Abstract
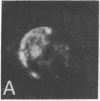
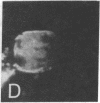
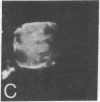
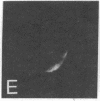
Surface immunofluorescence experiments using a human anti-i and two anti-I antisera have been performed on human peripheral blood lymphocytes. These are known to contain cold-reactive monoclonal IgM antibodies against the carbohydrate sequence: (formula: see text). A high proportion of B- and T-type lymphocytes express these I and i determinants. In the presence of anti-human immunoglobulin, the cold-reactive membrane-associated complexes of I-anti-I and i-anti-i become stabilized, and redistribution (with patching and capping) can be elicited at 37 degrees C. Dual fluorescence experiments have shown striking concordant staining of I or i (fluorescein) caps and patches with concanavalin A (rhodamine) reactive sites on normal and leukemic cells, suggesting that a proportion of I and i active structures of lymphocyte membranes are structurally associated or physiologically coupled with glycoproteins carrying oligosaccharides with branched mannosyl cores.
Full text
PDF

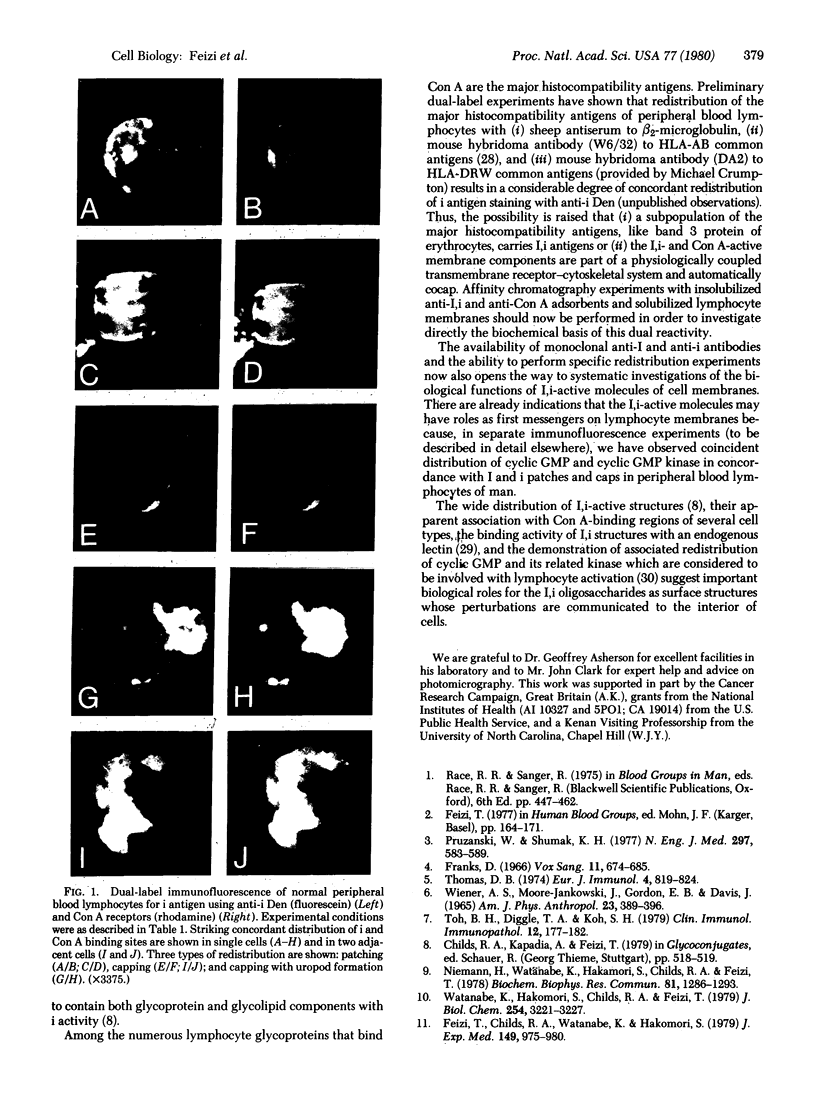

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Childs R. A., Feizi T. Calf heart lectin reacts with blood group Ii antigens and other precursor chains of the major blood group antigens. FEBS Lett. 1979 Mar 1;99(1):175–179. doi: 10.1016/0014-5793(79)80273-2. [DOI] [PubMed] [Google Scholar]
- Childs R. A., Feizi T., Fukuda M., Hakomori S. I. Blood-group-I activity associated with band 3, the major intrinsic membrane protein of human erythrocytes. Biochem J. 1978 Jul 1;173(1):333–336. doi: 10.1042/bj1730333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T., Childs R. A., Hakomori S. I., Powell M. E. Blood-group-Ii-active gangliosides of human erythrocyte membranes. Biochem J. 1978 Jul 1;173(1):245–254. doi: 10.1042/bj1730245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T., Childs R. A., Watanabe K., Hakomori S. I. Three types of blood group I specificity among monoclonal anti-I autoantibodies revealed by analogues of a branched erythrocyte glycolipid. J Exp Med. 1979 Apr 1;149(4):975–980. doi: 10.1084/jem.149.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T., Kabat E. A., Vicari G., Anderson B., Marsh W. L. Immunochemical studies on blood groups.XLIX. The I antigen complex: specificity differences among anti-I sera revealed by quantitative precipitin studies; partial structure of the I determinant specific for one anti-I serum. J Immunol. 1971 Jun;106(6):1578–1592. [PubMed] [Google Scholar]
- Franks D. Antigenic markers on cultured human cells I Ii, Tja, Donath-Landsteiner and "non-specific" autoantigens. Vox Sang. 1966 Nov-Dec;11(6):676–685. doi: 10.1111/j.1423-0410.1966.tb04268.x. [DOI] [PubMed] [Google Scholar]
- Kornfeld R. Structure of the oligosaccharides of three glycopeptides from calf thymocyte plasma membranes. Biochemistry. 1978 Apr 18;17(8):1415–1423. doi: 10.1021/bi00601a009. [DOI] [PubMed] [Google Scholar]
- Kościelak J., Miller-Podraza H., Krauze R., Piasek A. Isolation and characterization of poly(glycosyl)ceramides (megaloglycolipids) with A, H and I blood-group activities. Eur J Biochem. 1976 Dec;71(1):9–18. doi: 10.1111/j.1432-1033.1976.tb11083.x. [DOI] [PubMed] [Google Scholar]
