Abstract
Urinary excretion of lipocalin-type PGD2 synthase (L-PGDS), which converts PG H2 to PGD2, increases in early diabetic nephropathy. In addition, L-PGDS expression in the tubular epithelium increases in adriamycin-induced nephropathy, suggesting that locally produced L-PGDS may promote the development of CKD. In this study, we found that L-PGDS–derived PGD2 contributes to the progression of renal fibrosis via CRTH2-mediated activation of Th2 lymphocytes. In a mouse model, the tubular epithelium synthesized L-PGDS de novo after unilateral ureteral obstruction (UUO). L-PGDS-knockout mice and CRTH2-knockout mice both exhibited less renal fibrosis, reduced infiltration of Th2 lymphocytes into the cortex, and decreased production of the Th2 cytokines IL-4 and IL-13. Furthermore, oral administration of a CRTH2 antagonist, beginning 3 days after UUO, suppressed the progression of renal fibrosis. Ablation of IL-4 and IL-13 also ameliorated renal fibrosis in the UUO kidney. Taken together, these data suggest that blocking the activation of CRTH2 by PGD2 might be a strategy to slow the progression of renal fibrosis in CKD.
Kidney failure is a public health problem worldwide, with increasing incidence and prevalence, high costs, and poor outcomes. CKD is generally progressive, incurable, and ultimately fatal, although some patients resolve with little or no sequelae. Because current treatment is basically limited to slowing the progression to ESRD using angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor blockers, more efficient therapies with different or additional modes of action are clearly needed.
Regardless of disease etiology, tubulointerstitial fibrosis is the common pathway leading to ESRD in many kidney diseases and is regarded as a prognostic factor for renal function.1–3 It is noteworthy that some clinical trials are proving that antifibrotic therapies, such as pirfenidone against diabetic nephropathy,4 are also effective for CKD. Therefore, elucidating the etiological mechanism underlying renal fibrosis and developing novel therapeutic strategies remains a serious, unmet medical need.
Lipocalin-type PGD2 synthase (L-PGDS) is a secretary protein of the lipocalin superfamily that converts PG H2, a common precursor of prostanoids, to PGD2. Because the urinary excretion of L-PGDS increases in the early stage of diabetic nephropathy,5,6 as well as in patients with essential hypertension without any apparent renal injury,7 urinary L-PGDS may be an early diagnostic marker of renal injury in these patients. There is evidence indicating that, in the monkey kidney, L-PGDS is synthesized de novo in the loop of Henle, podocytes, and Bowman’s capsule of the glomeruli.8 Furthermore, L-PGDS gene expression in the tubular epithelium was increased in adriamycin-induced nephropathy.9 These findings suggest that, under conditions of tubulointerstitial stress, locally produced L-PGDS may be involved in the development of CKD. However, the precise pathophysiological significance of L-PGDS in the kidney remains to be determined.
PGD2 interacts with two receptors, the prostanoid DP1 receptor and the chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2). Activation of the DP1 receptor by PGD2 has been shown to produce vasodilation10 and bronchodilation.11 Furthermore, the DP1 receptor is expressed by certain leukocyte populations,12,13 including dendritic cells, where it controls various functions, including cytokine production. CRTH2 was originally identified as an orphan receptor expressed by Th2 lymphocytes. CRTH2 is not structurally related to the DP1 receptor and belongs to the family of chemokine receptors. Activation of CRTH2 by PGD2 plays an important role in allergic inflammation via the recruitment of Th2 lymphocytes and other leukocytes14 and, perhaps more importantly, by driving the production of the Th2 cytokines IL-4, IL-5, and IL-13.15
Results
De Novo Synthesis of L-PGDS in the Tubular Epithelium after Unilateral Ureteral Obstruction
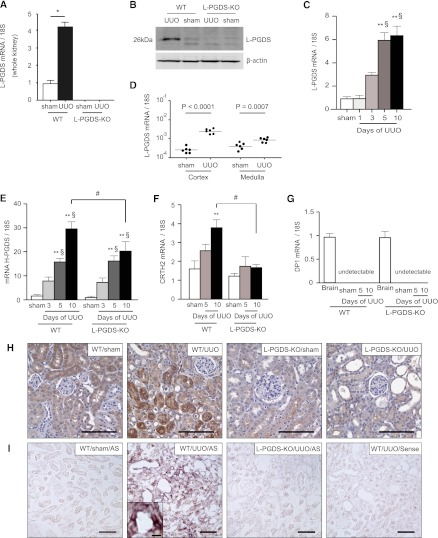
Unilateral ureteral obstruction (UUO) was used to induce primary tubular epithelial injury without any exogenous toxin or the “uremic” environment. The expression of L-PGDS at both the gene and protein levels was increased significantly in kidneys of wild-type (WT) mice 5 days after UUO (Figure 1, A and B). Ten days after UUO, the mRNA expression of L-PGDS remained significantly elevated (Figure 1C). There was greater induction of L-PGDS mRNA in the cortex than in the medulla (Figure 1D). Although mRNA levels of hematopoietic PG D2 synthase (H-PGDS) increased with UUO in WT mice, the induction of H-PGDS mRNA expression was suppressed in L-PGDS–knockout (KO) mice on day 10 after UUO (Figure 1E). Immunohistochemical analysis revealed a marked increase in L-PGDS immunoreactivity in the tubular epithelium, but not in the glomeruli, of obstructed kidneys from WT mice (Figure 1H). In situ hybridization with an L-PGDS antisense RNA probe revealed positive signals in tubules in WT mice 10 days after UUO; these signals were not observed in sham-operated WT mice or in L-PGDS-KO mice subjected to UUO (Figure 1I). These results indicate that L-PGDS is synthesized de novo in the tubular epithelium after UUO. Furthermore, double staining with lotus tetragonolobus lectin, a proximal tubule marker, localized the increased L-PGDS immunoreactivity to lotus tetragonolobus lectin-negative nonproximal tubules (Supplemental Figure 1).
Figure 1.
L-PGDS is synthesized de novo in the tubular epithelium of kidneys from mice subjected to UUO. (A) Relative changes in L-PGDS mRNA levels in kidneys from sham-operated rats and in obstructed kidneys from WT and L-PGDS-KO 5 days after UUO (n=8 mice in each group; *P<0.001). (B) Obstructed kidneys were harvested and subjected to Western blotting with antibodies against L-PGDS and β-actin (control). (C) Time course of change in L-PGDS mRNA expression in the cortex of UUO kidneys (n=5 in each group). **P<0.05 versus sham; §P<0.05 versus day 1 (one-way ANOVA). (D) Five days after UUO, obstructed kidneys were divided macroscopically into the cortex and medulla and then subjected to quantitative RT-PCR to determine L-PGDS mRNA expression in the cortex and medulla (n=6 in each group). (E–G) Relative changes in (E) H-PGDS, (F) CRTH2, and (G) DP1 mRNA expression after UUO (n=5 in each group). Brain samples were used as a positive control. **P<0.05 versus sham (one-way ANOVA); #P<0.05 (two-sided t test); §P<0.05 versus day 1 (one-way ANOVA). Data are the mean ± SD. (H) Immunostaining for L-PGDS in the cortex 10 days after UUO or sham operation. (I) In situ hybridization of L-PGDS. Scale bars, 100 μm in H and I; 10 μm in inset.
