Abstract
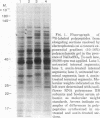
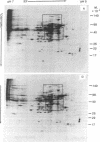
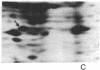
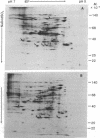
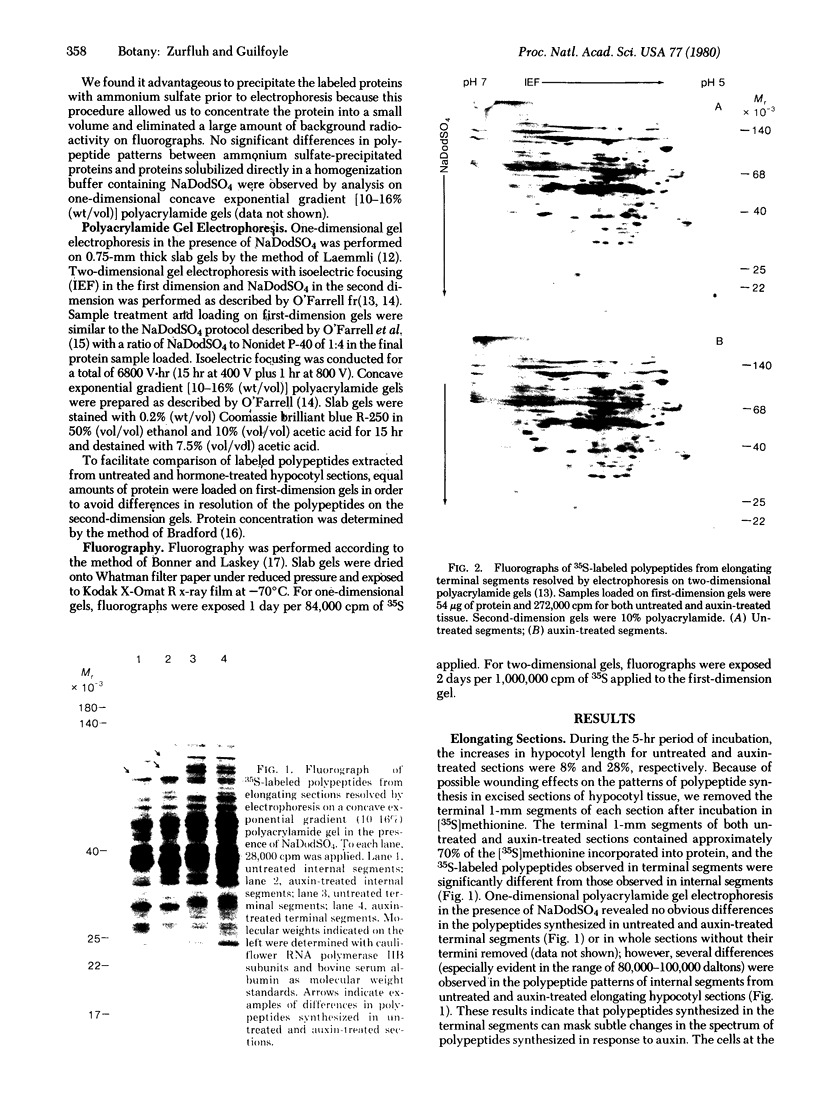
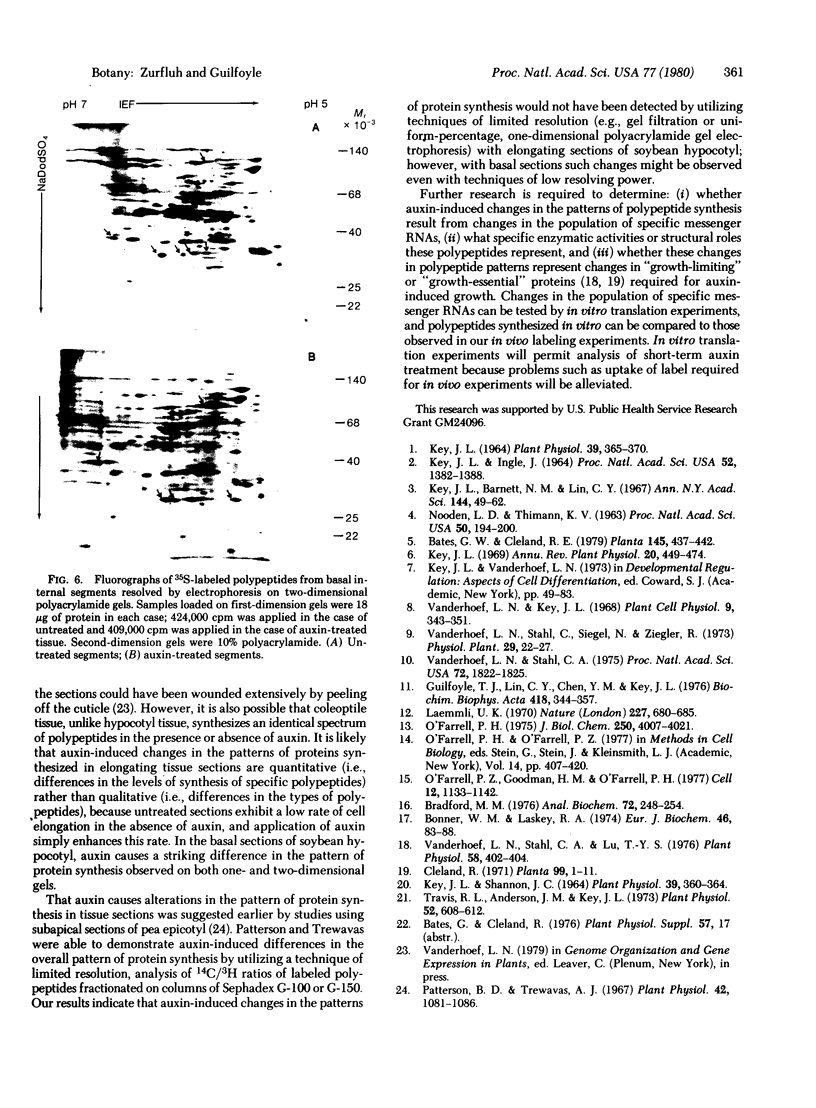
The patterns of protein synthesis in elongating and mature (basal) sections of soybean hypocotyl were examined after incubation in a medium containing auxin (auxin-treated) or a medium lacking auxin (untreated). The hypocotyl sections (1.2 cm) were labeled with [35S]methionine, and polypeptide patterns were analyzed by one- and two-dimensional polyacrylamide gel electrophoresis. Auxin treatment altered the pattern of protein synthesis in both elongating and basal soybean hypocotyl sections. Excision of terminal segments from incubated sections was required to clearly observe auxin-induced changes in the synthesis of polypeptides. Polypeptides synthesized in terminal segments, possibly in response to wounding, can mask subtle changes in the spectrum of polypeptides synthesized in response to auxin. Cytokinin treatment caused a decrease in [35S]methionine incorporation into polypeptides and altered the pattern of protein synthesis in untreated and auxin-treated elongating hypocotyl sections.
Keywords: wounding; 2,4-dichlorophenoxyacetic acid; cytokinin; two-dimensional gel electrophoresis
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Guilfoyle T. J., Lin C. Y., Chen Y. M., Key J. L. Purification and characterization of RNA polymerase I from a higher plant. Biochim Biophys Acta. 1976 Feb 5;418(3):344–357. doi: 10.1016/0005-2787(76)90296-3. [DOI] [PubMed] [Google Scholar]
- Key J. L., Barnett N. M., Lin C. Y. RNA and protein biosynthesis and the regulation of cell elongation by auxin. Ann N Y Acad Sci. 1967 Aug 9;144(1):49–62. doi: 10.1111/j.1749-6632.1967.tb34000.x. [DOI] [PubMed] [Google Scholar]
