Abstract
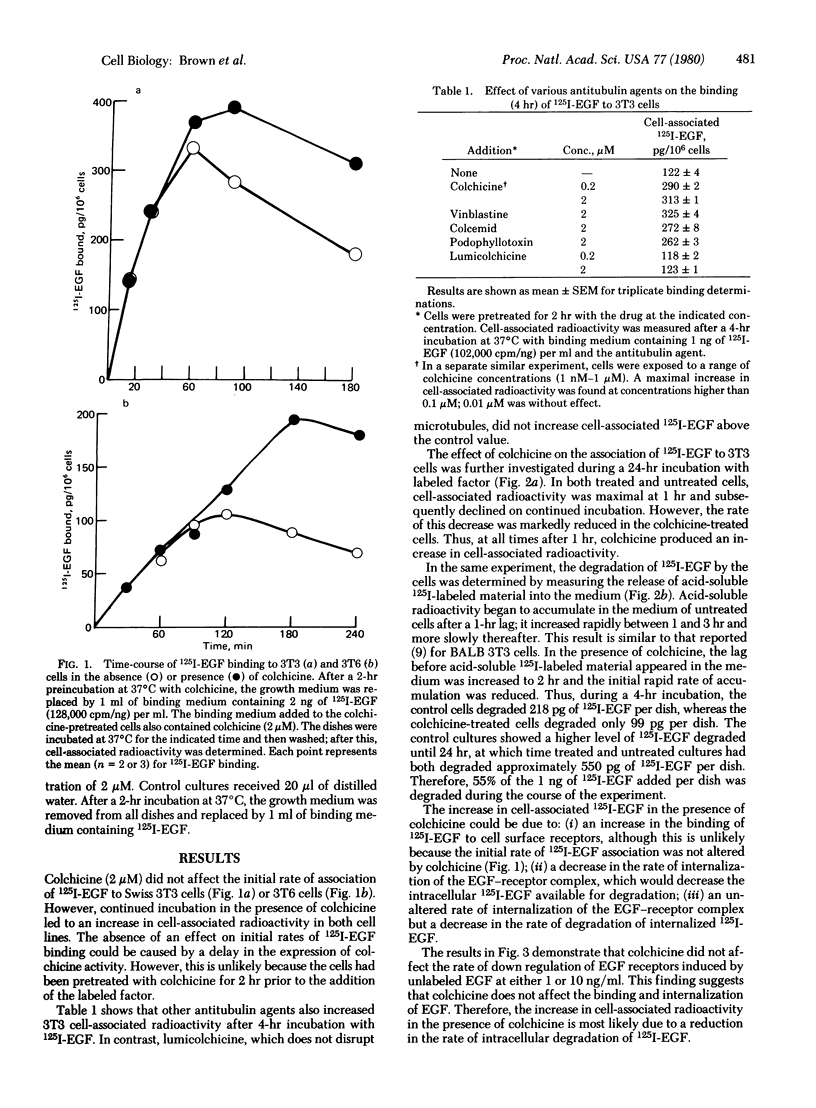
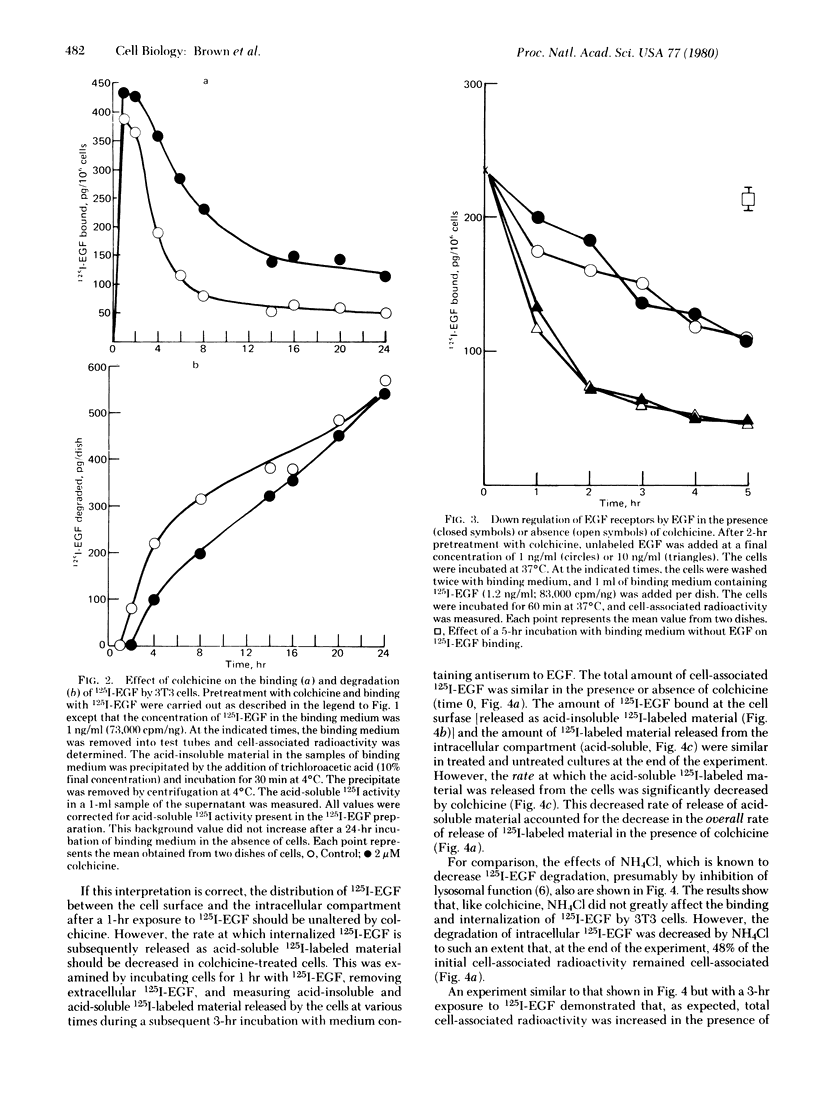
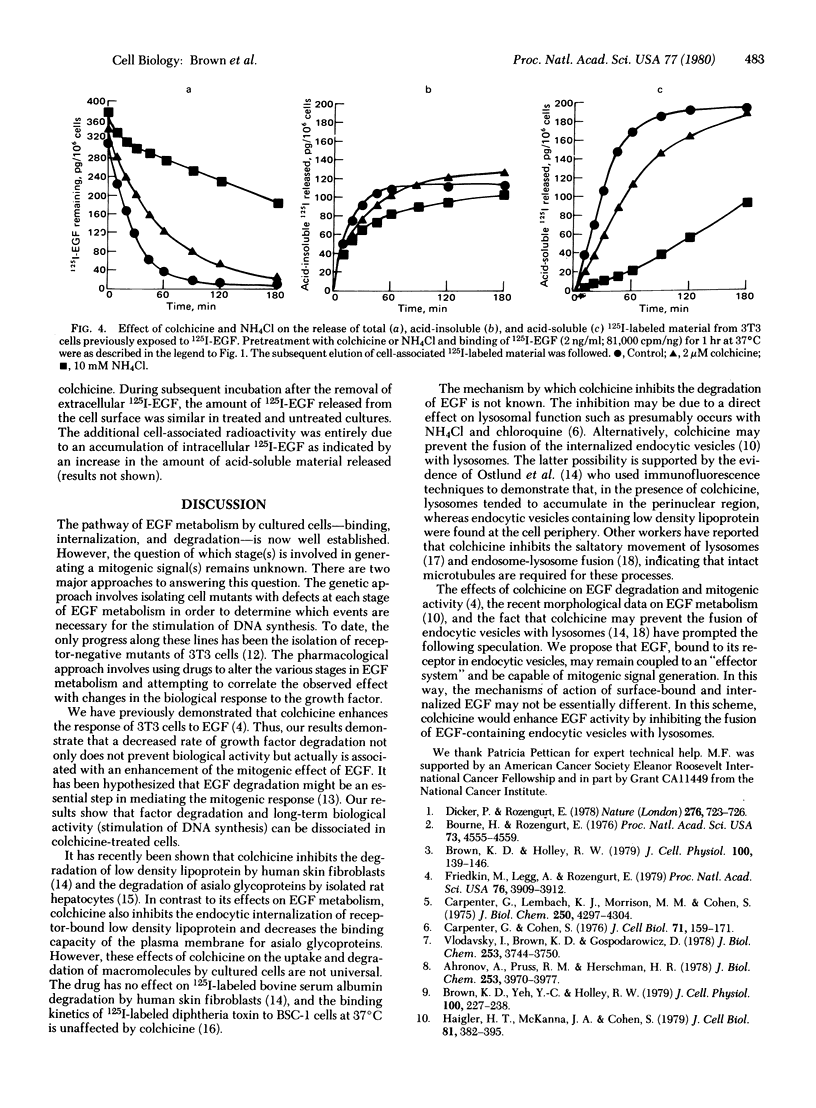
Colchicine (2 microM) did not affect the initial rate of association of 125I-labeled epidermal growth factor (125I-EGF) to Swiss 3T3 cells but continued incubation (up to 24 hr) led to an increase in cell-associated radioactivity. The effect is also produced by Colcemid, vinblastine, and podophyllotoxin but not by lumicolchicine. Disruption of microtubules with colchicine does not alter the rate of "down regulation" of EGF receptors, suggesting the binding and internalization of the factor proceed unchanged. However, colchicine markedly decreases the rate of appearance of acid-soluble radioactivity from cells either incubated continuously with 125I-EGF for 24 hr or exposed to the radioactive peptide for only 1 or 3 hr. The results indicate that colchicine decreases the rate of degradation of internalized 125I-EGF. Because antitubulin agents enhance the mitogenic effect of EGF our results suggest that peptide degradation can be dissociated from the long-term biological effect.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonov A., Pruss R. M., Herschman H. R. Epidermal growth factor. Relationship between receptor regulation and mitogenesis in 3T3 cells. J Biol Chem. 1978 Jun 10;253(11):3970–3977. [PubMed] [Google Scholar]
- Bourne H. R., Rozengurt E. An 18,000 molecular weight polypeptide induces early events and stimulates DNA synthesis in cultured cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4555–4559. doi: 10.1073/pnas.73.12.