Suppression of Tubulointerstitial Fibrosis in L-PGDS–Deficient Mice
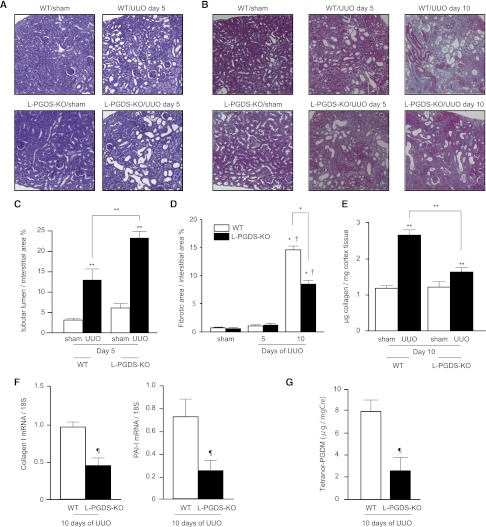
Although the dilation of tubular lumens in obstructed kidneys 5 days after UUO was more severe in L-PGDS-KO than WT mice (Figure 2, A and C), interstitial collagen deposition on Azan-stained sections (Figure 2B) was significantly lower in L-PGDS-KO than WT mice (8.48%±0.40% versus 14.66%±0.41%, respectively; Figure 2D). Consistent with this, the mRNA expression of collagen I and plasminogen activator inhibitor-1, as markers of fibrosis, was suppressed in L-PGDS-KO mice compared with WT mice (Figure 2F). Quantification of soluble collagen content revealed significantly less collagen deposition in obstructed kidneys of L-PGDS-KO mice compared with WT mice (1.636±0.103 versus 2.658±0.158 μg, respectively; Figure 2E). Ten days after UUO, urinary levels of 11,15-dioxo-9α-hydroxy-2,3,4,5-tetranorprostan-1,20-dioic acid (tetranor-PGDM), a stable metabolite of PGD2, were significantly lower in the dilated urinary tract of L-PGDS-KO mice compared with WT mice (2.627±1.199 versus 8.023±1.032 ng/mg creatinine [mgCr], respectively; Figure 2G).
Figure 2.
Attenuation of renal fibrosis in L-PGDS-KO mice. Representative images of renal cortical sections. (A) Periodic acid–Schiff stain demonstrating dilation of the tubular lumen in affected kidneys. (B) Azan stain showing interstitial collagen deposition (blue) in kidneys from mice subjected to UUO. (C and D) Quantification of (C) tubule dilation and (D) collagen deposition. Data are the mean ± SD (n=5 in each group). *P<0.001 (two-sided t test); **P<0.05 versus sham; †P<0.05 versus day 5 (one-way ANOVA). #P<0.05; (E) Soluble collagen content in the cortex; (F) relative cortical mRNA expression of collagen I and plasminogen activator inhibitor-1, as determined by RT-PCR; and (G) urinary tetranor-PGDM (corrected by urinary creatinine concentration) in the dilated ureter of WT and L-PGDS-KO mice after UUO. Data are the mean ± SD (n=5–12). **P<0.05 versus sham; ¶P<0.05 versus WT mice (two-sided t test).
It is well known that macrophages play a key role in the induction and progression of renal fibrosis.16 Immunohistochemical analysis showed that, 5 days after UUO, the number of infiltrating F4/80-positive macrophages in the cortex was comparable between WT and L-PGDS-KO mice (94.0±12.7 versus 83.7±8.1, respectively; Supplemental Figure 2, A and B). Consistent with this, there were no significant differences between WT and L-PGDS-KO mice in terms of the mRNA expression of F4/80 (Supplemental Figure 2C) and CD68 (data not shown). Fluorescence-activated cell sorting (FACS) analysis revealed that the number of CD11b-positive cells, as a percentage of total cells in the cortex, was comparable between WT and L-PGDS-KO mice at all time points evaluated after UUO (Supplemental Figure 2D).
We next characterized the macrophage subsets. Although M2 macrophages are known to be involved in tissue fibrosis, reduction in M2 macrophage number was not apparent in L-PGDS-KO mice compared with WT mice at 3, 5, and 7 days after UUO (Supplemental Figure 2E; representative FACS results are shown in Supplemental Figure 2F). This result suggested that the fibrosis-resistant phenotype observed in L-PGDS-KO mice cannot be explained satisfactorily by the classic macrophage theory.
Suppression of Cortical Infiltration and Activation of Th2 Cells in L-PGDS-KO Mice
We examined the mRNA expression of two distinct PGD2 receptors, namely DP1 and CRTH2. In agreement with previous findings, DP1 mRNA expression in the kidney was below the measurement threshold at any time after UUO (Figure 1G). In contrast, CRTH2 mRNA expression was markedly enhanced by UUO in WT mice, but not in L-PGDS-KO mice (Figure 1F). Because CRTH2 is predominantly expressed on Th2 lymphocytes, this finding suggests the possibility that L-PGDS deficiency suppresses the infiltration of Th2 cells into the tubulointerstitium in obstructed kidneys.
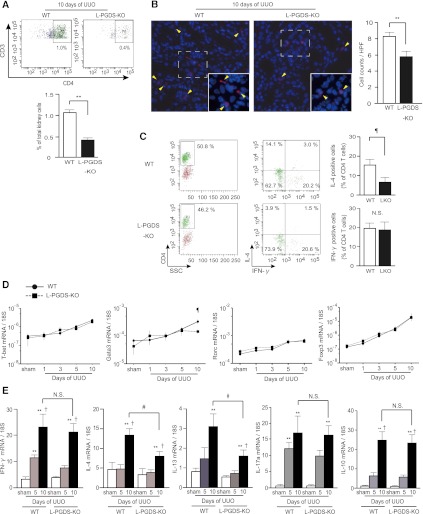
Flow cytometry analysis revealed a decrease in the number of infiltrating CD3+CD4+ lymphocytes, as a percentage of all cortical cells, 10 days after UUO in L-PGDS-KO compared with WT mice (0.46%±0.17% versus 1.07%±0.07%, respectively; Figure 3A). Immunofluorescence analysis demonstrated that the number of CD4+ T cells infiltrating into the interstitium in obstructed kidneys was significantly lower in L-PGDS-KO mice than in WT mice (5.61±0.51 versus 8.29±0.32, respectively; Figure 3B). To further characterize the infiltrating T cell subsets, we isolated CD4+ T cells from the cortex using magnetic-activated cell sorting (MACS) and stained them for intracellular cytokines. To note, although the percentage of IFN-γ–producing CD4+ T cells was not affected in L-PGDS-KO compared with WT mice (19.9%±2.6% versus 19.5%±3.7%, respectively), the number of IL-4–producing CD4+ T cells was significantly lower in L-PGDS-KO than WT mice (5.6%±3.4% versus 15.2%±2.8%, respectively; Figure 3C).
Figure 3.