- Key J. L., Ingle J. REQUIREMENT FOR THE SYNTHESIS OF DNA-LIKE RNA FOR GROWTH OF EXCISED PLANT TISSUE. Proc Natl Acad Sci U S A. 1964 Dec;52(6):1382–1388. doi: 10.1073/pnas.52.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L. Ribonucleic Acid and Protein Synthesis as Essential Processes for Cell Elongation. Plant Physiol. 1964 May;39(3):365–370. doi: 10.1104/pp.39.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Shannon J. C. Enhancement by Auxin of Ribonucleic Acid Synthesis in Excised Soybean Hypocotyl Tissue. Plant Physiol. 1964 May;39(3):360–364. doi: 10.1104/pp.39.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Noodén L. D., Thimann K. V. EVIDENCE FOR A REQUIREMENT FOR PROTEIN SYNTHESIS FOR AUXIN-INDUCED CELL ENLARGEMENT. Proc Natl Acad Sci U S A. 1963 Aug;50(2):194–200. doi: 10.1073/pnas.50.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Patterson B. D., Trewavas A. J. Changes in the pattern of protein synthesis induced by 3-indolylacetic Acid. Plant Physiol. 1967 Aug;42(8):1081–1086. doi: 10.1104/pp.42.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Anderson J. M., Key J. L. Influence of auxin and incubation on the relative level of polyribosomes in excised soybean hypocotyl. Plant Physiol. 1973 Dec;52(6):608–612. doi: 10.1104/pp.52.6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Stahl C. A., Lu T. Y. Two elongation responses to auxin respond differently to protein synthesis inhibition. Plant Physiol. 1976 Sep;58(3):402–404. doi: 10.1104/pp.58.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Stahl C. A. Separation of two responses to auxin by means of cytokinin inhibition. Proc Natl Acad Sci U S A. 1975 May;72(5):1822–1825. doi: 10.1073/pnas.72.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]