Abstract
Cells in tissues are surrounded by the extracellular matrix (ECM), a gel-like material of proteins and polysaccharides that are synthesized and secreted by cells. Here we propose that the ECM can be isolated from porcine adipose tissue and holds great promise as a xenogeneic biomaterial for tissue engineering and regenerative medicine. Porcine adipose tissue is easily obtained in large quantities from commonly discarded food waste. Decellularization protocols have been developed for extracting an intact ECM while effectively eliminating xenogeneic epitopes and minimally disrupting the ECM composition. Porcine adipose tissue was defatted by homogenization and centrifugation. It was then decellularized via chemical (1.5 M sodium chloride and 0.5% sodium dodecyl sulfate) and enzymatic treatments (DNase and RNase) with temperature control. After decellularization, immunogenic components such as nucleic acids and α-Gal were significantly reduced. However, abundant ECM components, such as collagen (332.9±12.1 μg/mg ECM dry weight), sulfated glycosaminoglycan (GAG, 85±0.7 μg/mg ECM dry weight), and elastin (152.6±4.5 μg/mg ECM dry weight), were well preserved in the decellularized material. The biochemical and mechanical features of a decellularized ECM supported the adhesion and growth of human cells in vitro. Moreover, the decellularized ECM exhibited biocompatibility, long-term stability, and bioinductivity in vivo. The overall results suggest that the decellularized ECM derived from porcine adipose tissue could be useful as an alternative biomaterial for xenograft tissue engineering.
Introduction
The acute shortage of human tissue and organs for the use in surgical applications has prompted significant research of alternatives. The use of animal tissue, particularly porcine tissue, provides potential for resolving the critical shortage of human tissue.1 Although several immune barriers need to be overcome, many researchers have focused on whole-porcine tissues or tissue derivatives, because their biochemical profile is similar to that of human organs.2,3 For successful use in clinical applications, these xenogeneic materials should be decellularized to remove xenogeneic antigens, such as α-Gal epitopes (Galα1-3, Galβ1-4, GlcNAc-R), DNA, and cellular components. These xenogeneic antigens may contribute to both compatibility problems in vitro and adverse host responses in vivo.4 Typically, decellularization involves a combination of physical, chemical, and enzymatic treatments. Optimal decellularization protocols should consider tissue characteristics such as thickness, density, cellularity, and lipid content.5 A number of decellularized porcine matrices have been studied,6–12 and some have received the regulatory approval for use in human patients using tissues from the dermis (Strattice™, Lifecell Corp.; Zimmer Collagen Repair Patch™, Zimmer Inc.), small intestine submucosa (SurgiSIS®, Cook Biotech, Inc.; Restore®, DePuyOrthopaedics, Inc.), urinary bladder (MatriStem®, ACell, Inc.), and heart valves (Synergraft®, CryoLife, Inc.).13,14
Herein, we demonstrate that the decellularized porcine extracellular matrix (ECM) derived from adipose tissue has a great potential as a xenogeneic material for use in xenograft tissue engineering. Porcine adipose tissue is one of the most abundant animal tissues.15 More than 6.8 million tons of porcine adipose tissue are produced in the world, and significant quantities of inedible adipose tissue are routinely discarded as food industry waste.16 Recycling porcine adipose tissue as a biomaterial may have economic benefits.
Adipose tissue contains various ECMs, including collagen type I–VI, laminin, fibronectin, elastin, and glycosaminoglycans (GAGs).10,17 It secretes a variety of bioactive molecules that regulate numerous cellular processes, including inflammation, cell growth, and differentiation.18–20 Our group reported that ECMs derived from human adipose tissue could be useful as a scaffold material for the adhesion, growth, and differentiation of human cells.21–25Although human adipose tissue may provide an ideal biomaterial, there are some concerns about the limitation of tissue supply and manufacturing scale-up for industrial production. In the present study, we present porcine adipose tissue as an alternative source of human tissue and focus on ECM extraction from porcine adipose tissue with the effective removal of potential xenogeneic epitopes and composition preservation for the ECM use in xenografts.
Materials and Methods
Extraction of ECM from porcine adipose tissue
The ECM was prepared from porcine adipose tissue, according to a method22 reported previously by our group after a slight modification. Briefly, porcine adipose tissue was obtained from a local slaughter house and processed. After excising the skin and muscle, the resulting adipose tissues were kept at −70°C until use. The ECM extraction procedures were performed at 40°C to prevent congealability of the oil components. Porcine adipose tissues were chopped into 1×1×1-cm3 pieces and washed thoroughly with distilled water. Distilled water (100 mL) was added to the porcine adipose tissue (50 mg), which was homogenized for 5 min using a houseware blender with pulsation. The tissue suspension was then centrifuged at 1800 g for 5 min, and the upper layer of oil components was discarded. This process was repeated several times until the complete removal of oil components. For decellularization, the ECM suspension was treated with a buffered 1.5 M hyperosmolar solution of sodium chloride (NaCl; Sigma), 0.5% sodium dodecyl sulfate (SDS; Sigma), and an enzymatic mixture of 0.2% DNase (2000 U, Sigma) and 200 μg/mL RNase (Sigma) at 37°C for 1 day in a shaking water bath (Personal-11EX, TAITEC). The ECM suspension was centrifuged and thoroughly washed with distilled water at the end of each step to remove residual reagents. The final viscous ECM was either used immediately or stored in sterile distilled water at 4°C until further use.
Scanning electron microscopy
The porcine adipose tissue and ECM specimens were first fixed in a 0.2-M cacodylate buffer (Sigma) containing 2% paraformaldehyde and 2.5% glutaraldehyde for 1 week at room temperature. They were then fixed in 1% osmium tetroxide (Tokyo Chemical Industry) for 2 h. After dehydration through a graded ethanol series (from 50% to 100%), the samples were dried with an upper-critical-point CO2 dryer (HCP-2; Hitachi). The ECM scaffold seeded with cells was fixed in 4% paraformaldehyde at 4°C for 24 h, dehydrated, frozen at −70°C, and freeze-dried. After coating with platinum, all samples were observed by scanning electron microscopy (SEM; VEGA-II SBH, TESCAN) by sputtering at an accelerating voltage of 15 kV.
DNA quantification
For DNA quantification, DNA was isolated with a commercial extraction kit (G-spin Kit; iNtRON Biotechnology). The total DNA content was measured by absorption at 260 nm on a spectrophotometer (NanoDrop 1000; Thermo Fisher Scientific). All samples were normalized to the ECM dry weight.
