Abstract
This study was designed to further our understanding of a potentially significant clinical event of negative nasal airflow near the end of the respiratory pause (inhibition) to accommodate swallowing. This negative flow, referred to as “SNIF,” or swallow noninspiratory flow, occurs at the onset of airway reestablishment at the conclusion of the oropharyngeal swallow. Using simultaneous digital video fluoroscopic and nasal respiratory air-flow recordings on 82 healthy adults (21–97 years old), the objectives of this study were to determine (1) the frequency of occurrence of SNIF during a 5-ml natural cup-drinking task, (2) differences in SNIF occurrence by age group, and (3) the temporal relationship between SNIF and other swallowing events. Results revealed that for most participants SNIF was observed in both swallowing trials. There was a statistically significant difference in SNIF occurrence by age category, with SNIF observed less frequently in the oldest participants. The peak onset of SNIF is closely related to the first release of contact between the soft palate and tongue base with the posterior pharyngeal wall and opening of the laryngeal vestibule. Based on this, and in agreement with previous investigators, we suggest that this negative flow may be related to a partial vacuum established by the relaxation of pharyngeal contraction near the conclusion of the pharyngeal swallow. The more frequent occurrence of SNIF in younger adults and less in older adults suggests a reduction in pharyngeal pressure associated with healthy aging.
Keywords: Swallowing, Deglutition, Respiration, Breathing, Nasal airflow, Deglutition disorders
The coordination of swallowing with respiratory airflow for upper airway protection has been well investigated. Recent radiographic studies of oropharyngeal movements and bolus flow combined with nasal airflow have elucidated airflow events surrounding respiratory swallowing patterning [1–11]. The respiratory cessation/inhibition to accommodate swallowing (also referred to as deglutition apnea or swallowing-related respiratory pause) tends to occur most frequently at end expiratory lung volumes for solid boluses and spontaneously occurring swallows of saliva and other secretions during wakefulness and sleep, and at slightly higher volumes for liquid swallows [12–15]. Respiratory inhibition is a key airway protective event during swallowing and occurs on average 639 ms prior to oral bolus transport and continues an average of 1,125 ms beyond the first movement of the hyoid, marking initiation of the pharyngeal swallow [6]. Additional respiratory events have been recorded immediately before or early within the swallowing-related respiratory pause, including diaphragmatic activation and potential inspiratory efforts against a closed airway [16, 17].
Of particular relevance to the current investigation, brief negative (nadir) pressures have been detected in nasal airflow signals immediately before the termination of the swallowing-related respiratory pause in small studies of healthy younger and older adults [3–6, 9, 10, 18–20] and in other nonhuman species [21]. Given that this respiratory event occurs during the period of zero flow and before full reestablishment of the laryngeal airway following a swallow, it has been referred to as a “brief, non-respiratory inward airflow” (SNIF) event [9]. It has been postulated that this negative flow may be related to a partial vacuum established by the offset of pharyngeal contraction [9, 22]. The physiologic mechanisms responsible for these nonrespiratory flow events and their functional significance are largely unknown and have not been studied in a larger sample of adults across the aging continuum.
Based on our clinical experience, inward airflow occurring during the reestablishment of the airway after the swallow might have important mechanistic and/or diagnostic significance related to swallowing physiology and its disorders. The present investigation, therefore, was designed to determine (1) the frequency of occurrence of SNIF during a 5-ml natural cup-drinking task, (2) the relationship of SNIF with age, and (3) the temporal relationship between SNIF and other swallowing events.
Methods
Participants
Eighty-two healthy, adult volunteers, ranging in age from 21 to 97 years, participated in the investigation. Participants were put into one of four age groups: 21–40, 41–60, 61–80, and ≥81 years. Medical and surgical histories and medications were obtained via patient interview and written questionnaires. Participants were free from a history of oral, nasal, pharyngeal (including uvulopalatopharyngoplasty), laryngeal, and esophageal surgeries. Additional exclusion criteria were known history of dysphagia, hiatal hernia, chronic indigestion, gastroesophageal reflux disease (GERD), pulmonary disease, cancer of the head and neck, neurological disease, current medications with known effects on swallowing or breathing, and tobacco use during the past 10 years. Participants with a history of tonsillectomy, adenoidectomy, or sinus surgery were not excluded. All participants were drinking liquids and eating solid foods as part of a regular diet. In addition to age, sex and race were noted as potential covariates. The study protocol was approved by the institutional review board. All participants gave written informed consent for their participation in this study.
