Abstract
CD4+ Foxp3+ regulatory T cells inhibit the production of interferon-γ, which is the major mediator of protection against Mycobacterium tuberculosis infection. In this study, we evaluated whether the protection conferred by three different vaccines against tuberculosis was associated with the number of spleen and lung regulatory T cells. We observed that after homologous immunization with the 65 000 molecular weight heat-shock protein (hsp 65) DNA vaccine, there was a significantly higher number of spleen CD4+ Foxp3+ cells compared with non-immunized mice. Heterologous immunization using bacillus Calmette–Guérin (BCG) to prime and DNA-hsp 65 to boost (BCG/DNA-hsp 65) or BCG to prime and culture filtrate proteins (CFP)-CpG to boost (BCG/CFP-CpG) induced a significantly higher ratio of spleen CD4+/CD4+ Foxp3+ cells compared with non-immunized mice. In addition, the protection conferred by either the BCG/DNA-hsp 65 or the BCG/CFP-CpG vaccines was significant compared with the DNA-hsp 65 vaccine. Despite the higher ratio of spleen CD4+/CD4+ Foxp3+ cells found in BCG/DNA-hsp 65-immunized or BCG/CFP-CpG-immunized mice, the lungs of both groups of mice were better preserved than those of DNA-hsp 65-immunized mice. These results confirm the protective efficacy of BCG/DNA-hsp 65 and BCG/CFP-CpG heterologous prime-boost vaccines and the DNA-hsp 65 homologous vaccine. Additionally, the prime-boost regimens assayed here represent a promising strategy for the development of new vaccines to protect against tuberculosis because they probably induce a proper ratio of CD4+ and regulatory (CD4+ Foxp3+) cells during the immunization regimen. In this study, this ratio was associated with a reduced number of regulatory cells and no injury to the lungs.
Keywords: CD4+ Foxp3+, DNA vaccine, heterologous immunization, regulatory T cell, tuberculosis
Introduction
Effector CD4+ lymphocytes are divided into at least three different subpopulations: T helper type 1 (Th1), Th2 and Th17 cells. The functions of each of these groupings are highly specialized and are controlled by regulatory CD4+ T cells. Regulatory T cells are further classified into three different populations that inhibit either the innate or the adaptive immune response.1 Regulatory T1 cells secrete interleukin-10 (IL-10), Th3 cells secrete transforming growth factor-β (TGF-β) and CD4+ Foxp3+ T cells may play different roles.2–4 CD4+ Foxp3+ cells secrete IL-10 and/or TGF-β and suppress the activation of T lymphocytes, macrophages and dendritic cells.5–7 They also express high levels of cytotoxic T-lymphocyte antigen-4. This protein's interaction with the B7 molecule triggers the inhibition of antigen-presenting cell function.5,7,8 High levels of CD25, the α-chain of the high-affinity receptor for IL-2 that is expressed on the membrane surface of CD4+ Foxp3+ cells promotes the sequestration of IL-2 and inhibits the proliferation of T cells.9 There are many other mechanisms that CD4+ Foxp3+ cells use to inhibit T cells and antigen-presenting cells, such as cytotoxicity, the induction of the indoleamine 2,3-dioxygenase enzyme and CD39 and CD73 expression.8,10
Regulatory T cells were first described in autoimmune processes where the absence of CD25+ cells was associated with the development of autoimmune disease.11–14 The description of the Foxp3 transcription factor indicated that it was a key protein involved in the development, differentiation and characterization of regulatory T cells.15–18 Besides autoimmune diseases, CD4+ Foxp3+ cells were found to play a role in various other infectious diseases, such as tuberculosis. An increase in the number of circulating regulatory T cells in tuberculosis patients was first described in 2006.19,20 The increase in the frequency of these cells was directly associated with the inhibition of interferon-γ (IFN-γ) secretion. The treatment of patients infected with Mycobacterium tuberculosis resulted in a decrease in the number of regulatory T cells and restored the production of IFN-γ.20,21 In experimental tuberculosis model, the depletion of Foxp3+ cells in infected C57BL/6 mice resulted in fewer bacteria (measured as colony-forming units) in the lungs compared with mice with Foxp3+ cells.22 While C57BL/6 mice exhibited an increased magnitude of their Th1 response and a lower effector function of their regulatory T cells, BALB/c mice had a lower magnitude of Th1 response and effector function of their regulatory T cells that suppressed IL-2 and IFN-γ secretion. This finding suggested that regulatory T cells may potentially represent a susceptibility factor in tuberculosis.23 Recently, it was reported that regulatory T cells delayed the migration of effector CD4+ and CD8+ cells to the lungs of infected mice.24
Tuberculosis remains a priority for public health. Each year, 1·1 million individuals die from this disease.25 Intense efforts have been concentrated in the development of new vaccines against tuberculosis. During decades of research, an effective immune response against tuberculosis should be characterized by the induction of IFN-γ-producing Th1 cells. Recently, the immunogenicity of vaccines has also begun to be analysed to better characterize the participation of Th17 and regulatory T cells.26,27
For years, we have worked with an experimental model of M. tuberculosis infection in an attempt to study vaccines against tuberculosis. We have used protein and microbial adjuvant, DNA vaccine and prime-boost heterologous immunization to improve vaccine-mediated protection against M. tuberculosis infection.
In this study, we addressed the question of whether different vaccines designed to induce protection against experimental tuberculosis would be able to induce CD4+ Foxp3+ regulatory T cells. In addition, we compared the protective efficacy with the frequency of regulatory T cells in the lungs of immunized and challenged mice.
Materials and methods
Mice
Specific-pathogen-free, female BALB/c mice were bred in the Animal Facility of the School of Medicine of Ribeirão Preto, University of São Paulo, maintained under barrier conditions in a Level III biohazard laboratory and used at 6–8 weeks of age.