Immunohistochemistry and histological staining of ECM components
The decellularized ECM specimens were fixed in 4% paraformaldehyde at 4°C for 1 h. The specimens were embedded in paraffin (Merck) and sectioned to 10-μm thickness. The sections were deparaffinized and dehydrated in ethanol. To detect residual cellular components, sections were stained with 4,6-diamidino-2-phenylindole (DAPI; Thermo Scientific) and hematoxylin and eosin (H&E). Immunohistochemistry for the porcine antigenic epitope α-Gal was performed using Griffoniasimplicifolialectin-I isolectin B4 (Vector Laboratory) for 1 h at room temperature. Subsequently, the sections were incubated with HRP-conjugated streptavidin (Invitrogen, Life Technologies) for 30 min. To visualize cell nuclei, the sections were counterstained with hematoxylin. For collagen fiber staining, sections were first stained by a rapid Gomori's trichrome method. The samples were fixed in the Bouin's solution for 1 h at 56°C and stained with Weigert's iron hematoxylin for 10 min. The samples were washed and stained with the Gomori's trichrome solution for 20 min and then differentiated in a 0.5% acetic acid solution. For sulfated GAG staining, sections were stained with Weigert's iron hematoxylin for 10 min and washed in tap water for 5 min. The sections were subsequently stained with 0.001% fast green (Sigma) for 5 min and 0.1% safranin O (Sigma) for 5 min. The Fullner and Lillie's orcinol-new fuchsin method was used to stain elastic fibers in the ECM. Samples were stained with an orcinol-new fuchsin working solution for 15 min at 37°C. Stained sections were dehydrated in alcohol, cleared in xylene, mounted in Permount® (Fisher Scientific), and then observed with a fluorescence microscope (IX81; Olympus).
Acid-/pepsin-soluble collagen quantification
The acid-/pepsin-soluble collagen content in the decellularized ECM was measured using a Sircol soluble collagen assay kit (Biocolor). To extract acid-/pepsin-soluble collagen, the ECM specimens were digested with 0.5 M acetic acid containing 1% (w/v) pepsin (P7012; Sigma) for 24 h at room temperature. The soluble collagen was incubated with 1 mL Sircol dye reagent for 30 min at room temperature. The collagen–dye complex was precipitated by centrifugation at 10,000 g for 10 min, and the supernatant was removed. The pellets were dissolved in 1 mL alkali reagent, and the relative absorbance was measured in a 96-well plate at 540 nm using a microplate reader (PowerWave XS, Bio-Tek Instruments).
Sulfated GAG quantification
The sulfated GAG content in the decellularized ECM was measured using a Blyscan sulfated GAG assay kit (Biocolor). To extract sulfated GAG, the ECMs were digested with a 0.1 M phosphate buffer (pH 6.8) containing 125 μg/mL papain (Sigma), 10 mM cysteine hydrochloride (Sigma), and 2 mM EDTA (Sigma) for 48 h at 60°C. The suspension was centrifuged at 15,000 g for 30 min. The supernatant was collected and incubated with a 0.2 M sodium citrate buffer (pH 4.8) containing 0.2% (w/v) cetylpyridinium for 2 h at 37°C. After centrifugation at 10,000 g for 10 min, the precipitated sulfated GAG was dissolved in 2 M lithium chloride and re-precipitated by mixing with cold ethanol. After centrifugation, the pellet was drained and resuspended in distilled water. The extracted sulfated GAG was mixed with 1 mL Blyscan dye and shaken for 30 min. The precipitate was collected by centrifugation for 5 min and then dissolved in 1 mL dissociation reagent. The absorbance was measured in a 96-well plate at 656 nm using a microplate reader.
Soluble elastin quantification
The soluble elastin content in the decellularized ECM was measured using a Fastin elastin assay kit (Biocolor). To extract soluble elastin, the ECM was hydrolyzed with 0.25 M oxalic acid (Sigma) at 100°C for 1 h. The insoluble residues were separated by centrifugation. The supernatant was collected, and the sediment underwent an additional extraction under the same conditions. The extracted soluble elastin was mixed with 1 mL Fastin dye and stirred for 30 min. The precipitate was collected by centrifugation for 10 min and then dissolved in 250 μL dissociation reagent. The absorbance was measured in a 96-well plate at 513 nm using a microplate reader.
Fabrication and characterization of porcine ECM scaffolds
To evaluate physicomechanical properties, the ECM was fabricated into three-dimensional porous scaffolds. The decellularized ECM was suspended with distilled water, gently poured into a mold, frozen at −20°C, and lyophilized in a freeze dryer. The pore size and porosity of an ECM scaffold (20-mm diameter and 1-mm thickness) were determined using an automated mercury porosimeter (Autopore IV 9500; Micromeritics). Tensile tests were conducted with a universal tensile machine equipped with a 50-N static load cell (Model 5565; Instron). A specimen (20 mm×5 mm×1 mm, length×width×thickness) was pulled at a rate of 2.54 mm/min.26,27 Strains were calculated using cross-head displacement, and stresses were calculated by dividing force data by the cross-sectional area of the sample. Three specimens were measured and averaged.
In vitro biocompatibility tests of decellularized porcine ECM
For in vitro biocompatibility tests, human adipose-derived stem cells (hASCs) and normal human dermal fibroblasts (NHDFs) were cultured on the decellularized porcine ECM. NHDFs were purchased from PromoCell GmbH, and hASCs were obtained from the subcutaneous adipose tissue of healthy human females using a method described previously.28,29 Briefly, human adipose tissue was washed with phosphate-buffered saline (PBS) containing 5% penicillin/streptomycin (P/S; Gibco-BRL, Life Technologies). After removing the red blood cells,30 the tissue was digested in PBS supplemented with 0.01% (w/v) collagenase type II (Gibco-BRL) for 1 h at 37°C. The digested tissue was filtered through a 100-μm mesh to remove aggregated tissue and debris. The filtered suspension was centrifuged at 200 g for 7 min, and the resulting stromal vascular fraction pellet was washed several times in PBS. The hASCs and NHDFs were maintained in the Dulbecco's modified Eagle's medium (DMEM, Gibco-BRL) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL) and 1% P/S at 37°C under 5% CO2 until passage 6.
Porcine ECM scaffolds (5-mm diameter and 1-mm thickness) were sterilized by ethanol, washed with PBS, and pre-wetted with the serum-free DMEM medium in 24-well plates. A suspension of hASCs and NHDFs containing 2×104 cells/scaffold was seeded onto the ECM scaffolds in the DMEM supplemented with 10% FBS and 1% P/S, and grown for 5 days at 37°C with 5% CO2. The medium was replaced by a fresh medium every 3 days. Cell attachment and proliferation were analyzed using a WST-1 assay kit (Roche). For cell attachment measurement, scaffolds were washed three times with PBS to remove unattached cells at 6 h after seeding. The WST-1 reagent was added to each well containing a cell-seeded porcine ECM scaffold, and the plates were incubated for 1 h at 37°C under 5% CO2. The optical density was then measured at 440 nm using a microplate reader. The absorbance values of the initial total cells (Ct) and the attached cells into the scaffolds (Ca) were measured. The cell adhesion ratio was estimated using the following equation:
 |
The proliferation of cells in the ECM scaffolds was also analyzed through the absorbance of attached cells. The viability was performed using a commercially available Live/Dead® viability assay kit (Molecular Probes®, Life Technologies). The porcine ECM scaffolds containing cells (n=5) were transferred to new 12-well plates, washed with PBS, and stained for 20 min with 100 μL of the combined Live/Dead® reagents at 37°C under darkness. After staining, the porcine ECM scaffolds were observed using a fluorescence microscope equipped with a digital camera (IX81; Olympus). Green fluorescence caused by the reaction of calcein with intracellular esterase indicates live cells, whereas red fluorescence caused by ethidium homodimers that are bound to nucleic acids indicates dead cells.
