Abstract
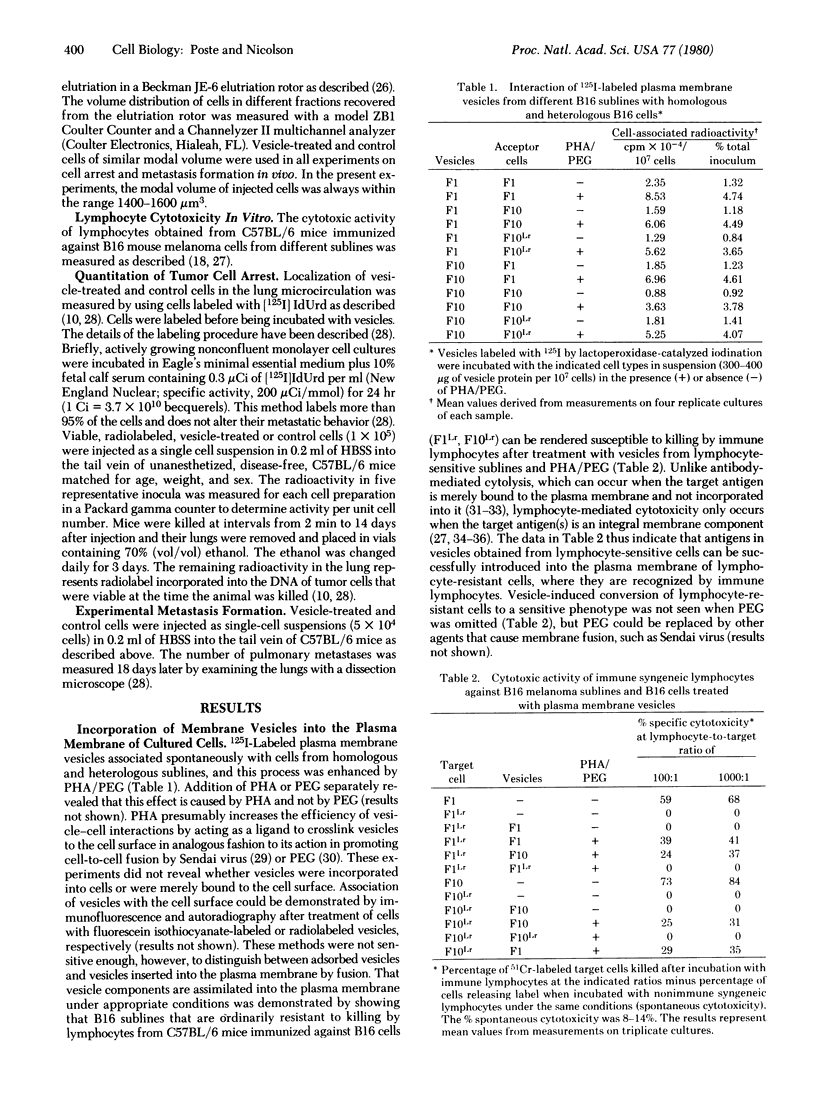
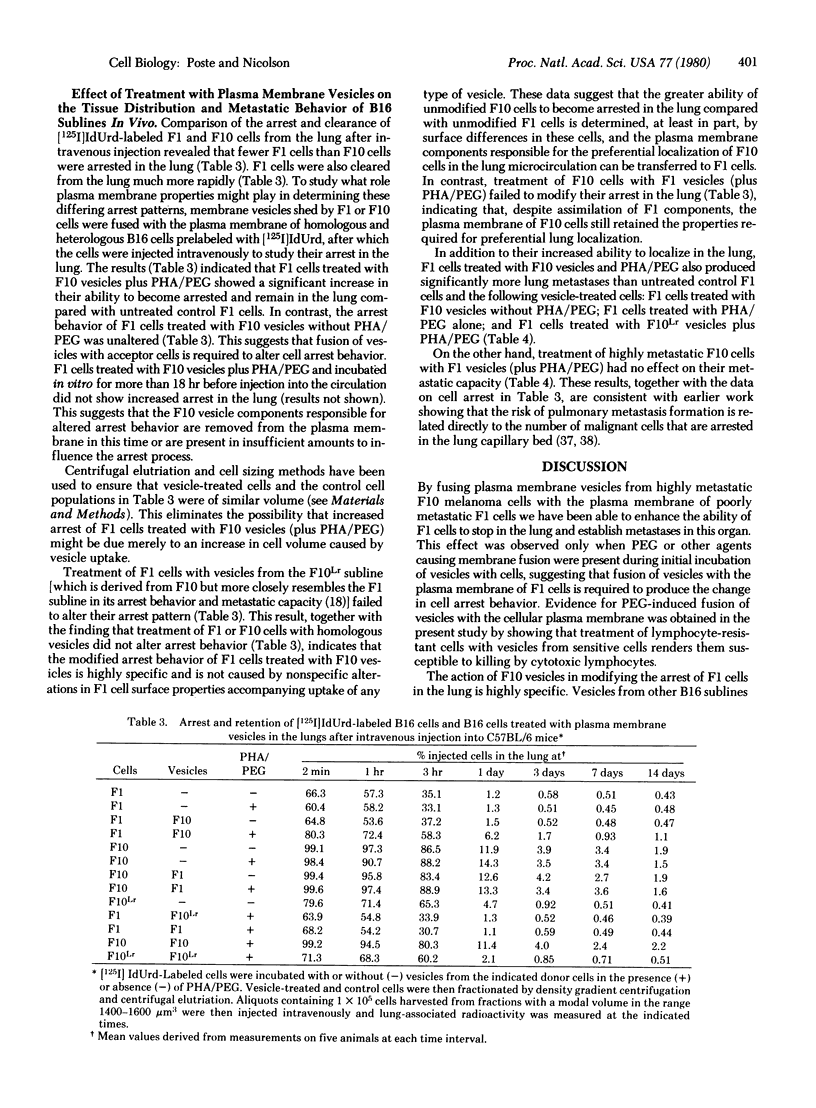
B16 mouse melanoma sublines in culture spontaneously shed intact plasma membrane vesicles. These vesicles can be fused with the plasma membrane of cells from homologous and heterologous B16 sublines by using polyethylene glycol and phytohemagglutinin-P. Fusion of vesicles from a highly metastatic subline (F10) that localizes exclusively in the lung with cells from a poorly metastatic subline (F1) significantly increased the ability of F1 cells to become arrested in the lung and form metastases in this organ. In contrast, fusion of F1 vesicles with F10 cells did not alter the ability of vesicle-modified cells to localize in the lung or form lung metastases. F10 vesicle-modified F1 cells reverted to their original arrest behavior and metastatic capacity after removal of F10 vesicle components from the plasma membrane. The changes in the arrest and metastatic behavior of F10 vesicle-modified F1 cells were highly highly specific. Vesicles from other B16 sublines that are poorly metastatic and show limited localization in the lung (F1, FLLr, and F10Lr) did not modify the arrest behavior and metastatic capacity of FU cells. These results suggest that the differences in the abilities of the F1 and F10 sublines to localize in the lung are determined by differences in cell surface properties.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown S., Teplitz M., Revel J. P. Interaction of mycoplasmas with cell cultures, as visualized by electron microscopy. Proc Natl Acad Sci U S A. 1974 Feb;71(2):464–468. doi: 10.1073/pnas.71.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson K. W., Beattie G., Nicolsin G. L. Selection and altered properties of brain-colonising metastatic melanoma. Nature. 1978 Apr 6;272(5653):543–545. doi: 10.1038/272543a0. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- DUNN T. B. Normal and pathologic anatomy of the reticular tissue in laboratory mice, with a classification and discussion of neoplasms. J Natl Cancer Inst. 1954 Jun;14(6):1281–1433. [PubMed] [Google Scholar]
- Fidler I. J. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975 Jan;35(1):218–224. [PubMed] [Google Scholar]
- Fidler I. J., Gersten D. M., Budmen M. B. Characterization in vivo and in vitro of tumor cells selected for resistance to syngeneic lymphocyte-mediated cytotoxicity. Cancer Res. 1976 Sep;36(9 PT1):3160–3165. [PubMed] [Google Scholar]