The use of animals in this study was approved by the Ethics Committee on Animal Experimentation, protocol no. 056/2007.
M. tuberculosis antigens
Mycobacterium tuberculosis culture filtrate proteins (CFP) were provided by Gilles Marchal, Pasteur Institute, Paris, France and sonicated M. tuberculosis (Mtb antigen) was obtained from H37Rv strain (ATCC 27294; American Type Culture Collection, Rockville, MD). Briefly, mycobacteria were cultured as previously described,28 heat-killed, sonicated for 15 min and centrifuged at 5000 g for 30 min. The supernatant was sterilized by filtration and the protein concentration was quantified with the Coomassie Plus assay kit (Pierce, Rockford, IL).
CpG oligodeoxynucleotides
The CpG oligodeoxynucleotides 1826 were synthesized by Invitrogen (Invitrogen Corp., Custom Primers, Carlsbad, CA) at a 1-μmol scale with a phosphorothioate link in all bases except the last one, according to the following sequence: 5′-TCC ATG ACG TTC CTG ACG TT-3′.
Plasmid construction and immunizations
The DNA vaccine pVAX-hsp 65 (i.e. 65 000 molecular weight heat-shock protein) (DNA-hsp 65) was derived from the pVAX vector (Invitrogen) and was constructed as described previously.28 For the DNA vaccination, 50 μg DNA-hsp 65 in 50 μl saline solution plus 12·5% sucrose was injected into each quadriceps muscle (a total of 100 μg per dose) for three doses at 15-day intervals. For the heterologous, prime-boost immunization regimen, a single dose of bacillus Calmette–Guérin [BCG; 2 × 105 colony-forming units (CFU)] in 100 μl pyrogen-free saline was administered by the subcutaneous (s.c.) route and after 15 days a single dose of the DNA-hsp 65 vaccine (100 μg) was administered by the intramuscular (i.m.) route. In the second strategy of prime-boost heterologous immunization, the mice received the first immunization with subcutaneous (s.c.) BCG, as described previously, and after 15 days received a single dose of s.c. CFP (50 μg) and CpG (50 μg) at a final volume of 100 μl. The dose of 50 μg CFP and 50 μg CpG was standardized previously.29 Control groups were as follows: a group that received an equal volume of saline; a group immunized with a single dose of s.c. BCG (2 × 105 CFU) and a group that received the pVAX plasmid without the hsp 65 gene.
To evaluate the number of CD4+ Foxp3+ cells in the spleens of different groups of immunized mice, recombinant hsp 65 protein was administered by the intravenous route 15 days after the completion of the immunization schedule with either the DNA-hsp 65 vaccine or the BCG/DNA-hsp 65 vaccine.
Bacteria and infection
The M. tuberculosis suspension was prepared and inoculated by the intratracheal route (1 × 105/mouse) as previously described.30
CFU determination
Thirty days after infection, the lower and medium right lobes of the lungs were cut into small pieces and digested with Liberase Blendzyme 2 (0·5 μg/ml; Roche, Indianapolis, IN) and deoxyribonuclease I (25 U/ml; GIBCO BRL, Grand Island, NY) diluted in incomplete RPMI-1640. Samples were incubated at 37° for 30 min and serial dilutions of the digested lobes were plated onto supplemented 7H11 agar media (BD Bioscience, Franklin Lakes, NJ).23 The number of CFU was determined 30 days after incubation at 37°.
Flow cytometry
The lung cell suspension was passed through a filter to remove pieces of non-digested lung, centrifuged and resuspended in complete RPMI-1640. Spleens were homogenized, and the spleen cell suspensions were resuspended in RPMI-1640 (Sigma-Aldrich, St Louis, MO). Lung (5 × 105 to 1 × 107) and spleen cells (1 × 106) were incubated for 40 min at 4° with Fc Block (1 μg/1 × 106 cells; BD Bioscience, Franklin Lakes, NJ). Cells were then incubated with anti-CD4 (clone RM4-5) for 30 min at 4° in total darkness. After staining for surface protein, the staining of Foxp3 was performed using the FITC anti-mouse/rat Foxp3 Staining kit (eBioscience, San Diego, CA) according to the manufacturer's instructions. The cells were analysed using flow cytometry with the FACSCanto™ (Becton Dickinson and Company-Immunocytometry Systems, San Jose, CA). The frequency of each cell population (CD4+ and CD4+ Foxp3+) was calculated in lymphocytes gated by size and granularity. The absolute number of the cell population (CD4+ or CD4+ Foxp3+) was calculated by the percentage of total events acquired in the samples and the total number of cells obtained from the Neubauer chamber.
Histology
For histopathological analysis, the right upper lobes of the lungs were fixed in 10% formalin, embedded in paraffin blocks, prepared routinely and then sectioned for light microscopy. Five-micrometre sections were stained with haematoxylin & eosin.
Delayed-type hypersensitivity (DTH)
Regulatory T cells (CD4+ CD25+) were purified from the spleen of non-immunized, immunized (15 days after immunization), infected (30 days after infection) or uninfected mice with the CD4+ CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The cells were resuspended (5 × 105 to 6 × 105 cells) with 50 μg sonicated M. tuberculosis (Mtb Ag) in 40 μl endotoxin-free sterile saline and injected in the right paw. As a control, mice were injected with 40 μl saline in the left paw or mice were injected only with Mtb antigen. The footpads were measured with a precision micrometer at 0, 24, 48 and 72 hr after the administration of antigen and CD4+ CD25+ cells. The Δ DTH was quantified by measuring the difference between the footpad thickness over time.