Infiltration of IL-4–producing Th2 cells is selectively suppressed in kidneys from L-PGDS-KO mice subjected to UUO. (A) Proportion of CD3+/CD4+ cells in WT and L-PGDS-KO mice on day 10 after UUO. Data are representative of two independent experiments (n=7 in each group). (B) Representative images of immunofluorescent signals for CD4 in the cortex of WT and L-PGDS-KO mice 10 days after UUO. Arrowheads (yellow) indicate CD4+ T cells infiltrating the interstitium. Insets are the extended images of the representative area surrounded by a dotted line. Quantitative comparisons are also shown (n=5 in each group). (C) Percentage of IFN-γ–producing Th1 cells and IL-4–producing Th2 cells in CD4+ T cells infiltrating into the cortex of WT and L-PGDS-KO mice. Data are representative of three independent experiments (n=5 in each group). Data are the mean ± SD. ¶P<0.05 versus WT (two-sided t test). (D and E) Induction of Th2-representative transcription factor Gata3 and the cytokines IL-4 and IL-13 is attenuated in kidneys from L-PGDS-KO mice subjected to UUO. (D) Time course of changes in the mRNA expression of the master transcriptional factors of Th cells, namely T-bet, GATA3, Rorc, and Foxp3, in WT and L-PGDS-KO mice after UUO. (E) Quantitative RT-PCR analysis of Th cytokine mRNA expression in the cortex 5 and 10 days after UUO. (Data show relative expression compared with that in sham-operated mice.) Data are the mean ± SD (n=5 in each group). **P<0.05 versus sham; †P<0.05 versus day 5 (one-way ANOVA); ¶P<0.05 versus WT (two-sided t test).
Next, we investigated the mRNA expression of the master transcriptional factors for T cell subsets, namely T-bet, Gata3, Rorc, and Foxp3. The expression of all these transcriptional factors increased with time after UUO. However, the expression of Gata3 in the obstructed kidneys 10 days after UUO was significantly lower in L-PGDS-KO compared with WT mice (Figure 3D). The gene expression of IFN-γ, IL-4, IL-13, IL-17, and IL-10 was increased in the obstructed kidneys of WT mice 10 days after UUO, but the increased expression of IL-4 and IL-13 was selectively blunted in the obstructed kidneys of L-PGDS-KO mice at the same time point (Figure 3E). These findings indicated that the deficiency in L-PGDS–derived PGD2 was associated with reduced Th2 lymphocyte numbers in UUO kidneys.
Amelioration of UUO-Induced Tubulointerstitial Fibrosis in CRTH2-KO Mice
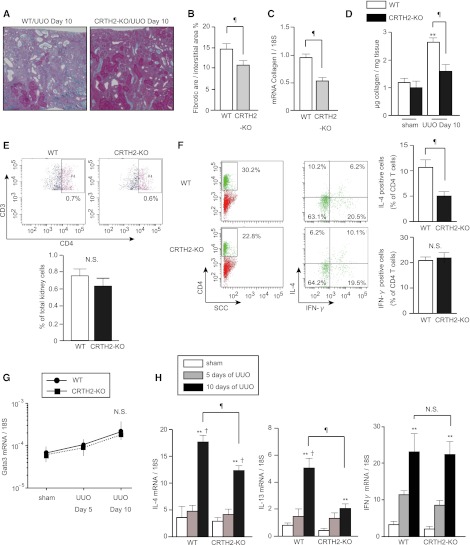
To investigate the role of CRTH2 in interstitial fibrosis, CRTH2-deficient (CRTH2-KO) mice were subjected to UUO. Azan staining revealed that interstitial collagen deposition was significantly suppressed in CRTH2-KO compared with WT mice 10 days after UUO (10.41%±0.67% versus 14.69%±1.36%, respectively; Figure 4, A and B). Consistent with these observations, Collagen I gene expression (Figure 4C) and soluble collagen content (1.588±0.257 versus 2.658±0.159 μg; Figure 4D) were lower in CRTH2-KO than WT mice.
Figure 4.
UUO-induced Th2 lymphocyte activation and interstitial fibrosis are blunted in CRTH2-KO mice. (A) Representative images of Azan staining of UUO kidneys from WT and CRTH2-KO mice. (B) Quantitative assessment of the fibrotic area. (C) Collagen I mRNA expression, as determined by quantitative RT-PCR. (Values show relative expression compared with that in WT mice.) (D) Comparison of soluble collagen content in the cortex of WT and CRTH2-KO mice. (E) Proportion of CD3+/CD4+ cells in WT and CRTH2-KO mice on day 10 after UUO. Data are representative of two independent experiments. (F) Percentage of IFN-γ–producing Th1 and IL-4–producing Th2 cells in CD4+ T cells infiltrating into the cortex of WT and CRTH2-KO mice. (Values are representative of three independent experiments.) Data are the mean ± SD (n=5 in each group). ¶P<0.05 versus WT; **P<0.05 versus sham (two-sided t test); †P<0.05 versus day 5 (one-way ANOVA). (G and H) Time course of changes in (G) Gata3 and (H) IL-4, IL-13, and IFN-γ mRNA expression in the cortex of WT and CRTH2-KO mice.
Although the number of infiltrating CD4+ T cells was comparable between CRTH2-KO and WT mice (Figure 4E), intracellular cytokine staining revealed that the number of infiltrating IL-4–positive Th2 cells, but not IFN-γ–positive Th1 cells, was significantly lower in CRTH2-KO compared with WT mice (4.68%±0.74% versus 11.28%±1.49%, respectively; Figure 4F). Interestingly, although gene expression of Gata3 10 days after UUO was comparable between WT and CRTH2-KO mice (Figure 4G), the expression of IL-4 and IL-13 was significantly suppressed 10 days after UUO in CRTH2-KO compared with WT mice (Figure 4H). There were no significant differences in IFN-γ expression between WT and CRTH2-KO mice. These findings indicated that PGD2-CRTH2 signaling contributes to the augmentation of Th2 cytokine production rather than the chemotaxis and differentiation of Th2 lymphocytes.