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Holley R. W. Epidermal growth factor and the control of proliferation of Balb 3T3 and benzo[a]pyrene-transformed Balb 3T3 cells. J Cell Physiol. 1979 Jul;100(1):139–146. doi: 10.1002/jcp.1041000114. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Yeh Y. C., Holley R. W. Binding, internalization, and degradation of epidermal growth factor by balb 3T3 and BP3T3 cells: relationship to cell density and the stimulation of cell proliferation. J Cell Physiol. 1979 Aug;100(2):227–238. doi: 10.1002/jcp.1041000204. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Lembach K. J., Morrison M. M., Cohen S. Characterization of the binding of 125-I-labeled epidermal growth factor to human fibroblasts. J Biol Chem. 1975 Jun 10;250(11):4297–4304. [PubMed] [Google Scholar]
- Das M., Fox C. F. Molecular mechanism of mitogen action: processing of receptor induced by epidermal growth factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2644–2648. doi: 10.1073/pnas.75.6.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Stimulation of DNA synthesis by tumour promoter and pure mitogenic factors. Nature. 1978 Dec 14;276(5689):723–726. doi: 10.1038/276723a0. [DOI] [PubMed] [Google Scholar]
- Freed J. J., Lebowitz M. M. The association of a class of saltatory movements with microtubules in cultured cells. J Cell Biol. 1970 May;45(2):334–354. doi: 10.1083/jcb.45.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedkin M., Legg A., Rozengurt E. Antitubulin agents enhance the stimulation of DNA synthesis by polypeptide growth factors in 3T3 mouse fibroblasts. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3909–3912. doi: 10.1073/pnas.76.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., McKanna J. A., Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J Cell Biol. 1979 May;81(2):382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolset S. O., Tolleshaug H., Berg T. The effects of colchicine and cytochalasin B on uptake and degradation of asialo-glycoproteins in isolated rat hepatocytes. Exp Cell Res. 1979 Aug;122(1):159–167. doi: 10.1016/0014-4827(79)90570-6. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., Bodel P. T. The dissociation by colchicine of phagocytosis from increased oxygen consumption in human leukocytes. J Clin Invest. 1967 May;46(5):786–796. doi: 10.1172/JCI105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B., Leppla S. H. Effects of lectins on the interaction of diphtheria toxin with mammalian cells. Exp Cell Res. 1979 Jun;121(1):95–101. doi: 10.1016/0014-4827(79)90448-8. [DOI] [PubMed] [Google Scholar]
- Ostlund R. E., Jr, Pfleger B., Schonfeld G. Role of microtubules in low density lipoprotein processing by cultured cells. J Clin Invest. 1979 Jan;63(1):75–84. doi: 10.1172/JCI109281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss R. M., Herschman H. R. Variants of 3T3 cells lacking mitogenic response to epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3918–3921. doi: 10.1073/pnas.74.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Vlodavsky I., Brown K. D., Gospodarowicz D. A comparison of the binding of epidermal growth factor to cultured granulosa and luteal cells. J Biol Chem. 1978 May 25;253(10):3744–3750. [PubMed] [Google Scholar]