Anti-CD25 treatment
Mice were injected intraperitoneally with 500 µg rat IgG1 anti-mouse CD25 monoclonal antibody (mAb) (PC61) or rat immunoglobulin G (immunoglobulin control), kindly donated by Dr João Santana da Silva (FMRP-USP). Either the PC61 mAb or the immunoglobulin control was administered 3 days before each immunization and 3 days before infection. Depletion was assessed using flow cytometry of peripheral blood samples after the second administration of the PC61 mAb.
Statistical analysis
All values are expressed as the mean ± standard error of the mean (SEM). The PRISMA software (Graph Pad software, Inc., San Diego, CA) was employed for all analyses. We used the Kolmogorov–Smirnov test and verified that the values were in a Gaussian distribution. Next we used one-way analysis of variance and Newman–Keuls multiple comparison test. A value of P < 0·05 was considered significant. The correlation between the ratio of CD4+/CD4+ Foxp3+ cells and CFU counts, and the number of CD4+ Foxp3+ cells and CFU counts were determined by the Pearson′s correlation coefficient.
Results
DNA-hsp 65 immunization, but not prime-boost strategies, induced the expansion of spleen CD4+ Foxp3+ cells
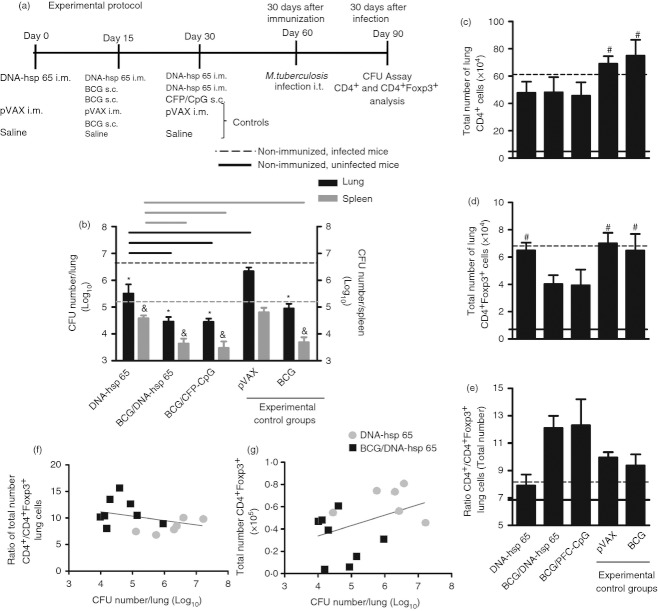
The experimental protocol in Fig. 1(a) describes the following three vaccines used: DNA-hsp 65, BCG/DNA-hsp 65 and BCG/CFP-CpG. Subcutaneous BCG was used as the gold standard. In addition to BCG, we used pVAX as an experimental control group. First, we evaluated the number of spleen CD4+ cells after immunization. DNA-hsp 65, BCG/DNA-hsp 65 and BCG/CFP-CpG vaccinations induced an increase in the number of CD4+ cells compared with non-immunized mice (black dotted line). In addition, the higher number of CD4+ cells induced by DNA-hsp 65 immunization was significant compared with the BCG-immunized group (Fig. 1b). There was also no difference in the frequency of spleen CD4+ cells (data not shown). Immunization with the DNA-hsp 65 vaccine stimulated an increase in the number of spleen CD4+ Foxp3+ cells compared with the non-immunized (grey dotted line), BCG prime DNA-hsp 65 boost and BCG groups (Fig. 1b). The frequency of these cells was similar among the different experimental groups (data not shown). We determined the ratio of CD4+ to CD4+ Foxp3+ cells and found that the heterologous prime-boost vaccination strategies (BCG/DNA-hsp 65 and BCG/CFP-CpG) elicited a significant increase in the ratio of CD4+/CD4+ Foxp3+ cells, but these regimens did not increase the total cell number (Fig. 1c) compared with other groups. Therefore, DNA- hsp 65 was the vaccine that predominantly induced a higher number of CD4+ Foxp3+ regulatory T cells, whereas the three vaccines DNA-hsp 65, BCG/DNA-hsp 65 and BCG/CFP-CpG induced a significant increase of spleen CD4+ cells. Indeed, the change in the ratio of CD4+/CD4+ Foxp3+ cells revealed that the heterologous prime-boost immunization was more effective in inducing a frequency of CD4+ cells significantly higher than the frequency of CD4+ Foxp3+ cells compared with non-immunized mice and the BCG-immunized group. Spleen cell cultures showed that the immunization with DNA-hsp 65, BCG/DNA-hsp 65 and BCG/CFP-CpG preferentially induced antigen-specific IFN-γ secretion instead of IL-10 (data not shown).
Figure 1.