Attenuation by the CRTH2 Antagonist CAY10471 of Tubulointerstitial Fibrosis
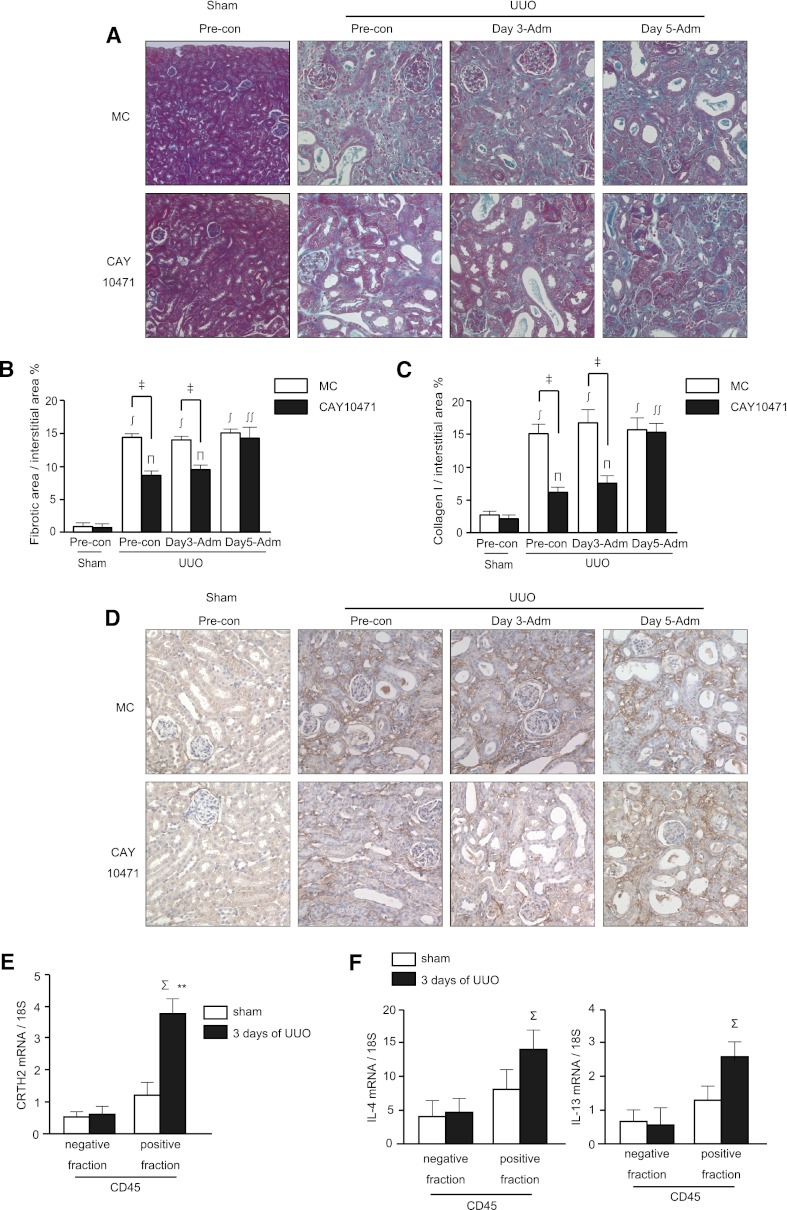
We next investigated whether pharmacological blockade of CRTH2 by CAY10471 could slow the progression of renal fibrosis in the obstructed kidneys. An increased area of fibrosis was observed in mice administered methyl cellulose (MC; vehicle) after UUO (0.45%±0.07% of sham versus 14.85%±0.48%, versus 14.44%±0.47%, versus 15.09%±0.45% after UUO with preconditioning, day 3 administration, and day 5 administration of MC, respectively) (Figure 5, A and B). Oral administration of CAY10471 beginning 4 days before UUO significantly attenuated interstitial collagen deposition in the cortex compared with the vehicle (MC)–treated group (8.40%±0.64% versus 14.85%±0.48%). Oral administration of CAY10471 from 3 days after UUO also significantly attenuated interstitial collagen deposition in the cortex compared with vehicle (MC) treatment (9.63%±0.74% versus 14.44%±0.47%). However, oral administration of CAY10471 beginning 5 days after UUO had little effect on interstitial collagen deposition in the cortex compared with vehicle (MC) treatment (14.61%±1.13% versus 15.09%±0.45%). Consistent with the Azan staining, collagen I deposition was significantly suppressed by CAY10471 administration beginning 4 days before UUO (4.22%±0.65% versus 15.02%±1.65% for vehicle control) and 3 days after UUO (7.80%±0.93% versus 17.03%±2.01% for vehicle control), but not at 5 days after UUO (15.46%±1.28% versus 15.53%±1.73% for vehicle control) (Figure 5, C and D).
Figure 5.
Oral administration of CRTH2 antagonist, CAY10471, attenuated the progression of tubulointerstitial fibrosis. (A) Representative images of Azan staining. The number of mice in the sham, preconditioning (CAY-10471 administration was started at 4 days before UUO), and day 3 administration and day 5 administration (CAY-10471 administration was started at 3 or 5 days after UUO, respectively) groups was 3, 6, 5, and 5, respectively. (B) Quantitative assessment of the fibrotic area. Data are the mean ± SD. ‡P<0.05 versus MC-fed; ∫P<0.001 versus sham of MC-fed; πP<0.01 versus sham of CAY10471; ∫∫P<0.05 versus UUO with preconditioning and day 3 administration of CAY10471 (two-sided t test, respectively). (C) Quantitative assessment of the collagen I-positive area. Data are the mean ± SD. ‡P<0.05 versus MC-fed; ∫P<0.001 versus sham of MC-fed; πP<0.01 versus sham of CAY10471; ∫∫P<0.05 versus UUO with preconditioning and day 3 administration of CAY10471 (two-sided t test, respectively). (D) Collagen I staining of paraffin sections. Representative images are shown. (E and F) Either the CRTH2 mRNA (E) or IL-4/IL-13 mRNA (F) expression was significantly increased at 3 days after UUO in CD45-positive fractions, but not in CD45-negative fractions (n=4 for each assay, ΣP<0.05 versus negative fraction).
An antifibrotic effect of CAY10471 was observed at day 3 after UUO, suggesting early CRTH2 activation. To determine which types of kidney cells express CRTH2, we sorted the leukocyte-rich fraction (CD45+ fraction) from other cells (CD45− fraction) from sham-operated and UUO kidney at 3 days after surgery (Supplemental Figure 5). UUO significantly increased the mRNA expression of CRTH2 in the CD45+ fraction, but not in the CD45− fraction (Figure 5E). Consistent with this, the mRNA expression levels of IL-4 and IL-13 were significantly increased in the cells obtained from the leukocyte-rich fraction, but not in those obtained from the other fraction (Figure 5F). These results indicated that CRTH2 is predominantly expressed and activated on infiltrating leukocytes, but not renal parenchymal cells in the UUO kidneys at this time point. Moreover, flow cytometry analysis confirmed that CRTH2 is expressed on CD4+ T cells (Supplemental Figure 6), and intracellular cytokine staining for IL-4 revealed that IL-4+ Th2 lymphocytes express CRTH2 (Supplemental Figure 7). These results indicated that CRTH2 expressed on TH2 lymphocytes in the obstructed kidney are the major target of CAY10471.
Attenuation of UUO-Induced Renal Fibrosis in IL-4–Deficient Mice and IL-13–Deficient Mice
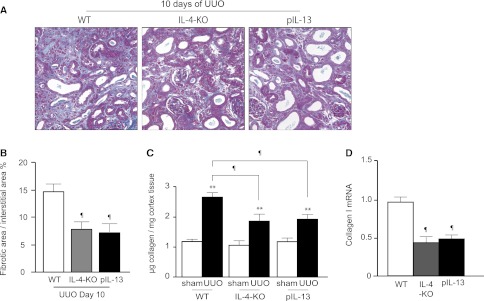
Finally, we examined the effect of IL-4 and IL-13 on renal fibrosis after UUO. IL-4–deficient mice (IL4-KO) and IL-13 distal promoter (CGRE)–deficient mice (pIL-13-KO) were subjected to UUO and the obstructed kidneys were harvested 10 days after UUO. Semiquantification of interstitial collagen deposition on Azan-stained sections revealed that the area of the cortex that stained positive for collagen was significantly reduced in both IL-4-KO and pIL-13-KO mice compared with WT mice (7.83%±0.70% and 7.22%±0.75% versus 14.69%±1.36%, respectively; n=5 in each group) (Figure 6, A and B). Consistent with these findings, quantification of soluble collagen content (Figure 6C) and collagen I mRNA expression (Figure 6D) in the obstructed kidneys 10 days after UUO were significantly attenuated in both IL-4-KO and pIL-13-KO mice compared with WT mice.