Instrumentation
Participants completed a modified video fluoroscopic procedure. All video fluoroscopic swallow studies (VFSS) were recorded with a digitally synchronized, dual-modality, video recording device with high temporal resolution (Digital Swallowing Workstation Model 7200, KayPEN-TAX Corp., Lincoln Park, NJ). The fluoroscopic unit (Philips Medical Systems, Amsterdam, the Netherlands) was equipped with a 1024-line video system. Video fluoroscopic recordings were made with a temporal resolution of 60 video fields (30 video frames) per second (16.67 ms per digital field). Testing was conducted in a standard fluoroscopy suite. Coning of the X-ray beam limited radiation exposure to the superior structures of the aerodigestive tract. The field of view was delimited anteriorly by the lips, superiorly by the nasal cavity, posteriorly by the cervical spine, and inferiorly by the PES (i.e., pharyngoesophageal segment, C5–C6) [23]. Participants were positioned in the lateral viewing plane while standing and self-administered two trials of 5-ml liquid boluses of barium sulfate contrast solution (Liquid Barosperse Barium Sulfate Suspension, catalog No. 179364, Lafayette Pharmaceuticals, Anaheim, CA) per graded medicine cup. This conservative volume was chosen to simulate a safe bolus size that was typically administered to patients with dysphagia during a VFSS. Participants were instructed to “drink the liquid in [their] usual manner” while limiting head and body motion other than that used during swallowing. No additional instructions (e.g., changes in timing, manner of swallowing) were given because the investigators’ aim was to analyze natural liquid swallowing behavior. The fluoroscope was activated by the radiologist during the participant’s self-administration of the contrast material into the oral cavity and remained activated until the bolus tail entered the esophagus through the PES. Radiation exposure times were 1 min or less for all participants.
Nasal pressure/flow was measured using a standard, 7-ft cannula coupled to a combination pressure transducer and thermistor proprietarily integrated into the Swallowing Signals Lab™ system provided by KayPENTAX, and was recorded with a sampling rate of 250 Hz. The nasal sensor on the Swallowing Signals Lab uses a Honeywell Micro Switch AWM2000 Series Microbridge Mass Airflow sensor to detect the direction and level of airflow at the nares. The microbridge chip is a mass flow sensor chip that uses a thermal transfer mechanism. The more mass flowing past the chip, the more heat is being transferred. By using a twin sensing element, directionality can also be determined. The sensor has two ports that allow air to flow through the sensor. The nasal cannula is connected to one side of the airflow sensor.
Air pressure and direction in the nasal cannula were automatically calibrated for temperature and pressure using proprietary Workstation software immediately prior to the study of each participant. When the nasal channel is calibrated, the software nulls any offsets and sets the displayed signal trace at midscale.
Data Analysis
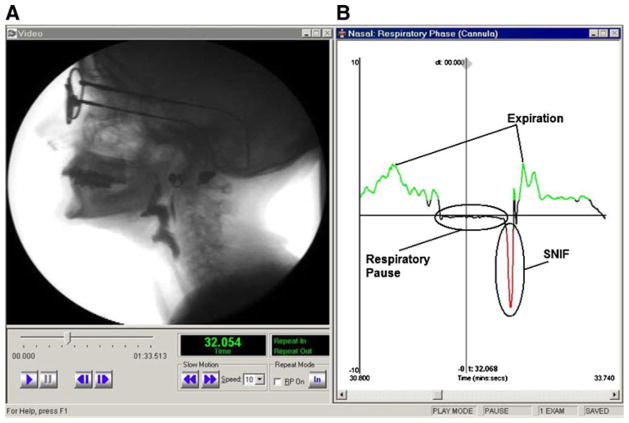
Continuous nasal flow was recorded continuously before, during, and after swallowing. This signal was used to record nasal airflow throughout all test swallows, the respiratory pause to accommodate swallowing, and detailed information regarding nonrespiratory flow events during the respiratory pause related to other swallowing events. A plateau in the respiratory signal along the abscissa indicated periods of zero flow, or respiratory pause. SNIF was operationally defined as the time of a peak in negative polarity of the nasal flow signal that occurred during but not before the cessation of the respiratory pause to accommodate swallowing. The peak in negative polarity was determined by analyzing the respiratory trace signal offline from data acquisition. One investigator (BMH) moved the cursor in the Swallowing Signals Lab software along the abscissa until the peak in SNIF was established by the software as the most negative polarity on the ordinate axis. Radiographically, the aero-digestive tract is returning to rest from full contraction of the oropharyngeal musculature immediately before the resumption of normal breathing during the oropharyngeal swallow (Fig. 1).