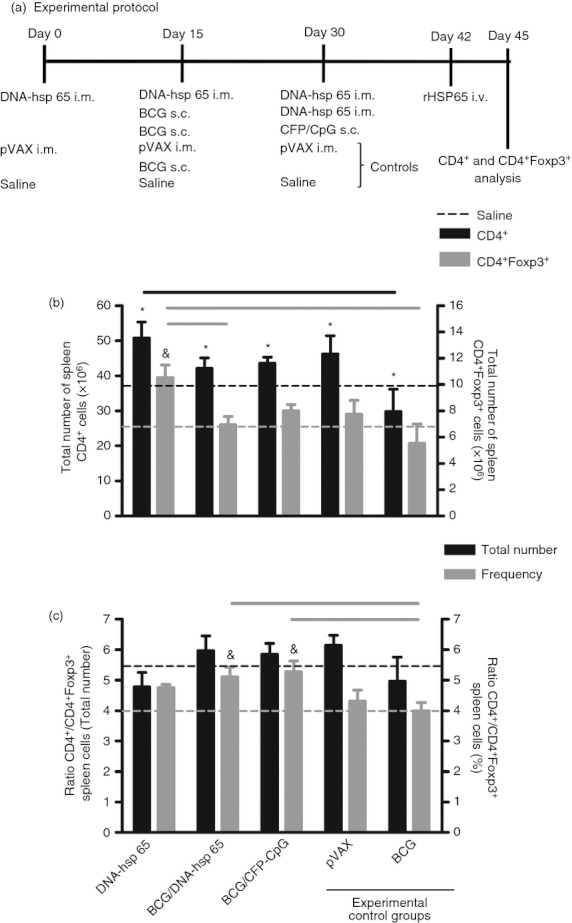
Total number and frequency of spleen CD4+ and CD4+ Foxp3+ cells from immunized mice. BALB/c mice were immunized with the DNA-heat-shock protein 65 (hsp 65), BCG/DNA-hsp 65 and BCG/CFP-CpG vaccines. Mice were immunized with BCG or injected with pVAX plasmid or saline as experimental controls. Fifteen days after the completion of the immunization schedule, the total number and frequency of effector (CD4+) and regulatory (CD4+Foxp3+) T cells were evaluated in the spleen (a). Spleen cells were first gated by size (forward scatter) and granularity (side scatter) and then by CD4 and Foxp3 expression. The results are expressed as the means ± SEM of individual animals analysed in each different group. Total number of spleen CD4+ cells and total number of spleen CD4+ Foxp3+ cells (b). Ratio of total CD4+/CD4+ Foxp3+ cells and ratio of the frequency of CD4+/CD4+ Foxp3+ cells (c). The total number of cells is representative of two independent experiments with the following experimental n: DNA-hsp 65, 6; BCG/DNA-hsp 65, 8; BCG/CFP-CpG, 6; pVAX, 7; BCG, 4. The cell frequency data are representative of three independent experiments (DNA-hsp 65, 8; BCG/DNA-hsp 65, 8; BCG/CFP-CpG, 6; pVAX, 10; BCG: 12). *P < 0·05 compared with non-immunized mice (black dotted line). &P < 0·05 compared with non-immunized mice (grey dotted line). Horizontal bars represent the significant difference between groups.
Lower numbers of lung CD4+ Foxp3+ cells were correlated with a stronger protection conferred by vaccination
Different groups of mice were immunized and challenged with M. tuberculosis according to the experimental protocol (Fig. 2a). In the lungs, all different vaccines studied were protective compared with the non-immunized, infected group (black dotted line; Fig. 2b). However, the BCG/DNA-hsp 65 and BCG/CFP-CpG immunizations induced a significant decrease in lung CFU counts compared with the DNA-hsp 65 vaccine. A similar reduction in CFU counts was also observed in the spleens of immunized and challenged mice compared with the non-immunized, infected mice group (grey dotted line; Fig. 2b). Next, we quantified the number of CD4+ and CD4+ Foxp3+ regulatory T cells in the lungs of immunized and challenged mice and verified that there was no significant difference in the number of both cell populations (Fig. 2c,d). Despite the lower number of CD4+ Foxp3+ cells in the lungs of BCG/DNA-hsp 65- and BCG/CFP-CpG-immunized mice, this was not significantly different from non-immunized, infected mice, DNA-hsp 65 immunized mice or BCG-immunized mice (Fig. 2d). We also determined the ratio of lung CD4+/CD4+ Foxp3+ cells and verified that no significant difference existed among the different groups, despite the higher ratio CD4+/CD4+ Foxp3+ cells observed in the lungs of BCG/DNA-hsp 65- and BCG/CFP-CpG-immunized groups. These results show a tendency of the prime-boost immunization strategies to expand and recruit more CD4+ cells than CD4+ Foxp3+ cells to the lungs of challenged mice (Fig. 2e). We also established correlations between the ratio of total number of lung CD4+/CD4+ Foxp3+ cells and CFU counts, and between the total number of lung CD4+ Foxp3+ cells and CFU counts. We observed a moderate negative correlation (Pearson′s correlation, r = −0·356, P = 0·212) between the ratio of CD4+/CD4+ Foxp3+ cells and CFU counts (Fig. 2f) and a moderate positive correlation (Pearson′s correlation, r = 0·404, P = 0·152) between CD4+ Foxp3+ cells and CFU counts (Fig. 2g). These data suggest that a lower number of lung CD4+ Foxp3+ cells is associated with the protection conferred by vaccines against tuberculosis.
Figure 2.
Number of colony-forming units (CFU) in the lung and spleen and correlations of CFU, CD4+/CD4+ Foxp3+ ratio and CD4+ Foxp3+ cells from immunized mice. Different groups of mice were challenged with 1 × 105 bacilli of Mycobacterium tuberculosis 30 days after immunization (a). Lung and spleen CFU counts were evaluated on day 30 post challenge (b). The total number of lung CD4+ (c) and total number of lung CD4+ Foxp3+ cells (d) are shown. The ratio of CD4+/CD4+ Foxp3+ cells (e), the ratio of lung CFU counts and CD4+/CD4+ Foxp3+ cells (f), the ratio of lung CFU counts and total number of CD4+Foxp3+ cells (g) are shown. The cell number is expressed as the mean ± SEM for individual animals. The CFU counts are expressed as Log10 of the mean ± SEM for individual animals. The data are representative of two independent experiments (DNA-heat-schock protein (hsp) 65, 7; BCG/DNA-hsp 65, 7; BCG/CFP-CpG, 8; pVAX, 4; BCG, 7). *P < 0·05 compared with non-immunized and infected mice (black dotted line); &P < 0·05 compared with non-immunized and infected mice (grey dotted line); #P < 0·05 compared with non-immunized, uninfected mice (solid line). Horizontal bars represent the significant difference between groups.