Figure 6.
The progression of renal fibrosis is attenuated in both IL-4 deficient and IL-13 deficient mice. (A) Representative images of Azan-stained sections of kidneys on day 10 after UUO in sham and UUO mice of WT, IL-4-KO, and pIL-13-KO. Quantification of (B) interstitial collagen deposition (blue), (C) soluble collagen content, and (D) relative collagen I mRNA expression 10 days after UUO in the cortex of WT, IL-4-KO, and pIL-13-KO mice (n=5 in each group). Data are the mean ± SD. **P<0.05 versus sham; ¶P<0.05 versus WT mice (two-sided t test).
Discussion
In this study, we demonstrated that L-PGDS, an enzyme responsible for PGD2 biosynthesis, is synthesized de novo in the tubular epithelium in the renal cortex after UUO. Consequently, locally produced PGD2 mediates the activation of Th2 lymphocytes, which directly promote fibrosis via the production of IL-4 and IL-13. UUO-induced tubulointerstitial fibrosis and the activation of Th2 lymphocytes were significantly ameliorated in L-PGDS-KO mice and CRTH2-KO mice, or after treatment with a CRTH2 antagonist. In addition, UUO-induced tubulointerstitial fibrosis was blunted in IL-4-KO and pIL-13-KO mice. These findings indicate that L-PGDS–derived PGD2 plays a pathologic role in the progression of renal fibrosis via CRTH2-mediated activation of Th2 lymphocytes. Th2 cell–produced IL-4 and IL-13 are critical mediators of renal fibrosis.
Advanced renal fibrosis in patients with CKD,17 as well as in animal models of renal fibrosis (including UUO),18–20 is invariably associated with tubulointerstitial infiltration of T lymphocytes. Recently, the pivotal role of CD4+ T cells in the progression of renal fibrosis was discovered using severe combined immunodeficient mice and a depleting anti-CD4 antibody.21 Furthermore, numerous studies in cytokine-deficient mice and using neutralizing antibodies have demonstrated that the development of the CD4+ Th2 cell response, including the local production of IL-4 and IL-13, is strongly associated with liver fibrosis,22,23 scleroderma,24 and pulmonary fibrosis.25–27 Stimulation of cultured fibroblasts with IL-4 or IL-13 triggers upregulation of the expression of the extracellular matrix proteins type I and III collagen.28–30 Despite this, to the best of our knowledge, this study is the first report demonstrating that CD4+ Th2 cells play a key role in renal fibrosis and that a CRTH2 antagonist can slow the progression of renal fibrosis by suppressing the Th2 cell response.
Ragolia et al.31 showed that L-PGDS KO mice developed glomerular hypertrophy, fibrosis, and basement membrane thickening on a low-fat diet, with exacerbation of these indices when the mice were moved to a high-fat diet. The results presented by this study are seemingly inconsistent with these reported renoprotective action of L-PGDS. Renal phenotype observed in L-PGDS-KO mice was secondary to the development of hyperglycemia due to insulin resistance developing with age. The magnitude of tubulointerstitial fibrosis is simply reflected by the different levels of glomerular injury. We clearly demonstrated that tubulointerstitial fibrosis caused by UUO was significantly suppressed in L-PGDS-KO mice compared with WT mice, despite the fact that the dilation of tubular lumens in obstructed kidneys after UUO was more severe in L-PGDS-KO mice than in WT mice. Because the mechanisms by which L-PGDS and PGD2 contribute to tubulointerstitial fibrosis are different, our results are not exactly comparable and not conflicting with the previous findings.
Zhang et al.32 demonstrate that PGD2 inhibits TGF-β1–induced epithelial to mesenchymal transition (EMT) in MDCK cells, and EMT is a major event in the pathogenesis of renal tubulointerstitial fibrosis. However, we could not see any difference in the expression levels of cadherin1 and vimentin, hallmarks of EMT, between the kidney of L-PGDS-KO and WT mice at 5 days and 10 days after UUO (Supplemental Figure 4).
In this study, we demonstrated that both the number of infiltrating CD3+ CD4+ lymphocytes and the gene expression of Gata3 in the UUO kidneys was significantly lower in L-PGDS-KO mice compared with WT mice, whereas both indices were comparable between CRTH2-KO and WT mice. The different phenotypes between L-PGDS-KO and CRTH2-KO mice indicated a CRTH2-independent function of PGD2 in the development of renal diseases. DP receptor deficiency is associated with a significant reduction in the concentration of TH2 cytokines and the extent of lymphocyte accumulation in the lung of a mouse model of asthma,11 and DP receptor antagonism alleviated asthma symptoms.33 By interacting with DP on splenic dendritic cells (DCs), PGD2 confers a more Th2-biased humoral immune response by suppressing the production of IL-12.34 Together, these findings suggested that although mRNA levels of DP1 in a whole kidney was below the measurement threshold, L-PGDS–synthesized PGD2 might regulate Th2 differentiation and proliferation by interacting with DP on kidney DCs. Recently, Snelgrove et al.35 clearly demonstrated the function of renal DCs in obstructed kidney by showing that renal DCs undergo morphologic changes and an increased capacity to induce T cell proliferation in the inflammatory milieu that exists after UUO. These findings support our speculation that PGD2-mediated activation of renal DCs promotes local Th2 responses in inflamed tubulointerstitium. By contrast, the extent of IL-4–producing CD4+ lymphocyte accumulation in UUO kidney was equally suppressed in both L-PGDS-KO and CRTH2-KO mice. Taken together, these findings indicated that PGD2-CRTH2 signaling contributes to the augmentation of Th2 cytokine production rather than the chemotaxis and differentiation of Th2 lymphocytes. The gene expression profiles of the C-C chemokine receptor type 3 ligands eotaxin and RANTES were not affected in L-PGDS-KO mice (Supplemental Figure 3). Thus, it seems likely that eosinophil accumulation is not affected by L-PGDS–derived PGD2 in UUO kidney.
Regardless of disease etiology, tubulointerstitial fibrosis is the common pathway leading to end stage renal failure in many kidney diseases and is seen as a prognostic factor for renal function.1–3 Our results suggest that the PGD2–CRTH2 pathway may be an effective target for therapeutic intervention in tubulointerstitial fibrosis, thereby ultimately slowing the progression to advanced renal failure and, more importantly, reducing the risk of cardiovascular events.
Whether this hypothesis also holds true for more selective antagonists remains to be confirmed in a diabetic nephropathy model and eventually needs to be tested in patients with various stages of diabetic nephropathy. A CRTH2 antagonist would fall in the category of immune modulation therapy, due to its potential effects on the innate immune response. However, clinical trials with CRTH2 antagonists thus far, which have been mainly for respiratory indications such as asthma, indicate no serious safety problems associated with a selective CRTH2 antagonist.