Fig. 1.
Simultaneous a videofluoroscopy and b respiratory trace data showing SNIF (screenshot from the Workstation)
We made a series of physiological measurements (as in our previous studies) to begin to describe and explain the functional significance, if any, of the SNIF signals [5, 6]. More specifically, the timing of SNIF with respect to three oropharyngeal swallowing events was measured: (1) onset of laryngeal vestibule opening, (2) release of tongue base from the posterior pharyngeal wall, and (3) release of the soft palate from the posterior pharyngeal wall. These three events were chosen because they occur near the termination of the respiratory pause to accommodate swallowing and our preliminary observations suggested that SNIF occurred in this time frame.
Onsets of all swallowing events were determined using the digital video recorder’s slow-motion and freeze-frame capabilities and measured in milliseconds using the digital display on the Workstation. The same investigator (BMH) made all measurements. An acceptable error rate was established as 2 video fields (1 video frame), or 33.3 ms [6]. The onset of oral bolus transport was established as the point from which all other swallowing events were referenced in time (t0). This is a highly stable event and is consistently related to the onset of the oropharyngeal swallowing [3, 5, 6]. Temporal averages were calculated for each event across all patients. For example, to determine the onset of laryngeal vestibule opening, we determined the first video field in which the separation of the arytenoid cartilages from the epiglottic petiole was viewed [5]. Following this determination, t0 was subtracted from the time at which the cartilages first separated. This analysis procedure was completed for all measures of both boluses presented to each participant.
Statistical Analysis
Frequencies and percentages of SNIF were used to describe swallows during the two 5-ml thin-liquid barium trials in normal participants. Differences in the incidence of SNIF by age group were determined using χ2 analysis. The relationship of SNIF timing to each of the three measured physiologic events was examined using Spearman’s ρ to address the non-normally distributed timing of the temporal events. An α level <0.05 was considered statistically significant.
Results
Demographics
The study sample consisted of 82 participants in four age groups: (1) 21–40 years (n = 21), (2) 41–60 years (n = 21), (3) 61–80 years (n = 19), and (4) ≥81 years (n = 21). Two participants were unable to follow the instructions to swallow the liquid in their usual manner and instead exhibited extraneous head and body movement, limiting the visual field for accurate interpretation. There were four additional subjects with temporal measures that indicated extreme outliers. These six subjects were excluded from the analyses. Thus, a total of 76 participants were included in further analyses: (1) 21–40 years (n = 21), (2) 41–60 years (n = 18), (3) 61–80 years (n = 17), and (4) ≥81 (n = 20).
Occurrence of SNIF in Two Trials
The majority of participants exhibited a SNIF during both trials (trial 1: n = 56, 74%; trial 2: n = 51, 67%). Some participants did not produce SNIF during either trial (trial 1: n = 20, 26%; trial 2: n = 25, 33%), and 11 participants (15%) produced SNIF during only one of the two trials (trial 1: n = 8, 11%; trial: n = 3, 4%).
SNIF Occurrence by Age Group
SNIF occurrence was significantly influenced by age (χ2 = 14.1, df = 6, P = 0.03). Older adults, ≥81 years, had fewer SNIF occurrences than younger age groups (Table 1). Moreover, the oldest age group demonstrated the greatest degree of inconsistency in SNIF occurrence between trials.
Table 1.