In vivo animal experiments
Six-week-old female Slc/ICR mice were anaesthetized by exposure to 97% CO2 for 20 s.31 After shaving the flanks (about 1 cm each), a viscous suspension (10 mg/mL, 1 mL) of decellularized ECM was injected subcutaneously into both flanks of female ICR mice using an 18-gauge needle. Animals were housed individually and fed standard mouse chow. At 1 and 6 weeks after injections, the mice were sacrificed, and the grafts were explanted. Immediately after harvest, the grafts were placed in 10% neutral buffered formalin (Sigma) for analysis. Eight mice per experimental group were analyzed.
Grafts were fixed in 4% paraformaldehyde, embedded in paraffin, and sliced at 6- to 10-μm thicknesses using a microtome. Sections were deparaffinized, dehydrated, and then stained with H&E for normal histological evaluation. To detect macrophages, histological and immunofluorescence staining was also performed using standardized protocols for Prussian blue (Sigma), toluidine blue (Sigma), and anti-CD68 (BioLegend). The sections for anti-CD68 were counterstained with 1 μg/mL DAPI for 1 min. To detect adipogenesis, grafts were fixed in 10% sucrose. After embedding in optimum cutting temperature (OCT) compounds (Tissue-Tek®, Miami, FL), samples were frozen at −70°C. The frozen samples were sliced into 10-μm sections using a cryostat, washed with distilled water and 30% isopropanol to remove the OCT compounds, and then stained with oil red O (Sigma) working solutions. The stained sections were observed with a fluorescence microscope.
Reverse transcription–polymerase chain reaction analysis
The total RNA was isolated from cells using an easy-BLUE™ Total RNA Extraction Kit (iNtRON Biotechnology), and RNA concentrations were quantified using a Nanodrop® ND-1000 spectrophotometer (Nanodrop Technologies). The extracted RNA was reverse-transcribed using the first-strand cDNA synthesis system with avian myeloblastosis virus (AMV) reverse transcriptase for polymerase chain reaction (PCR; iNtRON Biotechnology). The cDNA was used as a template for PCR analysis with primers specific for peroxisome-proliferative activated receptor gamma (PPARγ) (NM011146, forward: 5′-ACT GCC TAT GAG CAC TTC AC-3′, reverse: 5′-CAA TCG GAT GGT TCT TCG GA-3′), adipocyte fatty acid-binding protein (aP2) (NM0014425, forward: 5′-TCC TGG CCC AGT ATG AAG GAA ATC-3′, reverse: 5′-CTC TTT ATT GTG GTC GAC TTT CCA-3′), leptin (NM008493, forward: 5′-TGC TGC AGA TAG CCA ATG AC-3′, reverse:5′-GAG TAG AGT GAG GCT TCC AGG A-3′), adiponectin (NM009605, forward: 5′-CAA GGG AAC TTG TGC AGG-3′, reverse: 3′-CGT GAT GTG GTA AGA GAA GTA G-3′), and β-actin (NM007393, forward: 5′-TGG AAT CCT GTG GCA TCC ATG AAA C-3′, reverse: 5′-TAA AAC GCA GCT CAG TAA CAG TCC G-3′). Conventional PCR was performed using the TaKaRa PCR Thermal Cycler Dice™ (TaKaRa Bio) instrument under the following reaction profile: initial denaturation at 95°C for 5 min, 35–40 cycles of denaturation at 95°C for 1 min, annealing at 55°C–65°C for 1 min, polymerization at 72°C for 1 min, and extension at 72°C for 7 min. As an internal control, GAPDH expression was assessed using primers designed for the housekeeping gene. PCR products were electrophoresed in 1.5% agarose gels and analyzed using a gel imaging system (Gel DocXR; Bio-Rad Laboratories).
Statistical analysis
Experimental data were expressed as means±standard deviation (SD). Student's two-tailed t-test with SPSS 17.0 statistical software (SPSS) was used for comparison, and statistical significance was accepted at *p<0.01.
Results
Extraction of ECM from porcine adipose tissue
Xenogeneic ECM was extracted from discarded porcine adipose tissue and decellularized using a combined protocol of mechanical, chemical, and enzymatic treatments. After decellularization, a significant volume of viscous, white, fibrous matrix was collected (Fig. 1). Quantitatively, the matrix reproducibly represented between 8%–10% of the original tissue mass, depending on the specific tissue source. Oily and dense porcine adipose tissue was crushed by homogenization before treatment with chemical detergents and enzymes. In addition, extraction procedures were performed at 40°C to prevent the lipid congealability of porcine adipose tissue. As a result, the oil and cellular components were more effectively eliminated.
FIG. 1.
Macroscopic (A, B) and scanning electron microscopic (SEM) (C, D) images of native porcine adipose tissue (A, C) and decellularized extracellular matrix (ECM) (B, D). Scale bars represent 50 μm. Color images available online at www.liebertpub.com/tec
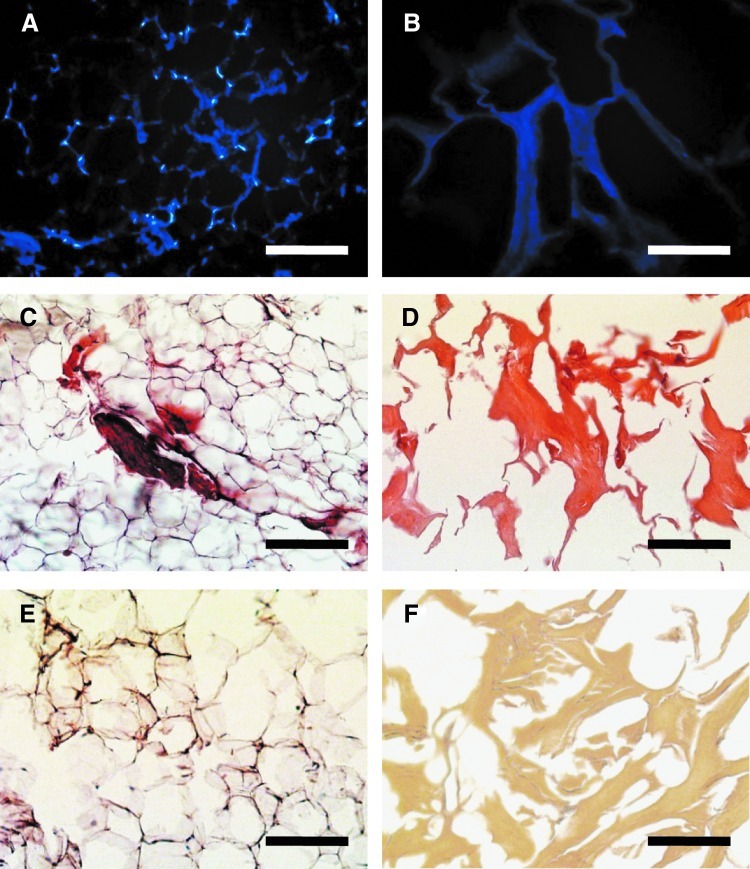
The goal of decellularization is to efficiently remove all cellular and nuclear materials, because xenogeneic cellular antigens may induce an inflammatory response or an immune-mediated rejection of the host tissue.5 To evaluate the decellularization efficiency, the tissue was stained with DAPI, H&E, and α-Gal before and after decellularization. The presence of DNA in ECM was also analyzed using a quantitative method. Compared to the native porcine adipose tissue (1173±175 ng/mg ECM), the DNA content of the decellularized ECM (43.2±3.23 ng/mg ECM) was significantly reduced, and the cellular components were effectively eliminated (Fig. 2). Moreover, α-Gal epitopes, which are of major clinical significance, were partially expressed in the native adipose tissue, but were not expressed in the decellularized ECM (Fig. 2E, F). The results demonstrate that decellularization significantly reduced the potential immunogenic components in the ECM.