Concise Methods
Animals and UUO
C57BL/6 mice were purchased from CLEA Japan (Tokyo, Japan). L-PGDS–deficient (L-PGDS-KO)36 and CRTH2-deficient (CRTH2-KO) mice were provided by Dr. Urade (Osaka Bioscience Institute, Osaka, Japan) and Dr. Nakamura (Tokyo Medical and Dental University, Tokyo, Japan), respectively. IL-4–deficient (IL4-KO) and IL-13 distal promoter (CGRE)–deficient (pIL13-KO)37 mice were provided by Dr. Kubo (RIKEN Yokohama Institute, Yokohama, Japan). All knockout mice used were bred on a C57BL/6 background. Male mice (aged 9–11 weeks) were used in all experiments. UUO was performed as described previously.38 At the appropriate time after UUO, mice were anesthetized and their kidneys perfused with ice-cold PBS and 4% paraformaldehyde via left ventricular puncture. Kidneys were then harvested and coronal slices were fixed with 4% paraformaldehyde before being embedded in paraffin for subsequent histologic and immunohistochemical evaluation. Samples of renal tissues were separated into the cortex and medulla, and these sections were snap-frozen in liquid nitrogen until biochemical analysis.
Gene Expression
Total RNA was isolated from the kidney cortex using the RNeasy Mini Kit (QIAGEN, Valencia, CA). cDNA was synthesized using the First-strand cDNA synthesis kit (Invitrogen, Carlsbad, CA). Quantitative real-time PCR was performed using the ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA). Predesigned gene-specific primer and probe sets (Taqman Gene Expression Assays, Applied Biosystems) were used. 18S ribosomal RNA was amplified as an internal control. All samples were run in triplicate.
Western Blotting
Kidneys were harvested and homogenized with lysis buffer (100 mM NaCl, 20 mM Tris-HCl, pH 7.4, 10 mM Na4P2O7, 1% deoxycholic acid, 10% glycerol, 5 mM EDTA, 1% Triton-X, 0.1% SDS and protease inhibitor cocktail [Nacalai Tesque, Kyoto, Japan]). Extracts from each sample were applied to SDS-PAGE. Rabbit anti-mouse L-PGDS polyclonal antibody was purchased from Cayman Chemical (Ann Arbor, MI). Protein expression was visualized using a horseradish peroxidase (HP)–conjugated secondary antibody and enhanced chemiluminescence (GE Healthcare, Little Chalfont, UK) and was detected using the LAS-3000 lumino-image analyzer (Fujifilm, Tokyo, Japan).
Histology
Periodic acid–Schiff and Azan staining were performed on 3-μm sections of paraffin-embedded kidneys. The dimensions of the tubular lumen, as well as interstitial collagen deposition relative to total interstitium, were analyzed quantitatively in 10 high-power fields of the cortical area per section using BZ-II Analyzer software (Keyence, Osaka, Japan) on a BIOREVO BZ-9000 microscope (Keyence). Briefly, the tonality of both the fibrosis area in Azan-stained sections (blue) and the collagen I deposition visualized with biotinylated rabbit anti-mouse collagen I antibody (ab21286; Abnova, Taiwan) followed by HP-conjugated streptavidin and diaminobenzidine (Bister) was determined with control sections as reference. The number of pixels with the predetermined color of tone were counted in each section, and then automatically converted into dimensions. In all experiments, glomerular area was subtracted from total cortical area.
Immunohistochemistry
Mouse kidneys were fixed in 10% formaldehyde and embedded in paraffin. Paraffin sections (4 μm) were mounted on glass slides, deparaffinized in xylene, and rehydrated in ethanol with increasing concentrations of water. The rehydrated sections were pretreated with 0.3% (v/v) H2O2 in methanol for 10 minutes at room temperature. Sections were then incubated for 20 minutes at room temperature with Protein Block Solution (DAKO, Glostrup, Denmark), for 16 hours at 4°C with rabbit anti-mouse L-PGDS polyclonal antibody (Cayman Chemical) or with rat anti-mouse F4/80 mAb (AbD Serotec, Kidlington, United Kingdom) in PBS containing 1% BSA, followed by 30 minutes of incubation at room temperature with peroxidase-labeled polymer (MAX-PO(R) or MAX-PO(Rat); Nichirei Bioscience, Tokyo, Japan). Lotus tetragonolobus lectin (B-1325; Vector Labs, CA) was used for double staining with L-PGDS. Immunoreactive signals were visualized as the streptavidin-biotinylated enzyme complex (SAB-PO(M); Nichirei). Sections were then counterstained with hematoxylin and observed under a BIOREVO BZ-9000 microscope (Keyence). Paraformaldehyde (2%)–fixed frozen kidney sections were used for immunofluorescence with phycoerythrin (PE)–conjugated anti-CD4 antibody (GK1.5; eBioscience, San Diego, CA) and 4′,6′-diamidino-2-phenylindole. The number of CD4+ cells infiltrating into the interstitium was counted in 10 high-power fields of the cortical area per section.
In Situ Hybridization
In situ hybridization was performed using our modified procedure, which is based on previous reports.39,40 Briefly, the digoxigenin (DIG)–labeled RNA probe for human L-PGDS was prepared as follows. The coding region of mouse L-PGDS was subcloned into pGEM-T Easy vector (Promega, Fitchburg, WI). DIG-labeled cRNA probes were synthesized using SP6 (antisense) or T7 (sense) RNA polymerase (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions and further purified using a Chroma Spin Column (BD Biosciences, San Jose, CA). The efficiency of DIG incorporation was determined by dot-blot analysis. Sections were treated with 10 μg/ml proteinase K for 15 minutes at 37°C, acetylated, and then incubated overnight at 70°C in hybridization buffer containing 0.5 μg/ml DIG-labeled riboprobes. Sections were then treated sequentially in 2× SSC/50% formamide for 20 minutes at 70°C three times, followed by 20 minutes at 70°C in 0.2× SSC for a further three times. The hybridized probe was detected using HP-conjugated anti-DIG antibody in a TSA-Plus DNP (dinitrophenol) AP System (Perkin-Elmer, Waltham, MA). Briefly, sections were incubated overnight at 4°C with HP-conjugated anti-DIG antibody (DakoCytomation, Denmark; 1:100 in blocking buffer). After three washes in TNT (0.1 M Tris-HCl, pH 7.5, 0.15 M NaCl, 0.05% Tween 20) for 5 minutes each time, sections were treated with 1:50 diluted TSA-Plus DNP reagents (Perkin-Elmer) for 10 minutes according to the manufacturer’s instructions, and the DIG signals were converted to DNP signals. After three washes in TNT for 5 minutes each time, sections were incubated overnight at 4°C with an alkaline phosphatase–conjugated anti-DNP antibody (1:100) in 1% blocking buffer. The chromogen reaction of alkaline phosphatase was performed with nitro-blue tetrazolium chloride/5-bromo-4-chloro-3′-indolylphosphatase p-toluidine salt (NBT/BCIP; Promega).
Quantification of Soluble Collagen
The total amount of soluble collagen was determined using a Sircol Collagen Assay Kit (Biocolor, Carrickfergus, UK) according to the manufacturer’s instructions. Briefly, 100 μl kidney cortex tissue lysate and 100 μl reference collagen standard were mixed with 1 ml Sircol dye for 30 minutes and then centrifuged at 15,000×g for 10 minutes to precipitate the collagen–dye complex. After decanting the suspension, the droplets were dissolved in 1 ml Sircol alkali reagent and vortexed. The absorbance of the solution was then read at 540 nm. All measurements were performed in triplicate. The amount of collagen was calculated from a standard curve constructed using the reference collagen standards.