SNIF occurrence by age group
| Age group (years) | SNIF | Inconsistent SNIF | Non-SNIF | Total |
|---|---|---|---|---|
| 21–40 | 15 (71%) | 3 (14%) | 3 (14%) | 21 |
| 41–60 | 14 (78%) | 3 (17%) | 1 (6%) | 18 |
| 61–80 | 10 (59%) | 4 (24%) | 3 (18%) | 17 |
| ≥81 | 9 (45%) | 1 (5%) | 10 (50%) | 20 |
| Total | 48 (63%) | 11 (15%) | 17 (22%) | 76 |
SNIF swallow noninspiratory flow for both 5-ml liquid barium swallow trials, Inconsistent SNIF swallow noninspiratory flow for 1 of 2 liquid barium swallow trials, Non-SNIF no swallow noninspiratory flow for either liquid barium swallow trial
Relationship of the Timing of SNIF with Other Specific Physiologic Swallowing Events
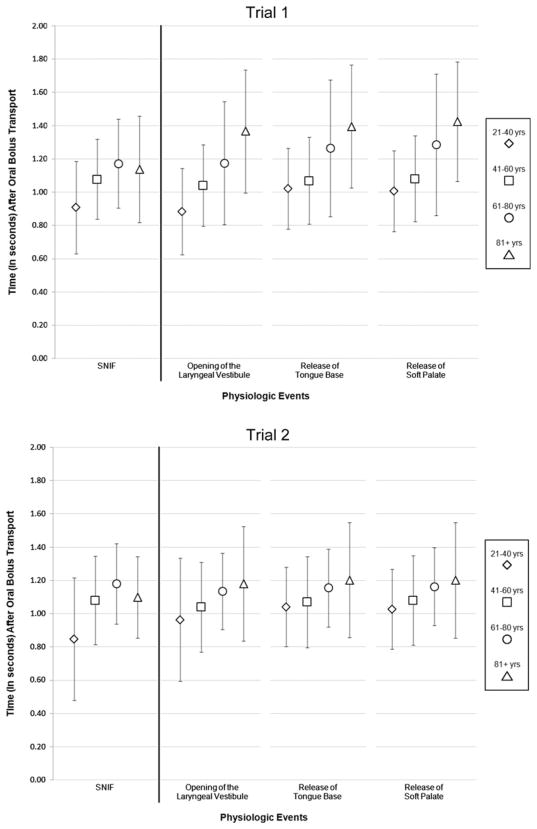
The main focus of this investigation was to determine the timing of SNIF relevant to key swallowing events. All measures were made relevant to the onset of oral bolus transport. There were no statistically significant differences (P > 0.05) between swallows for the onset of each physiologic event (i.e., laryngeal vestibule opening, release of tongue base contact from the posterior pharyngeal wall, release of the soft palate from the posterior pharyngeal wall) measured. Summarized in Table 2 are the key timing measures that were nearly simultaneous with the peak in nasal airflow for SNIF: first opening of the laryngeal vestibule and release of both the tongue base and soft palate from the posterior pharyngeal wall. The temporal order of events was onset opening of the laryngeal vestibule, peak SNIF, release of tongue base contact from the posterior pharyngeal wall, and then release of the soft palate from the posterior. Across the two trials, these events all occurred within a mean of 30 ms (SD = 49 ms) from peak SNIF, 16 ms from the margin of error for video resolution. The timing of the peak of SNIF was strongly positively correlated with the following physiologic events: Onset of laryngeal vestibule opening (trial 1: ρ = 0.992, P <0.0005; trial 2: ρ = 0.935, P <0.0005), onset of release of tongue base contact with the posterior pharyngeal wall (trial 1: ρ = 0.971, P <0.0005; trial 2: ρ = 0.962, P <0.0005), and onset of the release of the soft palate from the posterior pharyngeal wall (trial 1: ρ = 0.972, P <0.0005; trial 2: ρ = 0.961, P <0.0005). Mean onset times for each of the physiologic events varied by age group (Fig. 2); however, these differences were not statistically significant (trial 1: F = 0.637, df = 3, P = 0.596; trial 2: F = 1.111, df = 3, P = 0.357).
Table 2.
Physiologic swallow event timing
| Physiologic event | Trial 1a | Trial 2a |
|---|---|---|
| Opening of the laryngeal vestibule | 1084 (264) | 1047 (253) |
| SNIF | 1108 (266) | 1054 (264) |
| Tongue base release from the posterior pharyngeal wall | 1111 (269) | 1057 (253) |
| Soft palate release from the posterior pharyngeal wall | 1113 (272) | 1058 (256) |
Values are given as mean (standard deviation)
SNIF swallow noninspiratory flow
Onset times (in ms) after initiation of oral bolus transport
Fig. 2.