FIG. 2.
Representative microscopic images of native porcine adipose tissue (A, C, E) and decellularized ECM (B, D, F). Residual cellular components were stained by 4,6-diamidino-2-phenylindole (blue) (A, B) and hematoxylin and eosin (H&E) (cell nuclei, black spot; cytoplasm, pink) (C, D). The porcine antigenic epitope, α-Gal, was detected by immunohistochemistry with isolectin B4 (E, F, α-Gal; brown). Scale bars represent 200 μm. Color images available online at www.liebertpub.com/tec
Component analysis of decellularized porcine ECM
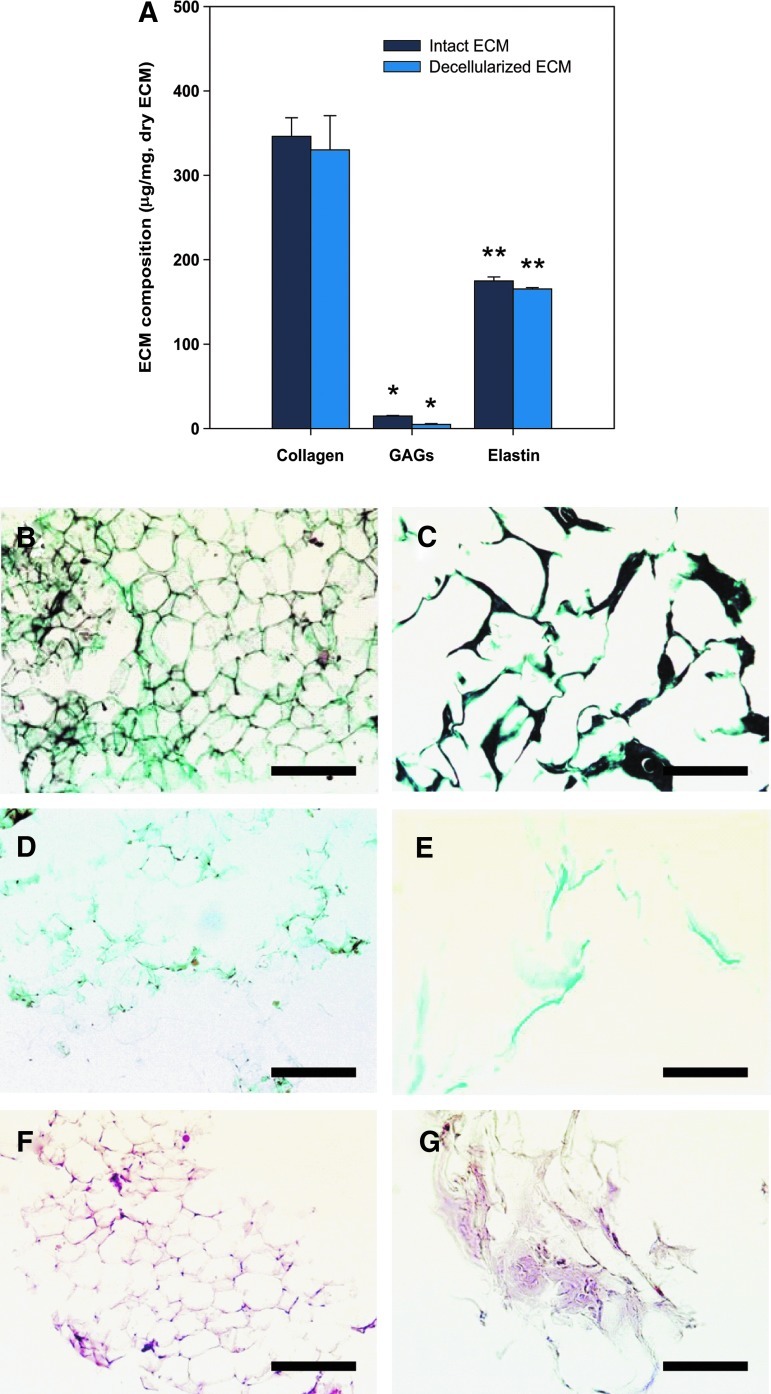
Representative biochemical and histological images of the decellularized ECM are shown in Figure 3. A large amount of proteins were preserved in the decellularized ECM during the decellularization process. The decellularized ECM was rich in acid-/pepsin-soluble collagen (332.9±12.1 μg/mg ECM dry weight) and soluble elastin (152.6±4.5 μg/mg ECM dry weight). A small amount of sulfated GAG (85±0.7 μg/mg ECM dry weight) was also found. The well-preserved collagen, sulfated GAG, and elastin were confirmed using Gomori's one-step trichrome, safranin-O, and orcinol-new fuchsin staining. This indicates that the decellularization protocol caused a minimal loss to the matrix.
FIG. 3.