BCG/DNA-hsp 65-immunized mice had better preserved lungs than DNA-hsp 65-, BCG/CFP-CpG- and BCG-immunized mice
We also evaluated the preservation of lung parenchyma in non-immunized, infected mice and in the different groups of immunized, challenged mice (Fig. 3). Experimental controls indicated that after 30 days of infection there was a multifocal and diffuse inflammation in the lungs of non-immunized, infected mice. The lungs of infected mice were characterized by the presence of foamy macrophages, few neutrophils and hyperplasia of the bronchus-associated lymphoid tissue (BALT), which indicated a higher number of lymphocytes compared with the uninfected group. The lungs of the DNA-hsp 65-vaccinated mice (DNA-hsp 65) were similar to the infected group and had a higher number of neutrophils compared with the lungs of infected mice. The lungs of the control immunization group, which received pVAX vector (pVAX) injection, were characterized by higher inflammation compared with the DNA-hsp 65-immunized mice. The lungs of the BCG/CFP-CpG-immunized mice (BCG/CFP-CpG) exhibited a discrete inflammatory infiltrate, reduction in BALT and a decrease in the number of foamy macrophages compared with the lungs of the infected group and the DNA-hsp 65-immunized mice. BCG-immunized mice were similar to the BCG/CFP-CpG-immunized mice. Mice immunized with BCG prime and DNA-hsp 65 boost (BCG/DNA-hsp 65) had the greatest preservation of the lungs: decreased BALT, a reduction in foamy macrophages and neutrophils and lower cellular influx compared with the infected group and the BCG-immunized group. These results suggest that while the BCG/DNA-hsp 65 vaccine induced a high ratio of spleen CD4+/CD4+ Foxp3+ cells, this ratio seems to be a key determinant in preventing disease progression and reducing tissue damage.
Figure 3.
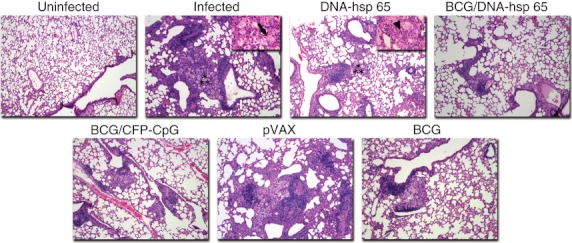
Histological analysis of lungs. Mice were vaccinated with DNA-heat-shock protein (hsp) 65, BCG/DNA-hsp 65 or BCG/CFP-CpG and challenged with Mycobacteirum tuberculosis as described in Fig. 2. Pathology was evaluated on the upper right lobe of the lung on day 30 post-challenge (original magnification, 100 ×). Asterisks show the areas represented in 400 × magnification (upper right quadrant). Arrow shows a foamy macrophage. Head arrow shows neutrophils.
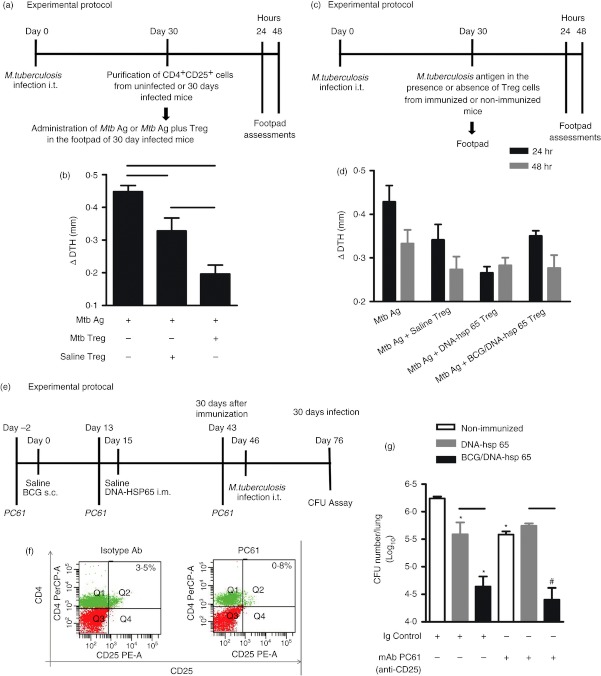
Protection induced by the BCG/DNA-hsp 65 vaccine was not affected by the depletion of CD25+ cells
To evaluate whether the number of CD4+ Foxp3+ cells that were induced by vaccination affected both the function of these cells in vivo and their protective efficacy, we performed a DTH assay to measure the activity of these cells. A group of mice infected with M. tuberculosis received Mtb antigen in the footpad 30 days after infection in the presence or absence of purified CD4+ CD25+ regulatory T cells isolated from uninfected (Saline Treg) or infected (Mtb Treg) mice (Fig. 4a). Forty-eight hours after antigen injection, or antigen and regulatory T cell administration, footpad oedema was measured. We observed that co-injection of Mtb antigen and regulatory T cells isolated from uninfected or infected mice suppressed oedema development. In addition, regulatory T cells from infected mice were more suppressive than regulatory T cells obtained from uninfected mice (Fig. 4b). Next, we performed a DTH assay with spleen CD4+ CD25+ cells that were obtained from non-immunized, DNA-hsp 65-immunized or BCG/DNA-hsp 65-immunized mice (Fig. 4c). We found that the administration of the Mtb antigen in the presence of regulatory T cells obtained from the DNA-hsp 65-immunized mice (Mtb Ag + DNA-hsp 65 Treg) displayed a tendency, although not significant, to decrease the footpad diameter when compared with the footpad oedema that was a result of the administration of Mtb antigen in the absence of regulatory T cells (Mtb Ag; Fig. 4d). The footpad diameter for mice that received Mtb antigen or Mtb antigen plus regulatory T cells that were obtained from saline-injected mice (Mtb Ag + Saline Treg) or mice that received Mtb antigen plus regulatory T cells obtained from the BCG/DNA-hsp 65 (Mtb Ag + BCG/DNA-hsp 65 Treg)-vaccinated mice were similar (Fig. 4d). There was no difference in the footpad diameters after 48 hr (Fig. 4d).