Onset of physiologic events by age group for each liquid bolus trial
Discussion
In agreement with previous investigations [3–6, 9, 10, 18–21], we found evidence of noninspiratory airflow (SNIF), negative pressures that occurred near the end of the pharyngeal swallow, before the end of the zero flow interval associated with the respiratory pause to accommodate swallowing. Our results demonstrate a link between SNIF and other physiologic swallowing events. Specifically, SNIF occurred nearly simultaneously with the release of the tongue base and soft palate from the posterior pharyngeal wall and with the onset of the laryngeal vestibule opening. The average time between events was only 16 ms within the margin of error, supporting the synchronous occurrence of these events. Simultaneous video fluoroscopic and airflow recordings confirmed the previously untested hypothesis that SNIF is a nonrespiratory phenomenon. In our study, it occurred at the onset of first separation of the arytenoids from the epiglottic petiole when the airway is partially or completely occluded. It is highly unlikely, therefore, that SNIF was due to inspiratory flow or a sudden drop in intratracheal pressure, as postulated in earlier studies [17, 24–26]. Moreover, the mid-portion or tail of a liquid bolus is often still passing through the hypopharynx at this time. An inspiratory event would clearly lead to penetration or aspiration of the passing bolus tail [3, 5, 6, 9, 11]. This finding is consistent with previous observations of similar nonrespiratory flow events recorded during infused or spontaneously occurring liquid swallows [9, 17, 22, 24–26]. There is some evidence of diaphragmatic activation and potential inspiratory efforts against a closed airway surrounding swallowing, but these occur before or very early within the swallowing-related respiratory pause and thus are unlikely to contribute to SNIF [16, 17].
Our data support the hypothesis that SNIF is a nonrespiratory event generated by pharyngeal pressure differentials at the offset of pharyngeal muscle contraction during the late stages of pharyngeal swallowing [9–11]. Our results showed a lower frequency of occurrence of SNIF in the oldest-age category (81≥ years) when compared with the younger participants. Moreover, SNIF most often occurred on only one trial in the oldest group. We tentatively suggest that this may represent lower and less stable oropharyngeal and laryngeal contraction pressures and pressure gradients during swallowing in the oldest age group. This finding is supported by previous evidence of significantly reduced pharyngeal contraction pressures measured by manometry in older individuals (in their 60s) compared to younger adults [27–29]. However, using video fluoroscopy with manometry, Shaw et al. [30] found increases in hypopharyngeal intrabolus pressures in old compared to younger adults. They postulated that the higher intrabolus pressures may represent a compensatory drive to overcome the downstream resistance offered by a reduced UES opening also found in older adults. The inclusion of manometric contact pressure recordings would be an ideal method to address the potential differences between the findings of Shaw et al. and those of the present investigation and to further test the hypothesis that SNIF represents a pressure gradient associated with release of primary pressure generating contact pressures that aid in bolus clearance during the oropharyngeal swallow.
In addition to the inconsistency of SNIF in our oldest-age group, we also found some instances of variable SNIF occurrence in young- and middle-age groups. These inconsistencies, and the fact that none of our participants demonstrated laryngeal penetration, aspiration, or a collection of pharyngeal residue, suggest that there is a range of normal pharyngeal contraction pressures for small-volume liquid swallowing. It is clear that future studies should include additional numbers of bolus trials, volumes, and viscosities to elucidate the potential underlying mechanism for occasional inconsistent production of SNIF within and across age groups. In fact, preliminary evidence has already shown that the combination of cervical accelerometry and nasal airflow has the potential to identify pharyngeal residue in individuals with dysphagia [31].
In working with human subjects, equating measures of physiologic events with kinematic relationships using video fluorographic data continues to be self-limiting. Current limits in temporal video resolution are determined largely by the amount of radiation required to produce an image of sufficient quality using higher frame rates for video capture. With some inconsistency, our data suggest the physiologic events measured temporally in this study (i.e., onset opening of the laryngeal vestibule, release of tongue base contact from the posterior pharyngeal wall, release of the soft palate from the posterior pharyngeal wall) all occurred at nearly the same time. A more precise determination of these timings will require advancements in the technology required to capture video data using radiographic equipment at resolutions better than 30 frames per second. Even so, the clinical implications of these small timing differences may not be significant.
If these nonrespiratory flow events were related to pharyngeal pressure changes, they may represent a distinct, easily accessible, and affordable clinical indicator of key swallowing pressures. They may also be useful as a visual feedback marker of pharyngeal effort in rehabilitation exercises to enhance bolus clearance and airway protection during swallowing rehabilitation.