Biochemical (A) and histological (B–G) analysis of native tissue and decellularized ECM, including acidic-/pepsin-soluble collagen, sulfate glycosaminoglycan (GAG), and soluble elastin. Histological observations of native (B, D, F) and decellularized ECM (C, E, G) for collagen (B, C), sulfated GAG (D, E), and elastin (F, G). All samples were normalized to ECM dry weight. Intact ECM represents the ECM isolated by physical treatments such as homogenization, centrifugation, and washing. Decellularized ECM represents the ECM obtained through the whole-decellularization procedure, including physical, chemical, and enzymatic treatments. Data are shown as means±standard deviations and are statistically significant at *,**p<0.01. Scale bars represent 200 μm. Color images available online at www.liebertpub.com/tec
In vitro biocompatibility of decellularized porcine ECM
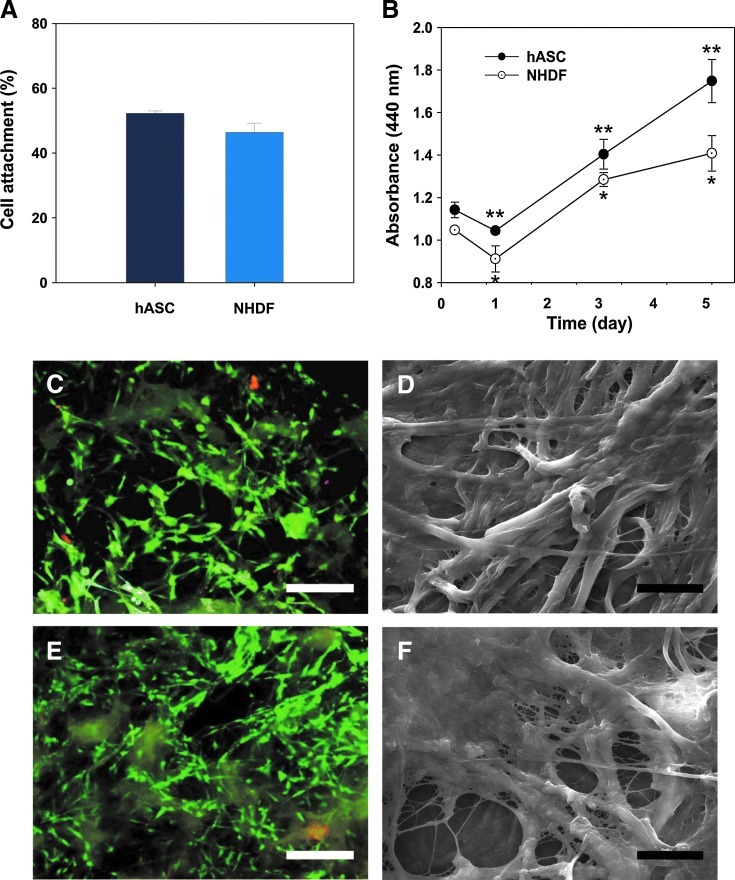
After extraction and decellularization from porcine adipose tissue, the xenogeneic ECM was lyophilized and fabricated into porous scaffolds (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tec). The xenogeneic ECM scaffolds had highly porous microstructures (mean pore size: 121.84±28.08 μm, porosity: 89.6%) and showed fair elasticity (tensile strength: 128.57±13.15 kPa) (Supplementary Fig. S2). From a physicomechanical viewpoint, the microstructures and mechanical strength are highly desirable in scaffolds used for tissue engineering and regenerative medicine. In vitro biocompatibility studies of porcine ECM scaffolds were carried out using hASCs and NHDFs. hASCs and NHDFs were cultured onto the ECM scaffold for 5 days (Fig. 4). The WST-1 assay indicated that both hASCs and NHDFs readily adhered to the surface of the decellularized porcine ECM scaffold within 6 h and continuously proliferated for 5 days (Fig. 4A, B). Furthermore, hASCs and NHDFs in porcine ECM scaffolds showed good viability and spreading, as confirmed by a Live/Dead assay and SEM (Fig. 4C–F). Most cells attached onto the ECM scaffolds stained green fluorescence, indicating that they were still viable 5 days postseeding. Red fluorescence, which indicates dead cells, was hardly observed (Fig. 4C, E). On day 5 after seeding, all cells were spread out on the ECM scaffolds and integrated with ECM scaffolds (Fig. 4D, F). These results imply that the xenogeneic ECM derived from porcine adipose tissue can support and promote cell growth.
FIG. 4.
In vitro biocompatibility test of decellularized porcine ECM scaffolds. Cells were seeded onto porcine ECM scaffolds and cultured for 5 days. The number of attached cells was measured 6 h postseeding (A), and grown cells were analyzed using the WST-1 assay kit (B). Data are shown as means±standard deviations and are statistically significant at p<0.01. Fluorescence (C, E) and SEM images (D, F) of human adipose-derived stem cells (hASCs) (C, D) and normal human dermal fibroblasts (NHDFs) (E, F) grown on porcine ECM scaffolds on day 5. Green fluorescence caused by calcein AM with intracellular esterase indicated live cells, whereas red fluorescence caused by ethidium homodimers bound to nucleic acids indicated dead cells. Scale bars represent 100 μm (yellow) and 20 μm (black). Color images available online at www.liebertpub.com/tec
In vivo animal study
To test cell infiltration, early inflammation, and bioinductivity in the host tissue, an ECM suspension was injected subcutaneously into the backs of Slc/ICR mice. The injected ECM was easily identified throughout the experimental period of 6 weeks. Two days after injection, the grafts had a white-yellowish appearance (Fig. 5A). At 4 weeks, new yellow tissue and blood vessels were observed, and the graft volume was virtually maintained (Fig. 5B). Overall, the ECM grafts exhibited high stability and compatibility with the surrounding tissues.
FIG. 5.