Figure 4.
Evaluation of the regulatory T cells in vivo after immunization with the DNA-heat-shock protein (hsp) 65 or BCG/DNA-hsp 65 vaccine. BALB/c mice were infected intratracheally with 1 × 105 bacilli. Thirty days after infection, 50 μg Mycobacterium tuberculosis antigen (Mtb Ag) in the presence or absence of CD4+ CD25+ cells, which were obtained from infected or uninfected mice, were administered in the right footpad. The left footpad received saline (a). Oedema measurement was performed 48 hr after the administration of antigen (b). BALB/c mice were infected, and 30 days after infection, they received 50 μg M. tuberculosis antigens in the right footpad in the presence or absence of CD4+ CD25+ cells that were obtained from immunized or non-immunized mice (c). Oedema measurements were performed at 24 and 48 hr (d) after antigen administration. BALB/c mice were immunized with BCG/DNA-hsp 65 and challenged 30 days after prime boost vaccination. Three days before immunization and challenge, the mice were treated with the monoclonal antibody (mAb) PC61 (e). Representative flow cytometry data demonstrating the percentages of CD4+ CD25+ lymphocytes in the blood of mice treated with 500 μg mAb PC61 during immunization (f). Colony-forming unit (CFU) counts were evaluated 30 days after challenge (g). The results are expressed as the mean ± SEM. The data are representative of two experiments. For the delayed type hypersensitivity (DTH) assay, with CD4+ CD25+ cells purified from uninfected or infected mice, seven mice were used. For the DTH assay, with CD4+ CD25+ cells purified from immunized or non-immunized mice, 8–14 mice were used. For the treatment with anti-CD25, eight or nine mice were used. Horizontal bars represent the significant difference between the groups. *P < 0·05 compared with non-immunized, infected and treated with immunoglobulin control mice; #P < 0·05 compared with mice non-immunized, infected and treated with mAb PC61.
A second assay that was used to deplete CD25+ cells using mAbs against CD25 (PC61) was employed to evaluate the role of the regulatory T cells induced by vaccination. Figure 4(e) shows a schematic of the experimental protocol used to deplete CD25+ cells before and during the immunization schedule. Figure 4(f) shows that treatment with the mAb PC61 reduced the frequency of CD4+ CD25+ cells in the peripheral blood. We observed a significant reduction in the number of CFU in the lungs of non-immunized, infected mice that were previously treated with PC61 compared with non-immunized, infected mice treated with a control antibody (Ig control; Fig. 4g). Therefore, depletion of CD25+ cells contributed to a reduction in the severity of infection. As expected, both DNA-hsp 65 and BCG/DNA-hsp 65-immunized mice previously treated with the control antibody were significantly protected, as confirmed by the significant reduction in the number of CFU compared with the untreated, non-immunized and infected groups. Although the treatment reduced the CFU numbers in the lungs compared with the non-immunized, infected mice, it was not different compared with the CFU counts in the lungs of treated and untreated BCG/DNA-hsp 65-immunized mice (Fig. 4g). These results suggest that the protection conferred by the BCG/DNA-hsp 65 vaccine was not dependent on CD25+ cells. It is likely that this vaccine stimulated the differentiation of IFN-γ-producing CD4+ cells more than CD4+ Foxp3+ cells.
Discussion
In this study, we demonstrated that heterologous immunization with BCG/DNA-hsp 65 or BCG/CFP-CpG was significantly more protective than DNA-hsp 65 immunization which induced a higher number of spleen CD4+ Foxp3+ cells.
The induction of regulatory T cells is an important feature of the immune response that should be addressed during the development of new vaccines against tuberculosis and other diseases. The induction of a high number or increased frequency of regulatory T cells during immunization will help to drive the expansion of these cells after an antigen is encountered or during infection with a pathogen. This process can interfere with the efficacy of the protective immune response. According to previous studies that evaluated the number and function of regulatory CD4+ CD25+ or CD4+ Foxp3+ cells obtained from the peripheral blood of patients with tuberculosis, these cells contribute to the increased progression of infection. This failure to control infection has been associated with regulatory T cells inhibiting IFN-γ secretion from effector cells.19,20,31 Therefore, pre-clinical assays used in the development of new vaccines against tuberculosis must take into account the differentiation and activation of all regulatory T cells, not only CD4+ Foxp3+ cells, but also regulatory T1 cells and Th3 cells. Additionally, immunization schedules need to be designed dependent on the type of immunization (homologous versus heterologous) and the number of doses to avoid the expansion of higher numbers of regulatory T cells that could affect the protective efficacy of the vaccination.
Heterologous immunization seems to represent an advantage over homologous immunization. Modifying the mechanism of release of the antigen to the immune system reinforces the induction/expansion of a highly specific antigen immune response.32,33 BCG prime may be a promising strategy because this vaccine is safe and induces no side-effects. Among the various research groups that study tuberculosis, there is a tendency to maintain the use of the BCG vaccine and to improve its immunogenic potential.33–35 Whole BCG bacteria may contribute to the induction of the inflammatory response and cytokine secretion that drives the differentiation of the cellular immune response. The boost with CFP plus CpG or DNA-hsp 65 could expand antigen-specific lymphocytes that can control infection after the M. tuberculosis challenge. This is an important issue to be addressed because we have previously shown that homologous vaccination with three doses of CFP plus CpG did not protected immunized mice; in spite of intense IFN-γ production, there was severe lung injury.29 Although protective, high levels of IFN-γ production need to be counterbalanced to minimize tissue inflammation and damage. Regulatory T cells, IL-10 and TGF-β have all been associated with the inhibition of the cellular immune response and inflammation. Heat-shock proteins are considered highly immunogenic antigens, but several studies have shown that these proteins have regulatory properties in autoimmune diseases.36,37 Previously, we treated mice with arthritis or diabetes with the DNA-hsp 65 construct and observed that the treatment impaired the progression of experimental disease. In addition, we found increased staining for CD25 in the pancreas of treated mice, suggesting that the DNA-hsp 65 vaccine could induce T regulatory cells in the pancreas.36,37 Therefore, the induction of a regulatory T-cell response may be important to avoid the tissue damage that characterizes chronic inflammatory diseases. Achievement of the proper ratio of effector (CD4+) and regulatory (CD4+ Foxp3+) cells during the immunization regimen will be challenging.