- Fidler I. J., Gersten D. M., Hart I. R. The biology of cancer invasion and metastasis. Adv Cancer Res. 1978;28:149–250. doi: 10.1016/s0065-230x(08)60648-x. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Nicolson G. L. Fate of recirculating B16 melanoma metastatic variant cells in parabiotic syngeneic recipients. J Natl Cancer Inst. 1977 Jun;58(6):1867–1872. doi: 10.1093/jnci/58.6.1867. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Nicolson G. L. Organ selectivity for implantation survival and growth of B16 melanoma variant tumor lines. J Natl Cancer Inst. 1976 Nov;57(5):1199–1202. doi: 10.1093/jnci/57.5.1199. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Selection of successive tumour lines for metastasis. Nat New Biol. 1973 Apr 4;242(118):148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- Forman J., Finkelstein R. A. Inability of choleragenoid-sensitized cells to induce T cell-mediated cytolysis. J Immunol. 1977 May;118(5):1655–1658. [PubMed] [Google Scholar]
- Grdina D. J., Peters L. J., Jones S., Chan E. Separation of cells from a murine fibrosarcoma on the basis of size. I. Relationship between cell size and age as modified by growth in vivo or in vitro. J Natl Cancer Inst. 1978 Jul;61(1):209–214. doi: 10.1093/jnci/61.1.209. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Wu E., Poste G. Measurement of the translational mobility of concanavalin A in glycerol-saline solutions and on the cell surface by fluorescence recovery after photobleaching. Biochim Biophys Acta. 1976 Apr 16;433(1):215–222. doi: 10.1016/0005-2736(76)90189-9. [DOI] [PubMed] [Google Scholar]
- KINSEY D. L. An experimental study of preferential metastasis. Cancer. 1960 Jul-Aug;13:674–676. doi: 10.1002/1097-0142(196007/08)13:4<674::aid-cncr2820130405>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Koszinowski U., Gething M. J., Waterfield M. T-cell cytotoxicity in the absence of viral protein synthesis in target cells. Nature. 1977 May 12;267(5607):160–163. doi: 10.1038/267160a0. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Nicolson G. L. Interactions of tumor cells with vascular endothelial cell monolayers: a model for metastatic invasion. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5704–5708. doi: 10.1073/pnas.76.11.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrle R., Wagner H., Röllinghoff M., Rott R. Influenza virus-specific T cell-mediated cytotoxicity: integration of the virus antigen into the target cell membrane is essential for target cell formation. Eur J Immunol. 1979 Feb;9(2):107–111. doi: 10.1002/eji.1830090203. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liotta L. A., Vembu D., Saini R. K., Boone C. In vivo monitoring of the death rate of artificial murine pulmonary micrometastases. Cancer Res. 1978 May;38(5):1231–1236. [PubMed] [Google Scholar]
- Mercer W. E., Schlegel R. A. Phytohemagglutinin enhancement of cell fusion reduces polyethylene glycol cytotoxicity. Exp Cell Res. 1979 May;120(2):417–421. doi: 10.1016/0014-4827(79)90403-8. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Brunson K. W., Fidler I. J. Specificity of arrest, survival, and growth of selected metastatic variant cell lines. Cancer Res. 1978 Nov;38(11 Pt 2):4105–4111. [PubMed] [Google Scholar]