Flow Cytometry
To quantitatively analyze the number of infiltrating leukocytes, renal cortex tissue was digested enzymatically and the suspension was analyzed by multicolor flow cytometry. Briefly, cortex tissues were dissected, placed in RPMI1640 medium containing 40 μg/ml Liberase TM (Roche) and 8.5 U/ml DNase I (Roche) for 25 minutes at 37°C, and then washed with serum-free RPMI1640 medium. After erythrocyte lysis according to the manufacturer’s instructions (1× RBC Lysis Buffer; eBioscience), cells were resuspended in FACS buffer (1% BSA/2 mM EDTA/PBS). Cell aliquots were labeled with anti-CD45-FITC, anti-CD3-APC, anti-CD3-FITC, anti-CD4-PE, anti-CD11b-FITC (eBioscience), anti-CD11c-PE, and anti-CD206-Alexafluor647 (Biolegend) for 30 minutes at 4°C, and then washed with FACS buffer. For intracellular staining, CD4+ T cells were collected by depletion of non-CD4+ cells using the MACS CD4+ T cell Isolation Kit II for Mouse (Miltenyi Biotech, Auburn, CA). Isolated CD4+ T cells were then incubated for 4.5 hours at 37°C with RPMI1640 containing 10% FBS, 1% penicillin–streptomycin, 50 ng/ml PMA, 1 μg/ml ionomycin, and Golgistop (Cytofix/Cytoperm Plus Kit with Golgistop; BD Biosciences, Franklin Lakes, NJ). Cells were then incubated for 5 minutes at 4°C with Mouse FcBlock (2.4G2; BD Biosciences), followed by incubation with PE-conjugated anti-CD4 antibody (GK1.5; eBioscience) for 30 minutes at 4°C. After fixation and permeabilization, cells were incubated for 30 minutes at 4°C with IL-4-APC (11B11; eBioscience) or IL-4-PE, and IFN-γ-FITC (XMG1.2; eBioscience) before being acquired using a FACS AriaII and analyzed with FACSDiva software (BD Biosciences).
We addressed CRTH2 expression using the MACS procedure described above. Briefly, the CD45-positive population was collected by “positive selection” using CD45 MicroBeads (Miltenyi Biotech, Auburn, CA) followed by FACS analysis with CD45-PE-Cy5.5 (clone 30-F11) and CD11b-FITC (clone M1–70) (eBioscience). For CRTH2 expression by FACS analysis, we used rabbit anti-CRTH2 polyclonal antibody (LS-B3209; LSBio, Seattle, WA) and Alexafluor 647 goat anti-rabbit IgG (A-21244; Molecular Probes) as a secondary antibody.
CAY10471 Treatment
CAY10471 (Cayman Chemical) was made up to a stock solution of 20 mg/ml in DMSO and was stored at –20°C until use. Just before use, CAY10471 was suspended in MC. Mice were treated with oral doses of 20 mg/kg CAY10471 twice daily. Control mice were dosed with an equivalent concentration of DMSO in MC.
Measurement of Urinary Tetranor-PGDM
Tetranor-PGDM is a metabolite of PGD2 that is detectable by mass spectrometry in the urine of mice and humans.41 In this study, the urine retained in the urinary duct was collected from WT and L-PGDS-KO mice 10 days after UUO, and the amount of tetranor-PGDM was determined by liquid chromatography–tandem mass spectrometry, as described previously.41 Creatinine concentrations were determined using a commercially available assay kit (Wako Pure Chemical, Osaka, Japan).
Statistical Analyses
The significance of differences between two mean values was determined using unpaired t tests. Multiple comparisons involving more than three groups were analyzed by ANOVA. P<0.05 was considered significant. Data were analyzed using Prism 5 software (GraphPad Software, San Diego, CA) and are given as the mean ± SEM.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was partially supported by the Molecular Nephrology Forum. This work was also supported by a PRESTO (Metabolism and Cellular Function) grant from the Japanese Science and Technology Agency (to M.S.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012020126/-/DCSupplemental.
References
- 1.Nath KA: Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Harris RC, Neilson EG: Toward a unified theory of renal progression. Annu Rev Med 57: 365–380, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bohle A, Strutz F, Müller GA: On the pathogenesis of chronic renal failure in primary glomerulopathies: A view from the interstitium. Exp Nephrol 2: 205–210, 1994 [PubMed] [Google Scholar]
- 4.Sharma K, Ix JH, Mathew AV, Cho M, Pflueger A, Dunn SR, Francos B, Sharma S, Falkner B, McGowan TA, Donohue M, Ramachandrarao S, Xu R, Fervenza FC, Kopp JB: Pirfenidone for diabetic nephropathy. J Am Soc Nephrol 22: 1144–1151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirawa N, Uehara Y, Ikeda T, Gomi T, Hamano K, Totsuka Y, Yamakado M, Takagi M, Eguchi N, Oda H, Seiki K, Nakajima H, Urade Y: Urinary prostaglandin D synthase (beta-trace) excretion increases in the early stage of diabetes mellitus. Nephron 87: 321–327, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Hamano K, Totsuka Y, Ajima M, Gomi T, Ikeda T, Hirawa N, Eguchi Y, Yamakado M, Takagi M, Nakajima H, Oda H, Seiki K, Eguchi N, Urade Y, Uehara Y: Blood sugar control reverses the increase in urinary excretion of prostaglandin D synthase in diabetic patients. Nephron 92: 77–85, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Hirawa N, Uehara Y, Yamakado M, Toya Y, Gomi T, Ikeda T, Eguchi Y, Takagi M, Oda H, Seiki K, Urade Y, Umemura S: Lipocalin-type prostaglandin d synthase in essential hypertension. Hypertension 39: 449–454, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Nagata N, Fujimori K, Okazaki I, Oda H, Eguchi N, Uehara Y, Urade Y: De novo synthesis, uptake and proteolytic processing of lipocalin-type prostaglandin D synthase, beta-trace, in the kidneys. FEBS J 276: 7146–7158, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Tsuchida T, Eguchi N, Eguchi Y, Numabe A, Nakajima H, Oda H, Seiki K, Hakamada-Taguchi R, Urade Y, Uehara Y: Lipocalin-type prostaglandin D synthase in urine in adriamycin-induced nephropathy of mice. Nephron Physiol 96: 42–51, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Giles H, Leff P, Bolofo ML, Kelly MG, Robertson AD: The classification of prostaglandin DP-receptors in platelets and vasculature using BW A868C, a novel, selective and potent competitive antagonist. Br J Pharmacol 96: 291–300, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, Sugimoto Y, Kobayashi T, Ushikubi F, Aze Y, Eguchi N, Urade Y, Yoshida N, Kimura K, Mizoguchi A, Honda Y, Nagai H, Narumiya S: Prostaglandin D2 as a mediator of allergic asthma. Science 287: 2013–2017, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Gosset P, Pichavant M, Faveeuw C, Bureau F, Tonnel AB, Trottein F: Prostaglandin D2 affects the differentiation and functions of human dendritic cells: Impact on the T cell response. Eur J Immunol 35: 1491–1500, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Tanaka K, Hirai H, Takano S, Nakamura M, Nagata K: Effects of prostaglandin D2 on helper T cell functions. Biochem Biophys Res Commun 316: 1009–1014, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, Ichimasa M, Sugamura K, Nakamura M, Takano S, Nagata K: Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med 193: 255–261, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue L, Gyles SL, Wettey FR, Gazi L, Townsend E, Hunter MG, Pettipher R: Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol 175: 6531–6536, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Chevalier RL, Forbes MS, Thornhill BA: Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75: 1145–1152, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Robertson H, Ali S, McDonnell BJ, Burt AD, Kirby JA: Chronic renal allograft dysfunction: The role of T cell-mediated tubular epithelial to mesenchymal cell transition. J Am Soc Nephrol 15: 390–397, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Strutz F, Neilson EG: The role of lymphocytes in the progression of interstitial disease. Kidney Int Suppl 45: S106–S110, 1994 [PubMed] [Google Scholar]