Macroscopic appearance (A, B) and histological examination (C–F) of grafts in mice. An ECM suspension (10 mg/mL, 1 mL) was injected subcutaneously into the backs of female Slc/ICR mice (6-week old, n=10). Two days (A, C, E) and 4 weeks (B, D, F) after injection, the mice were sacrificed and the grafts were carefully explanted. The sections of the ECM graft were stained with H&E. Scale bars represent 0.5 cm (blue), 200 μm (black), and 100 μm (red), respectively. Color images available online at www.liebertpub.com/tec
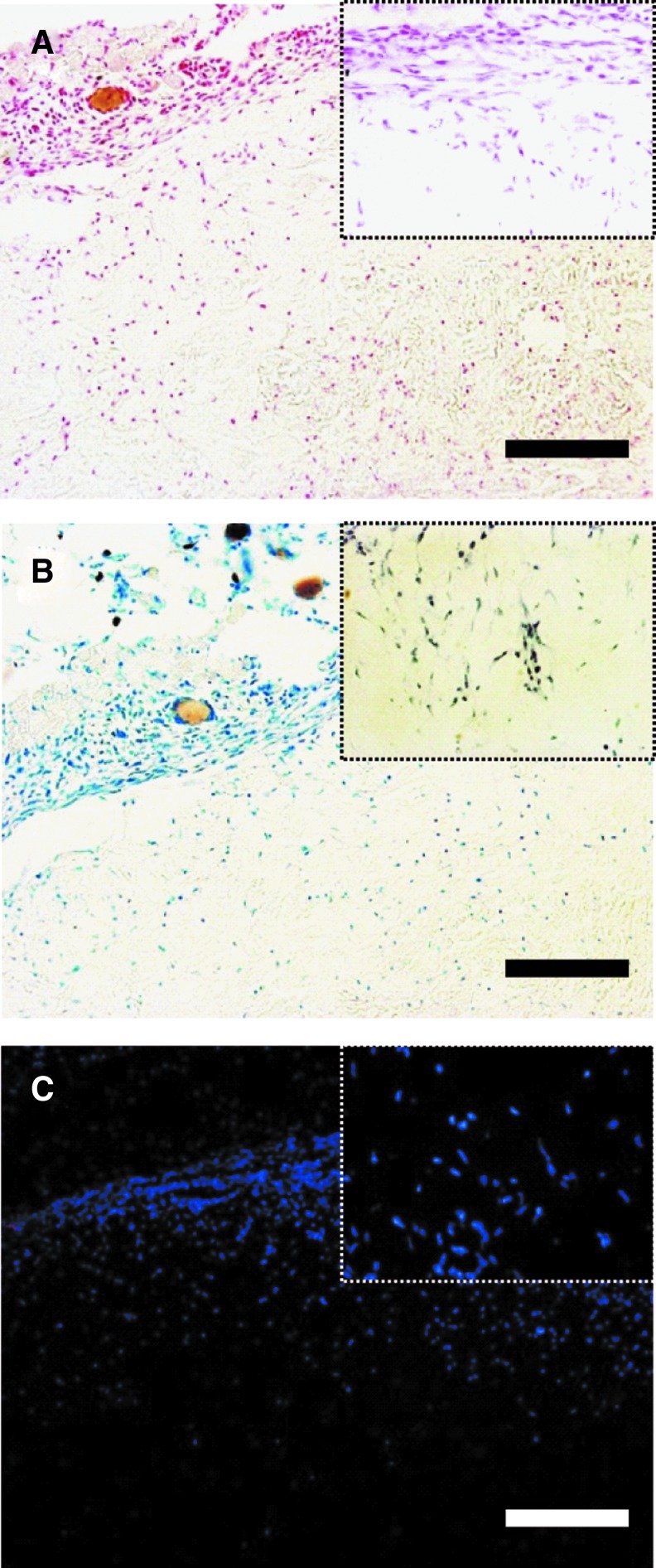
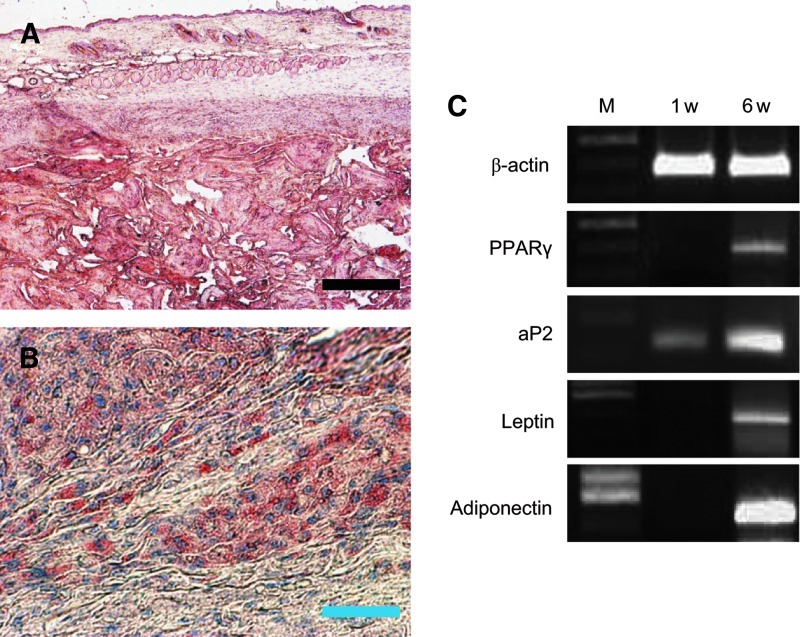
The H&E stain shows that host cells infiltrated into the xenogeneic ECM grafts (Fig. 5C–F). A number of host cells found at the center of the ECM grafts had no signs of inflammatory response or tissue necrosis. The results of the histological (Prussian blue and toluidine blue stain) and immunofluorescence (CD68) evaluations concretely showed that activated macrophages were hardly detected in the xenogeneic ECM graft (Fig. 6). Interestingly, 6 weeks after injection, newly formed adipose tissue was observed in the grafts. Oil-red O staining showed mature adipocytes with accumulated lipid droplets in the ECM grafts (Fig. 7A, B). The mouse adipogenic genes, PPAR, aP2, leptin, and adiponectin, were strongly expressed in the ECM grafts (Fig. 7C). Oil-red O staining and reverse transcription–PCR analysis of the graft confirmed that the xenogeneic ECM could provide biochemical cues for the promotion of specific differentiation in the host mouse cells, particularly adipogenesis.
FIG. 6.
Histological and immunofluorescence examinations of decellularized ECM grafts 2 days after injection. Prussian blue (A), toluidine blue (B), and anti-CD68 (C) stains were used to detect activated macrophages. The denoted areas show the magnified image. The scale bar represents 100 μm. Color images available online at www.liebertpub.com/tec
FIG. 7.
Adipogenic differentiation assessment in the decellularized porcine ECM graft using histological examination (A, B) and reverse transcription–polymerase chain reaction (C). An ECM suspension (10 mg/mL, 1 mL) was injected subcutaneously into the backs of the mice. After 6 weeks, grafts were stained by oil red O. Numerous oil drops were observed in the ECM grafts. The scale bars represent 200 μm (black) and 30 μm (blue). Adipogenic-specific genes were analyzed using mouse-origin primers designed for peroxisome-proliferative activated receptor gamma (PPARγ), adipocyte fatty acid-binding protein (aP2), leptin, and adiponectin. β-actin was used as an internal control. Color images available online at www.liebertpub.com/tec
Discussion
Decellularized ECM functions not only as a supporting material but also as a regulator of cellular behaviors, including cell survival, proliferation, morphogenesis, and differentiation.32Decellularized ECM is a useful matrix for tissue engineering, because it can mimic the compositions, microstructure, and biomechanical properties of the native ECM.14,33 Porcine tissues, including small intestine submucosa (SIS), bladder, dermis, pulmonary valves, adipose tissue, and tendons, have been used to overcome the supply and manufacturing limitations of human sources.6–11,34 However, the ECM derived from porcine tissues may raise some concerns associated with immune reaction, as well as xenogeneic disease transmission. The residual xenogeneic epitopes, such as cellular components and α-Gal epitopes, may be a major obstacle to its utility as a surgical material for tissue regeneration.35 Therefore, new and more efficient decellularization techniques should be established to eliminate xenogeneic epitopes.