- Krusius T., Finne J., Rauvala H. The poly(glycosyl) chains of glycoproteins. Characterisation of a novel type of glycoprotein saccharides from human erythrocyte membrane. Eur J Biochem. 1978 Dec 1;92(1):289–300. doi: 10.1111/j.1432-1033.1978.tb12747.x. [DOI] [PubMed] [Google Scholar]
- Lecomte J., Feizi T. A common idiotype on human macroglobulins with anti-I and anti-i specificity. Clin Exp Immunol. 1975 May;20(2):287–302. [PMC free article] [PubMed] [Google Scholar]
- Moore J. O., Logue G. L. I and i antigens on normal and leukemic leukocytes. Cancer. 1978 Jul;42(1):140–148. doi: 10.1002/1097-0142(197807)42:1<140::aid-cncr2820420124>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Niemann H., Watanabe K., Hakomori S. Blood group i and I activities of "lacto-N-norhexaosylceramide" and its analogues: the structural requirements for i-specificities. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1286–1293. doi: 10.1016/0006-291x(78)91275-5. [DOI] [PubMed] [Google Scholar]
- Ogata S., Muramatsu T., Kobata A. Fractionation of glycopeptides by affinity column chromatography on concanavalin A-sepharose. J Biochem. 1975 Oct;78(4):687–696. doi: 10.1093/oxfordjournals.jbchem.a130956. [DOI] [PubMed] [Google Scholar]
- Pruzanski W., Farid N., Keystone E., Armstrong M., Greaves M. F. The influence of homogeneous cold agglutinins on human B and T lymphocytes. Clin Immunol Immunopathol. 1975 Jul;4(2):248–257. doi: 10.1016/0090-1229(75)90060-4. [DOI] [PubMed] [Google Scholar]
- Pruzanski W., Shumak K. H. Biologic activity of cold-reacting autoantibodies (second of two parts). N Engl J Med. 1977 Sep 15;297(11):583–589. doi: 10.1056/NEJM197709152971105. [DOI] [PubMed] [Google Scholar]
- Shumak K. H., Rachkewich R. A., Greaves M. F. I and i antigens on normal human T and B lymphocytes and on lymphocytes from patients with chronic lymphocytic leukemia. Clin Immunol Immunopathol. 1975 Jul;4(2):241–247. doi: 10.1016/0090-1229(75)90059-8. [DOI] [PubMed] [Google Scholar]
- Strom T. B., Lundin A. P., Carpenter C. B. The role of cyclic nucleotides in lymphocyte activation and function. Prog Clin Immunol. 1977;3:115–153. [PubMed] [Google Scholar]
- Toh B. H., Diggle T. A., Koh S. H. Ii blood group antigens on fibroblast cell surfaces. Clin Immunol Immunopathol. 1979 Feb;12(2):177–182. doi: 10.1016/0090-1229(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Hakomori S. I., Childs R. A., Feizi T. Characterization of a blood group I-active ganglioside. Structural requirements for I and i specificities. J Biol Chem. 1979 May 10;254(9):3221–3228. [PubMed] [Google Scholar]
- Watanabe K., Laine R. A., Hakomori S. I. On neutral fucoglycolipids having long, branched carbohydrate chains: H-active and I-active glycosphingolipids of human erythrocyte membranes. Biochemistry. 1975 Jun 17;14(12):2725–2733. doi: 10.1021/bi00683a026. [DOI] [PubMed] [Google Scholar]
- Wiener A. S., Moor-Jankowski J., Gordon E. B., Davis J. The blood factors I and i in primates including man, and in lower species. Am J Phys Anthropol. 1965 Dec;23(4):389–396. doi: 10.1002/ajpa.1330230412. [DOI] [PubMed] [Google Scholar]
- Yount W. J., Utsinger P. D., Hutt L. M., Buchanan P. D., Korn J. H., Fuller C. R., Logue M., Pagano J. S. Subpopulations of human lymphoblastoid cell lines. Correlation with the expression of surface receptors and content of Epstein-Barr virus genome. Scand J Immunol. 1976;5(6-7):795–810. doi: 10.1111/j.1365-3083.1976.tb03029.x. [DOI] [PubMed] [Google Scholar]