The first study to show that regulatory T cells could influence the immunogenicity and protective efficacy of a vaccine was carried out using a herpes simplex virus vaccine. In this study, Toka et al.38 demonstrated that the depletion of CD25+ cells by the administration of an anti-CD25 mAb (PC61) before DNA vaccine immunization increased the specific CD8+ T-cell response and decreased the viral load better than immunized mice treated with a control antibody. An increase in the antigen-specific CD8+ cells in CD25-depleted mice was observed in mice immunized with a DNA vaccine against hepatitis B virus and in mice vaccinated against human papillomavirus.39,40 In tuberculosis, the role of T regulatory cells induced by immunization has been evaluated with the BCG vaccine. Jaron et al.41 demonstrated that after BCG immunization, regulatory T cells were recruited to the lymph nodes. The depletion of CD25+ cells with mAb PC61 increased the production of IFN-γ and slightly reduced the CFU counts in the lungs compared with untreated mice. On the contrary, Quinn et al.42 described that the treatment with mAb against CD25 before BCG immunization did not protect mice against M. tuberculosis challenge. These results indicate that the role of regulatory T cells during BCG immunization is still controversial and needs to be investigated further. In this study, we used the BCG vaccine as an experimental control. Our efforts were focused on the DNA-hsp 65, BCG/DNA-hsp 65 and BCG/CFP-CpG vaccines against tuberculosis. The frequency, number and role of regulatory T cells in mice immunized with these vaccines have not been evaluated previously. Prime-boost regimens (BCG/DNA-hsp 65 and BCG/CFP-CpG) induced a higher ratio of CD4+/CD4+ Foxp3+ cells in the spleen compared with non-vaccinated mice and BCG-vaccinated mice. In addition, the positive correlation between the number of regulatory T cells and CFU counts suggests that the protective efficacy of vaccines against tuberculosis is associated with a decreased number of CD4+ Foxp3+ cells.
Although we observed that CD4+ CD25+ cells transferred from DNA-hsp 65-immunized mice had a tendency to suppress the DTH response compared with CD4+ CD25+ cells transferred from BCG/DNA-hsp 65-immunized mice or from mice that did not receive regulatory T cells, these differences were not significant. In addition, although the depletion of CD25+ cells from non-immunized mice induced a significant reduction in the lung CFU count compared with non-immunized mice treated with a control antibody, the depletion of CD25+ cells did not affect the protective efficacy of the BCG/DNA-hsp 65 immunization. This result is probably associated with the increased ratio of CD4+/CD4+ Foxp3+ cells in the spleen that was induced after vaccination and in the lungs after challenge. This leads to two hypotheses: (i) the BCG/DNA-hsp 65 vaccine is a weak inducer of regulatory T cells or (ii) this vaccine is a strong inducer of CD4+ cells and the ratio between these two cell types counterbalances the protection and lung injury.
Finally, these results confirm the protective efficacy of the prime-boost vaccines, BCG/DNA-hsp 65 and BCG/CFP-CpG, and the DNA-hsp 65 homologous vaccine. They also indicate that these prime-boost regimens are promising strategies for the development of a new vaccine against tuberculosis because they induce an effective cellular immune response that is associated with a reduced number of regulatory cells and decreased lung injury.
Acknowledgments
We would like to thank Fabiana Rossetto de Morais for help with flow cytometry; Rita de Cassia Aguiar Tinasi for BCG donation; and Izaira Tincani Brandão and Ana Paula Masson for general technical assistance. This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grant 2007/02695-5.
Disclosures
The authors declare no conflict of interest.