- Onuigbo W. I. Organ selectivity in human cancer metastasis. A review. Oncology. 1974;30(4):294–303. doi: 10.1159/000224968. [DOI] [PubMed] [Google Scholar]
- Ozato K., Henney C. S. Studies on lymphocyte-mediated cytolysis. XII. Hapten transferred to cell surfaces by interaction with liposomes is recognized by antibody but not by hapten-specific H-2 restricted cytotoxic T cells. J Immunol. 1978 Dec;121(6):2405–2412. [PubMed] [Google Scholar]
- Parks R. C. Organ-specific metastasis of a transplantable reticulum cell sarcoma. J Natl Cancer Inst. 1974 Mar;52(3):971–973. doi: 10.1093/jnci/52.3.971. [DOI] [PubMed] [Google Scholar]
- Petitou M., Tuy F., Rosenfeld C., Mishal Z., Paintrand M., Jasnin C., Mathe G., Inbar M. Decreased microviscosity of membrane lipids in leukemic cells: two possible mechanisms. Proc Natl Acad Sci U S A. 1978 May;75(5):2306–2310. doi: 10.1073/pnas.75.5.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgrim H. I. The kinetics of the organ-specific metastasis of a transplantable reticuloendothelial tumor. Cancer Res. 1969 Jun;29(6):1200–1205. [PubMed] [Google Scholar]
- Pollard M., Luckert P. H. Transplantable metastasizing prostate adenocarcinomas in rats. J Natl Cancer Inst. 1975 Mar;54(3):643–649. [PubMed] [Google Scholar]
- Raz A., Barzilai R., Spira G., Inbar M. Oncogenicity and immunogenicity associated with membranes isolated from cell-free ascites fluid of lymphoma-bearing mice. Cancer Res. 1978 Aug;38(8):2480–2485. [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Studzinski G. P., Gierthy J. F., Cholon J. J. An autoradiographic screening test for mycoplasmal contamination of mammalian cell cultures. In Vitro. 1973 May-Jun;8(6):466–472. doi: 10.1007/BF02615948. [DOI] [PubMed] [Google Scholar]
- Sugamura K., Shimizu K., Bach F. H. Involvement of fusion activity of ultraviolet light-inactivated Sendai virus in formation of target antigens recognized by cytotoxic T cells. J Exp Med. 1978 Jul 1;148(1):276–287. doi: 10.1084/jem.148.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarbaker E. V., Cohen A. M., Ketcham A. S. Do metastases metastasize? Ann Surg. 1971 Aug;174(2):161–166. doi: 10.1097/00000658-197108000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T., Matter A., Vogel K., Burger M. M. Liver-colonizing melanoma cells selected from B-16 melanoma. Int J Cancer. 1979 Jun 15;23(6):854–857. doi: 10.1002/ijc.2910230618. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk W. J., Emmelot P., Hilkmann H. A., Hilgers J., Feltkamp C. A. Rigid plasma-membrane-derived vesicles, enriched in tumour-associated surface antigens (MLr), occurring in the ascites fluid of a murine leukaemia (GRSL). Int J Cancer. 1979 Jan 15;23(1):62–70. doi: 10.1002/ijc.2910230112. [DOI] [PubMed] [Google Scholar]