- 19.Anders HJ, Vielhauer V, Frink M, Linde Y, Cohen CD, Blattner SM, Kretzler M, Strutz F, Mack M, Gröne HJ, Onuffer J, Horuk R, Nelson PJ, Schlöndorff D: A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J Clin Invest 109: 251–259, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eis V, Luckow B, Vielhauer V, Siveke JT, Linde Y, Segerer S, Perez De Lema G, Cohen CD, Kretzler M, Mack M, Horuk R, Murphy PM, Gao JL, Hudkins KL, Alpers CE, Gröne HJ, Schlöndorff D, Anders HJ: Chemokine receptor CCR1 but not CCR5 mediates leukocyte recruitment and subsequent renal fibrosis after unilateral ureteral obstruction. J Am Soc Nephrol 15: 337–347, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Tapmeier TT, Fearn A, Brown K, Chowdhury P, Sacks SH, Sheerin NS, Wong W: Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int 78: 351–362, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Cheever AW, Williams ME, Wynn TA, Finkelman FD, Seder RA, Cox TM, Hieny S, Caspar P, Sher A: Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J Immunol 153: 753–759, 1994 [PubMed] [Google Scholar]
- 23.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA: An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest 104: 777–785, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong C, Wong C, Roberts CR, Teh HS, Jirik FR: Anti-IL-4 treatment prevents dermal collagen deposition in the tight-skin mouse model of scleroderma. Eur J Immunol 28: 2619–2629, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Blease K, Jakubzick C, Westwick J, Lukacs N, Kunkel SL, Hogaboam CM: Therapeutic effect of IL-13 immunoneutralization during chronic experimental fungal asthma. J Immunol 166: 5219–5224, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Keane MP, Gomperts BN, Weigt S, Xue YY, Burdick MD, Nakamura H, Zisman DA, Ardehali A, Saggar R, Lynch JP, 3rd, Hogaboam C, Kunkel SL, Lukacs NW, Ross DJ, Grusby MJ, Strieter RM, Belperio JA: IL-13 is pivotal in the fibro-obliterative process of bronchiolitis obliterans syndrome. J Immunol 178: 511–519, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kolodsick JE, Toews GB, Jakubzick C, Hogaboam C, Moore TA, McKenzie A, Wilke CA, Chrisman CJ, Moore BB: Protection from fluorescein isothiocyanate-induced fibrosis in IL-13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts. J Immunol 172: 4068–4076, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Fertin C, Nicolas JF, Gillery P, Kalis B, Banchereau J, Maquart FX: Interleukin-4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cell Mol Biol 37: 823–829, 1991 [PubMed] [Google Scholar]
- 29.Doucet C, Brouty-Boyé D, Pottin-Clémenceau C, Canonica GW, Jasmin C, Azzarone B: Interleukin (IL) 4 and IL-13 act on human lung fibroblasts. Implication in asthma. J Clin Invest 101: 2129–2139, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiggelman AM, Boers W, Linthorst C, Sala M, Chamuleau RA: Collagen synthesis by human liver (myo)fibroblasts in culture: Evidence for a regulatory role of IL-1 beta, IL-4, TGF beta and IFN gamma. J Hepatol 23: 307–317, 1995 [PubMed] [Google Scholar]
- 31.Ragolia L, Palaia T, Hall CE, Maesaka JK, Eguchi N, Urade Y: Accelerated glucose intolerance, nephropathy, and atherosclerosis in prostaglandin D2 synthase knock-out mice. J Biol Chem 280: 29946–29955, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Zhang A, Dong Z, Yang T: Prostaglandin D2 inhibits TGF-beta1-induced epithelial-to-mesenchymal transition in MDCK cells. Am J Physiol Renal Physiol 291: F1332–F1342, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Arimura A, Yasui K, Kishino J, Asanuma F, Hasegawa H, Kakudo S, Ohtani M, Arita H: Prevention of allergic inflammation by a novel prostaglandin receptor antagonist, S-5751. J Pharmacol Exp Ther 298: 411–419, 2001 [PubMed] [Google Scholar]
- 34.Faveeuw C, Gosset P, Bureau F, Angeli V, Hirai H, Maruyama T, Narumiya S, Capron M, Trottein F: Prostaglandin D2 inhibits the production of interleukin-12 in murine dendritic cells through multiple signaling pathways. Eur J Immunol 33: 889–898, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Snelgrove SL, Kausman JY, Lo C, Lo C, Ooi JD, Coates PT, Hickey MJ, Holdsworth SR, Kurts C, Engel DR, Kitching AR: Renal dendritic cells adopt a pro-inflammatory phenotype in obstructive uropathy to activate T cells but do not directly contribute to fibrosis. Am J Pathol 180: 91–103, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Eguchi N, Minami T, Shirafuji N, Kanaoka Y, Tanaka T, Nagata A, Yoshida N, Urade Y, Ito S, Hayaishi O: Lack of tactile pain (allodynia) in lipocalin-type prostaglandin D synthase-deficient mice. Proc Natl Acad Sci U S A 96: 726–730, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka S, Motomura Y, Suzuki Y, Yagi R, Inoue H, Miyatake S, Kubo M: The enhancer HS2 critically regulates GATA-3-mediated Il4 transcription in T(H)2 cells. Nat Immunol 12: 77–85, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Kaneto H, Morrissey J, Klahr S: Increased expression of TGF-beta 1 mRNA in the obstructed kidney of rats with unilateral ureteral ligation. Kidney Int 44(2313–321, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Liang F, Hatanaka Y, Saito H, Yamamori T, Hashikawa T: Differential expression of gamma-aminobutyric acid type B receptor-1a and -1b mRNA variants in GABA and non-GABAergic neurons of the rat brain. J Comp Neurol 416: 475–495, 2000 [PubMed] [Google Scholar]
- 40.Komatsu Y, Watakabe A, Hashikawa T, Tochitani S, Yamamori T: Retinol-binding protein gene is highly expressed in higher-order association areas of the primate neocortex. Cereb Cortex 15: 96–108, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Song WL, Wang M, Ricciotti E, Fries S, Yu Y, Grosser T, Reilly M, Lawson JA, FitzGerald GA: Tetranor PGDM, an abundant urinary metabolite reflects biosynthesis of prostaglandin D2 in mice and humans. J Biol Chem 283: 1179–1188, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.