References
- 1.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–69. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson C, Powrie F. Regulatory T cells. Curr Opin Pharmacol. 2004;4:408–14. doi: 10.1016/j.coph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Mandapathil M, Whiteside TL. Targeting human inducible regulatory T cells (Tr1) in patients with cancer: blocking of adenosine-prostaglandin E2 cooperation. Expert Opin Biol Ther. 2011;11:1203–14. doi: 10.1517/14712598.2011.581225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kushwah R, Hu J. Role of dendritic cells in the induction of regulatory T cells. Cell Biosci. 2011;1:20. doi: 10.1186/2045-3701-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–11. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shevach EM. Biological functions of regulatory T cells. Adv Immunol. 2011;112:137–76. doi: 10.1016/B978-0-12-387827-4.00004-8. [DOI] [PubMed] [Google Scholar]
- 8.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24:242–8. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 9.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–62. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 10.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccirillo CA, Thornton AM. Cornerstone of peripheral tolerance: naturally occurring CD4+CD25+ regulatory T cells. Trends Immunol. 2004;25:374–80. doi: 10.1016/j.it.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Maloy KJ, Antonelli LR, Lefevre M, Powrie F. Cure of innate intestinal immune pathology by CD4+CD25+ regulatory T cells. Immunol Lett. 2005;97:189–92. doi: 10.1016/j.imlet.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Tang Q, Adams JY, Tooley AJ, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long SA, Buckner JH. CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J Immunol. 2011;187:2061–6. doi: 10.4049/jimmunol.1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 16.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 17.Hori S, Sakaguchi S. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect. 2004;6:745–51. doi: 10.1016/j.micinf.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev. 2011;241:260–8. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006;173:803–10. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro-Rodrigues R, Resende CT, Rojas R, Toossi Z, Dietze R, Boom WH, Maciel E, Hirsch CS. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144:25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Zhou B, Li M, et al. CD4+CD25+FoxP3+ regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease. Clin Immunol. 2007;123:50–9. doi: 10.1016/j.clim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, Bevan MJ, Urdahl KB. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007;204:2159–69. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paula MO, Fonseca DM, Wowk PF, Gembre AF, Fedatto PF, Sérgio CA, Silva CL, Bonato VL. Host genetic background affects regulatory T-cell activity that influences the magnitude of cellular immune response against Mycobacterium tuberculosis. Immunol Cell Biol. 2011;89:526–34. doi: 10.1038/icb.2010.116. [DOI] [PubMed] [Google Scholar]
- 24.Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med. 2010;207:1409–20. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Report – Global Tuberculosis Control. Geneva: World Health Organization; 2011. [Google Scholar]
- 26.Fonseca DM, Silva CL, Wowk PF, Paula MO, Ramos SG, Horn C, Marchal G, Bonato VL. Mycobacterium tuberculosis culture filtrate proteins plus CpG oligodeoxynucleotides confer protection to Mycobacterium bovis BCG-primed mice by inhibiting interleukin-4 secretion. Infect Immun. 2009;77:5311–21. doi: 10.1128/IAI.00580-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berod L, Puttur F, Huehn J, Sparwasser T. Tregs in infection and vaccinology: heroes or traitors? Microb Biotechnol. 2011;5:260–9. doi: 10.1111/j.1751-7915.2011.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonato VL, Lima VM, Tascon RE, Lowrie DB, Silva CL. Identification and characterization of protective T cells in hsp65 DNA-vaccinated and Mycobacterium tuberculosis-infected mice. Infect Immun. 1998;66:169–75. doi: 10.1128/iai.66.1.169-175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fonseca DM, Silva CL, Paula MO, Soares EG, Marchal G, Horn C, Bonato VL. Increased levels of interferon-γ primed by culture filtrate proteins antigen and CpG-ODN immunization do not confer significant protection against Mycobacterium tuberculosis infection. Immunology. 2007;121:508–17. doi: 10.1111/j.1365-2567.2007.02597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonato VL, Gonçalves ED, Soares EG, Santos-Júnior RR, Sartori A, Coelho-Castelo AA, Silva CL. Immune regulatory effect of pHSP65 DNA therapy in pulmonary tuberculosis: activation of CD8+ cells, interferon-γ recovery and reduction of lung injury. Immunology. 2004;113:130–8. doi: 10.1111/j.1365-2567.2004.01931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 2011;4:288–93. doi: 10.1038/mi.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider J, Gilbert SC, Blanchard TJ, et al. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 33.Rouanet C, Locht C. Boosting BCG to protect against TB. Expert Rev Respir Med. 2010;4:339–48. doi: 10.1586/ers.10.25. [DOI] [PubMed] [Google Scholar]
- 34.Lu J, Wang C, Zhou Z, et al. Immunogenicity and protective efficacy against murine tuberculosis of a prime-boost regimen with BCG and a DNA vaccine expressing ESAT-6 and Ag85A fusion protein. Clin Dev Immunol. 2011;2011:617892. doi: 10.1155/2011/617892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerrero GG, Locht C. Recombinant HBHA boosting effect on BCG-induced immunity against Mycobacterium tuberculosis infection. Clin Dev Immunol. 2011;2011:730702. doi: 10.1155/2011/730702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos-Junior RR, Sartori A, De Franco M, et al. Immunomodulation and protection induced by DNA-hsp65 vaccination in an animal model of arthritis. Hum Gene Ther. 2005;16:1338–45. doi: 10.1089/hum.2005.16.1338. [DOI] [PubMed] [Google Scholar]
- 37.Santos Júnior RR, Sartori A, Bonato VL, Coelho Castelo AA, Vilella CA, Zollner RL, Silva CL. Immune modulation induced by tuberculosis DNA vaccine protects non-obese diabetic mice from diabetes progression. Clin Exp Immunol. 2007;149:570–8. doi: 10.1111/j.1365-2249.2007.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toka FN, Suvas S, Rouse BT. CD4+CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J Virol. 2004;78:13082–9. doi: 10.1128/JVI.78.23.13082-13089.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furuichi Y, Tokuyama H, Ueha S, Kurachi M, Moriyasu F, Kakimi K. Depletion of CD25+CD4+T cells (Tregs) enhances the HBV-specific CD8+ T cell response primed by DNA immunization. World J Gastroenterol. 2005;11:3772–7. doi: 10.3748/wjg.v11.i24.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narayan S, Choyce A, Linedale R, et al. Epithelial expression of human papillomavirus type 16 E7 protein results in peripheral CD8 T-cell suppression mediated by CD4+CD25+ T cells. Eur J Immunol. 2009;39:481–90. doi: 10.1002/eji.200838527. [DOI] [PubMed] [Google Scholar]
- 41.Jaron B, Maranghi E, Leclerc C, Majlessi L. Effect of Attenuation of Treg during BCG Immunization on Anti-Mycobacterial Th1 Responses and Protection against Mycobacterium tuberculosis. PLoS ONE. 2008;3:e2833. doi: 10.1371/journal.pone.0002833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quinn KM, Rich FJ, Goldsack LM, Lisle GW, Buddle BM, Delahunt B, Kirman JR. Accelerating the secondary immune response by inactivating CD4+CD25+ T regulatory cells prior to BCG vaccination does not enhance protection against tuberculosis. Eur J Immunol. 2008;38:695–705. doi: 10.1002/eji.200737888. [DOI] [PubMed] [Google Scholar]