Contributor Information
Martin B. Brodsky, Department of Physical Medicine and Rehabilitation, Johns Hopkins University, Baltimore, MD, USA.
David H. McFarland, Faculties of Medicine, Université de Montréal and McGill University, Montréal, QC, Canada.
Yvonne Michel, Daniel Island, SC, USA.
Suzanne B. Orr, Accent on Communication, Atlanta, GA, USA.
Bonnie Martin-Harris, Email: harrisbm@musc.edu, Department of Otolaryngology-Head and Neck Surgery, Evelyn Trammell Institute for Voice and Swallowing, Medical University of South Carolina, 135 Rutledge Avenue, P.O. Box 250550, Charleston, SC 29425, USA.
References
- 1.Brodsky MB, McFarland DH, Dozier TS, Blair J, Ayers C, Michel Y, Gillespie MB, Day TA, Martin-Harris B. Respiratory-swallow phase patterns and their relationship to swallowing impairment in patients treated for oropharyngeal cancer. Head Neck. 2010;32:481–9. doi: 10.1002/hed.21209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dozier TS, Brodsky MB, Michel Y, Walters BC, Jr, Martin-Harris B. Coordination of swallowing and respiration in normal sequential cup swallows. Laryngoscope. 2006;116:1489–93. doi: 10.1097/01.mlg.0000227724.61801.b4. [DOI] [PubMed] [Google Scholar]
- 3.Martin BJ, Logemann JA, Shaker R, Dodds WJ. Coordination between respiration and swallowing: Respiratory phase relationships and temporal integration. J Appl Physiol. 1994;76:714–23. doi: 10.1152/jappl.1994.76.2.714. [DOI] [PubMed] [Google Scholar]
- 4.Martin BJW. The influence of deglutition on respiration. Evanston, IL: Northwestern University; 1991. [Google Scholar]
- 5.Martin-Harris B, Brodsky MB, Michel Y, Ford CL, Walters B, Heffner J. Breathing and swallowing dynamics across the adult lifespan. Arch Otolaryngol Head Neck Surg. 2005;131:762–70. doi: 10.1001/archotol.131.9.762. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Harris B, Brodsky MB, Price CC, Michel Y, Walters B. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: single liquid swallows. J Appl Physiol. 2003;94:1735–43. doi: 10.1152/japplphysiol.00806.2002. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Harris B, Michel Y, Castell DO. Physiologic model of oropharyngeal swallowing revisited. Otolaryngol Head Neck Surg. 2005;133:234–40. doi: 10.1016/j.otohns.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 8.Palmer JB, Hiiemae KM. Eating and breathing: Interactions between respiration and feeding on solid food. Dysphagia. 2003;18:169–78. doi: 10.1007/s00455-002-0097-9. [DOI] [PubMed] [Google Scholar]
- 9.Paydarfar D, Gilbert RJ, Poppel CS, Nassab PF. Respiratory phase resetting and airflow changes induced by swallowing in humans. J Physiol (Lond) 1995;483:273–88. doi: 10.1113/jphysiol.1995.sp020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perlman AL, Ettema SL, Barkmeier J. Respiratory and acoustic signals associated with bolus passage during swallowing. Dysphagia. 2000;15:89–94. doi: 10.1007/s004550010006. [DOI] [PubMed] [Google Scholar]
- 11.Perlman AL, He X, Barkmeier J, Van Leer E. Bolus location associated with videofluoroscopic and respirodeglutometric events. J Speech Lang Hear Res. 2005;48:21–33. doi: 10.1044/1092-4388(2005/003). [DOI] [PubMed] [Google Scholar]
- 12.McFarland DH, Lund JP, Gagner M. Effects of posture on the coordination of respiration and swallowing. J Neurophysiol. 1994;72:2431–7. doi: 10.1152/jn.1994.72.5.2431. [DOI] [PubMed] [Google Scholar]
- 13.McFarland DH, Lund JP. Modification of mastication and respiration during swallowing in the adult human. J Neurophysiol. 1995;74:1509–17. doi: 10.1152/jn.1995.74.4.1509. [DOI] [PubMed] [Google Scholar]
- 14.McFarland DH, Lund JP. An investigation of the coupling between respiration, mastication, and swallowing in the awake rabbit. J Neurophysiol. 1993;69:95–108. doi: 10.1152/jn.1993.69.1.95. [DOI] [PubMed] [Google Scholar]