A number of decellularization protocols have been developed to maximize the decellularization effect while minimizing any adverse effect on the composition, biological activity, integrity, and biomechanical properties of the remaining ECM.13 Recently, other groups reported methods for decellularization from human and porcine adipose tissue.10,35 They utilized solvents for lipid removal such as isopropanol, in addition to physical, chemical, and enzymatic treatment. In this study, ECM was isolated from porcine adipose tissue by simple pulverization under high temperature and effectively decellularized after a relatively short exposure to NaCl, SDS, and enzymes. Unlike formless human adipose tissue, porcine adipose tissue cannot be easily decellularized, because it is thick and dense. In addition, at low temperatures, lipids trapped within the adipose tissue may congeal, resulting in adverse effects on decellularization and lyophilization. The complete disruption of adipose tissue via homogenization at an appropriate temperature can improve the efficiency of decellularization. Chemical and enzymatic agents are required to remove cellular remnants and xenogeneic epitopes that adhere to ECM proteins.36 However, the use of chemical and enzymatic agents raises some concerns, due to the potential for the presence of residual cytotoxic agents in the decellularized ECM. In addition, any combination of chemical and enzymatic agents may not be able to completely remove cell components from a tissue or organ. Most commercially available biological scaffold materials contain a trace amount of remnant DNA, despite their use in clinical treatments.37
The α-Gal epitope is found in high density as a cell surface molecule in most species, with the notable exceptions of humans and Old World monkeys. It is the primary cause of rejection of xenogeneic organ transplants. In the present study, the optimized decellularization protocol was highly effective at removing DNA, cellular components, and the α-Gal epitope (Fig. 2). Moreover, the fibrous structure of the decellularized ECM was similar to that observed in intact ECM surrounding adipocytes (Fig. 1). These results support the finding that our decellularization protocol effectively eliminates xenogeneic epitopes and preserves the fibrous structure of the native ECM. Reducing the decellularization agents and processing time could be beneficial in terms of the costs and scalability of the approach.
Human and porcine adipose tissues have previously been reported as containing many ECM components, including collagen I, III, IV, and VII; elastin; laminin; fibronectin; and various bioactive molecules.10,22,38 The retention of ECM composition was a central goal during decellularization. Biochemical and histological analysis clearly showed that abundant ECM components, such as total collagen, sulfated GAGs, and elastin, were preserved in the decellularized porcine ECM (Fig. 3). In particular, sulfated GAGs, including heparin, sequester various growth factors such as basic fibroblast growth factor, insulin-like growth factor, and transforming growth factor-β, and also protect from denaturation.39 Thus, their presence in the decellularized ECM may be important in the retention of naturally present cytokines. The preserved ECM components would provide important biochemical signals for cell adhesion, proliferation, migration, and differentiation, and thus would be favorable for tissue repair and remodeling.40,41
After decellularization, the porcine ECM was fabricated into three-dimensional scaffolds with a variety of shapes, such as round dishes, sheets, microspheres, hollow tubes, and gels, by lyophilization.23,24 This is a well-established method used to stabilize the bioactivity of functional proteins and to make the scaffold easier to handle in physicomechanical and cellular biological evaluations (Supplementary Fig. S1). The scaffolds had highly porous structures and exhibited an elastic behavior (Supplementary Fig. S2). The results observed in physicomechanical testing suggest that decellularized ECM has the mechanical integrity necessary for use as an in vitro cell culture substrate. In vitro and in vivo experiments were performed to assess the biocompatibility of decellularized ECM (Figs. 4–7). The biochemical composition of the decellularized porcine ECM can support the adhesion and proliferation of hASCs and NHDFs. In the animal model, the macroscopic appearance of the decellularized ECM grafts was well conserved, and host cells infiltrated into grafts without an immune response. Histological staining and gene expression results 6 weeks after injection clearly indicate that decellularized ECM provides a favorable microenvironment for the adipogenic differentiation of host cells. Several groups have reported controlling adipogenesis using various chemical additives and paracrine signals.42 On the basis of our results, we suggest that decellularized ECM has a significant impact on cell fate and tissue reconstruction.
Conclusion
Porcine adipose tissue, which is easily obtained from food waste, was successfully decellularized through a protocol designed to extract an intact ECM. The xenogeneic epitopes, such as nucleic acid, cellular components, and α-Gal epitopes, were effectively removed while preserving ECM composition. Decellularized xenogeneic ECM provided a suitable substrate for the adhesion and proliferation of human cells in vitro and also showed potential as a biocompatible material for in vivo tissue reconstruction. The decellularized ECM derived from porcine adipose tissue could serve as a xenogeneic biomaterial and has a great potential for use in regenerative medicine.
Supplementary Material
Acknowledgments
This work was supported by the Basic Science Research Program (Grant No. 2009-0075546) and the Bio & Medical Technology Development Program (Grant No. 2011-0019774) through the National Research Foundation of Korea (NRF) funded by the Korean government (MEST). This work was also supported by the Seoul R&BD Program (Grant No. SS110011).
Disclosure Statement
No competing financial interests exist.
References
- 1.Ekser B. Cooper D.K.C. Overcoming the barriers to xenotransplantation: prospects for the future. Expert Rev Clin Immunol. 2010;6:219. doi: 10.1586/eci.09.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y.G. Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007;7:519. doi: 10.1038/nri2099. [DOI] [PubMed] [Google Scholar]
- 3.Cooper D.K.C. Ayares D. The immense potential of xenotransplantation in surgery. Int J Surg. 2011;9:122. doi: 10.1016/j.ijsu.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Badylak S.F. Gilbert T.W. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert T.W. Sellaro T.L. Badylak S.F. Decellularization of tissues and organs. Biomaterials. 2006;27:3675. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 6.de la Fuente S.G. Gottfried M.R. Lawson D.C. Harris M.B. Mantyh C.R. Pappas T.N. Evaluation of porcine-derived small intestine submucosa as a biodegradable graft for gastrointestinal healing. J Gastrointest Surg. 2003;7:96. doi: 10.1016/S1091-255X(02)00050-1. [DOI] [PubMed] [Google Scholar]
- 7.Reing J.E. Brown B.N. Daly K.A. Freund J.M. Gilbert T.W. Hsiong S.X., et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials. 2010;31:8626. doi: 10.1016/j.biomaterials.2010.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang B. Zhang Y. Zhou L. Sun Z. Zheng J. Chen Y., et al. Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering. Tissue Eng Part C Methods. 2010;16:1201. doi: 10.1089/ten.TEC.2009.0311. [DOI] [PubMed] [Google Scholar]
