetic unit. Brookhaven Symp Biol. 1976 Jun 7;(28):132–148. [PubMed] [Google Scholar]
- Thorne S. W., Newcomb E. H., Osmond C. B. Identification of chlorophyll b in extracts of prokaryotic algae by fluorescence spectroscopy. Proc Natl Acad Sci U S A. 1977 Feb;74(2):575–578. doi: 10.1073/pnas.74.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers N. W., Alberte R. S., Lewin R. A., Thornber J. P., Britton G., Goodwin T. W. Photosynthetic unit size, carotenoids, and chlorophyll-protein composition of Prochloron sp., a prokaryotic green alga. Proc Natl Acad Sci U S A. 1978 May;75(5):2301–2305. doi: 10.1073/pnas.75.5.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman F. A. Ultrastructural Comparison of Cyanidium caldarium Wild Type and III-C Mutant Lacking Phycobilisomes. Plant Physiol. 1979 Feb;63(2):375–381. doi: 10.1104/pp.63.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]