- 15.Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol. 1956;19:44–60. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]
- 16.Hårdemark Cedborg AI, Sundman E, Bodén K, Hedström HW, Kuylenstierna R, Ekberg O, Eriksson LI. Co-ordination of spontaneous swallowing with respiratory airflow and diaphragmatic and abdominal muscle activity in healthy adult humans. Exp Physiol. 2009;94:459–68. doi: 10.1113/expphysiol.2008.045724. [DOI] [PubMed] [Google Scholar]
- 17.Vantrappen G, Hellemans J. Studies on the normal deglutition complex. Dig Dis Sci. 1967;12:255–66. doi: 10.1007/BF02233643. [DOI] [PubMed] [Google Scholar]
- 18.Hirst LJ, Ford GA, Gibson GJ, Wilson JA. Swallow-induced alterations in breathing in normal older people. Dysphagia. 2002;17:152–61. doi: 10.1007/s00455-001-0115-3. [DOI] [PubMed] [Google Scholar]
- 19.McConnel FM. Analysis of pressure generation and bolus transit during pharyngeal swallowing. Laryngoscope. 1988;98:71–8. doi: 10.1288/00005537-198801000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Sokol EM, Heitmann P, Wolf BS, Cohen BR. Simultaneous cineradiographic and manometric study of the pharynx, hypo-pharynx, and cervical esophagus. Gastroenterology. 1966;51:960–74. [PubMed] [Google Scholar]
- 21.Feroah TR, Forster HV, Fuentes CG, Lang IM, Beste D, Martino P, Pan L, Rice T. Effects of spontaneous swallows on breathing in awake goats. J Appl Physiol. 2002;92:1923–35. doi: 10.1152/japplphysiol.01079.2000. [DOI] [PubMed] [Google Scholar]
- 22.Atkinson M, Kramer P, Wyman S, Ingelfinger F. The dynamics of swallow. I. Normal pharyngeal mechanisms. J Clin Invest. 1957;36:581–98. doi: 10.1172/JCI103457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahrilas PJ, Dodds WJ, Dent J, Logemann JA, Shaker R. Upper esophageal sphincter function during deglutition. Gastroenterology. 1988;95:52–62. doi: 10.1016/0016-5085(88)90290-9. [DOI] [PubMed] [Google Scholar]
- 24.Odanaka T. Studies on the swallow respiration. J Physiol Soc Jpn. 1952;14:114–9. [Google Scholar]
- 25.Yamamoto T. Experimental studies for swallowing respiration. Otologica (Kyoto) 1956;49:346–61. [Google Scholar]
- 26.Kawasaki M, Ogura JH, Takenouchi S. Neurophysiologic observations of normal deglutition. I. Its relationship to the respiratory cycle. Laryngoscope. 1964;74:1747–65. doi: 10.1288/00005537-196412000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Butler S, Stuart A, Wilhelm E, Rees C, Williamson J, Kritchevsky S. The effects of aspiration status, liquid type, and bolus volume on pharyngeal peak pressure in healthy older adults. Dysphagia. doi: 10.1007/s00455-010-9290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier-Ewert HK, Van Herwaarden MA, Gideon RM, Castell JA, Achem S, Castell DO. Effect of age on differences in upper esophageal sphincter and pharynx pressures between patients with dysphagia and control subjects. Am J Gastroenterol. 2001;96:35–40. doi: 10.1111/j.1572-0241.2001.03448.x. [DOI] [PubMed] [Google Scholar]
- 29.Tracy JF, Logemann JA, Kahrilas PJ, Jacob P, Kobara M, Krugler C. Preliminary observations on the effects of age on oropharyngeal deglutition. Dysphagia. 1989;4:90–4. doi: 10.1007/BF02407151. [DOI] [PubMed] [Google Scholar]
- 30.Shaw DW, Cook IJ, Gabb M, Holloway RH, Simula ME, Panagopoulos V, Dent J. Influence of normal aging on oral-pharyngeal and upper esophageal sphincter function during swallowing. Am J Physiol Gastrointest Liver Physiol. 1995;268:G389–96. doi: 10.1152/ajpgi.1995.268.3.G389. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, Steele CM, Chau T. Classification of healthy and abnormal swallows based on accelerometry and nasal airflow signals. Artif Intell Med. 2011;52:17–25. doi: 10.1016/j.artmed.2011.03.002. [DOI] [PubMed] [Google Scholar]