- 9.Bayrak A. Tyralla M. Ladhoff J. Schleicher M. Stock U.A. Volk H.D., et al. Human immune responses to porcine xenogeneic matrices and their extracellular matrix constituents in vitro. Biomaterials. 2010;31:3793. doi: 10.1016/j.biomaterials.2010.01.120. [DOI] [PubMed] [Google Scholar]
- 10.Brown B.N. Freund J.M. Han L. Rubin J.P. Reing J.E. Jeffries E.M., et al. Comparison of three methods for the derivation of a biologic scaffold composed of adipose tissue extracellular matrix. Tissue Eng Part C Methods. 2011;17:411. doi: 10.1089/ten.tec.2010.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeken C.R. White A.K. Bachman S.L. Ramshaw B.J. Cleveland D.S. Loy T.S., et al. Method of preparing a decellularized porcine tendon using tributyl phosphate. J Biomed Mater Res B Appl Biomater. 2011;96:199. doi: 10.1002/jbm.b.31753. [DOI] [PubMed] [Google Scholar]
- 12.Wainwright J.M. Czajka C.A. Patel U.B. Freytes D.O. Tobita K. Gilbert T.W., et al. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods. 2010;16:525. doi: 10.1089/ten.tec.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crapo P.M. Gilbert T.W. Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badylak S.F. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 15.De Ugarte D.A. Ashjian P.H. Elbarbary A. Hedrick M.H. Future of fat as raw material for tissue regeneration. Ann Plast Surg. 2003;50:215. doi: 10.1097/01.SAP.0000029661.38066.15. [DOI] [PubMed] [Google Scholar]
- 16.Hass M.J. Animal fats. In: Shahidi F., editor. Bailey's Industrial Oil and Fat Products. 6th. New York: John Wiley & Sons, Inc.; 2005. pp. 161–212. [Google Scholar]
- 17.Haraida S. Nerlich A.G. Wiest I. Schleicher E. Löhrs U. Distribution of basement membrane components in normal adipose tissue and in benign and malignant tumors of lipomatous origin. Mod Pathol. 1996;9:137. [PubMed] [Google Scholar]
- 18.Trujillo M.E. Scherer P.E. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- 19.Harunaga J.S. Yamada K.M. Cell-matrix adhesions in 3D. Matrix Biol. 2011;30:363. doi: 10.1016/j.matbio.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauney J. Volloch V. Progression of human bone marrow stromal cells into both osteogenic and adipogenic lineages is differentially regulated by structural conformation of collagen I matrix via distinct signaling pathways. Matrix Biol. 2009;28:239. doi: 10.1016/j.matbio.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi J.S. Kim B.S. Kim J.D. Choi Y.C. Lee E.K. Park K., et al. In vitro expansion of human adipose-derived stem cells in a spinner culture system using human extracellular matrix powders. Cell TissueRes. 2011;345:415. doi: 10.1007/s00441-011-1223-5. [DOI] [PubMed] [Google Scholar]
- 22.Choi J.S. Kim B.S. Kim J.Y. Kim J.D. Choi Y.C. Yang H.J., et al. Decellularized extracellular matrix derived from human adipose tissue as a potential scaffold for allograft tissue engineering. J Biomed Mater Res A. 2011;97:292. doi: 10.1002/jbm.a.33056. [DOI] [PubMed] [Google Scholar]
- 23.Choi J.S. Yang H.J. Kim B.S. Kim J.D. Kim J.Y. Yoo B., et al. Human extracellular matrix (ECM) powders for injectable cell delivery and adipose tissue engineering. J Control Release. 2009;139:2. doi: 10.1016/j.jconrel.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 24.Choi J.S. Yang H.J. Kim B.S. Kim J.D. Lee S.H. Lee E.K., et al. Fabrication of porous extracellular matrix scaffolds from human adipose tissue. Tissue Eng Part C Methods. 2010;16:387. doi: 10.1089/ten.TEC.2009.0276. [DOI] [PubMed] [Google Scholar]
- 25.Choi J.S. Kim B.S. Kim J.D. Choi Y.C. Lee H.Y. Cho Y.W. In vitro cartilage tissue engineering using adipose-derived extracellular matrix scaffolds seeded with adipose-derived stem cells. Tissue Eng Part A. 2012;18:80. doi: 10.1089/ten.tea.2011.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caliari S.R. Ramirez M.A. Harley B.A. The development of collagen-GAG scaffold-membrane composites for tendon tissue engineering. Biomaterials. 2011;32:8990. doi: 10.1016/j.biomaterials.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daamen W.F. van Moerkerk H.T. Hafmans T. Buttafoco L. Poot A.A. Veerkamp J.H., et al. Preparation and evaluation of molecularly-defined collagen-elastin-glycosaminoglycan scaffolds for tissue engineering. Biomaterials. 2003;24:4001. doi: 10.1016/s0142-9612(03)00273-4. [DOI] [PubMed] [Google Scholar]
- 28.Van R.L. Bayliss C.E. Roncari D.A. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J Clin Invest. 1976;58:699. doi: 10.1172/JCI108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bunnell B.A. Flaat M. Gagliardi C. Patel B. Ripoll C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods. 2008;45:115. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuk P.A. Zhu M. Ashjian P. De Ugarte D.A. Huang J.I. Mizuno H., et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed B. Varon J. Chait B.T. Kreek M.J. Carbon dioxide-induced anesthesia results in a rapid increase in plasma levels of vasopressin. Endocrinology. 2009;150:2934. doi: 10.1210/en.2008-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song J.J. Ott H.C. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med. 2011;17:424. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Mosser G. Anglo A. Helary C. Bouligand Y. Giraud-Guille M.M. Dense tissue-like collagen matrices formed in cell-free conditions. Matrix Biol. 2006;25:3. doi: 10.1016/j.matbio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Schenke-Layland K. Riemann I. Opitz F. Konig K. Halbhuber K.J. Stock U.A. Comparative study of cellular and extracellular matrix composition of native and tissue engineered heart valves. Matrix Biol. 2004;23:113. doi: 10.1016/j.matbio.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Xu H. Wan H. Zuo W. Sun W. Owens R.T. Harper J.R., et al. A porcine-derived acellular dermal scaffold that supports soft tissue regeneration: removal of terminal galactose-alpha-(1,3)-galactose and retention of matrix structure. Tissue Eng Part A. 2009;15:1807. doi: 10.1089/ten.tea.2008.0384. [DOI] [PubMed] [Google Scholar]
- 36.Stone K.R. Ayala G. Goldstein J. Hurst R. Walgenbach A. Galili U. Porcine cartilage transplants in the cynomolgus monkey. III. Transplantation of alpha-galactosidase-treated porcine cartilage. Transplantation. 1998;65:1577. doi: 10.1097/00007890-199806270-00007. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert T.W. Freund J.M. Badylak S.F. Quantification of DNA in biologic scaffold materials. J Surg Res. 2009;152:135. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flynn L.E. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials. 2010;31:4715. doi: 10.1016/j.biomaterials.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 39.Taipale J. Keski-Oja J. Growth factors in the extracellular matrix. FASEB J. 1997;11:51. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- 40.Hoshiba T. Lu H. Kawazoe N. Chen G. Decellularized matrices for tissue engineering. Expert Opin Biol Ther. 2010;10:1717. doi: 10.1517/14712598.2010.534079. [DOI] [PubMed] [Google Scholar]
- 41.Nelson C.M. Tien J. Microstructured extracellular matrices in tissue engineering and development. Curr Opin Biotechnol. 2006;17:518. doi: 10.1016/j.copbio.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Hemmrich K. von Heimburg D. Cierpka K. Haydarlioglu S. Pallua N. Optimization of the differentiation of human preadipocytes in vitro. Differentiation. 2005;73:28. doi: 10.1111/j.1432-0436.2005.07301003.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.